The Effect of Selected Fruit (Apple, Bitter Orange and Grape) Juice Concentrates Used as Osmotic Agents on the Osmotic-Dehydration Kinetics and Physico-Chemical Properties of Pomegranate Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Preparation of Osmotic Solutions
2.3. Osmodehydration Process
2.4. Mass Transfer Kinetics
2.5. Physico-Chemical Analyses
2.5.1. Dry Matter
2.5.2. pH and Water Activity
2.5.3. Brix Degree
2.5.4. Color
2.5.5. Total Phenols
2.5.6. Antioxidant Activity
2.6. Hardness Analysis
2.7. Viscosity Analysis
2.8. Hedonic Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Kinetics of Osmotic Dehydration
3.2. Evolution of Physico-Chemical Properties of PSs after OD
3.3. Hardness of PSs
3.4. Evolution of Physico-Chemical Properties of Osmotic Solutions after OD
3.5. Apparent Viscosity of Apple, Bitter-Orange and Grape Juices
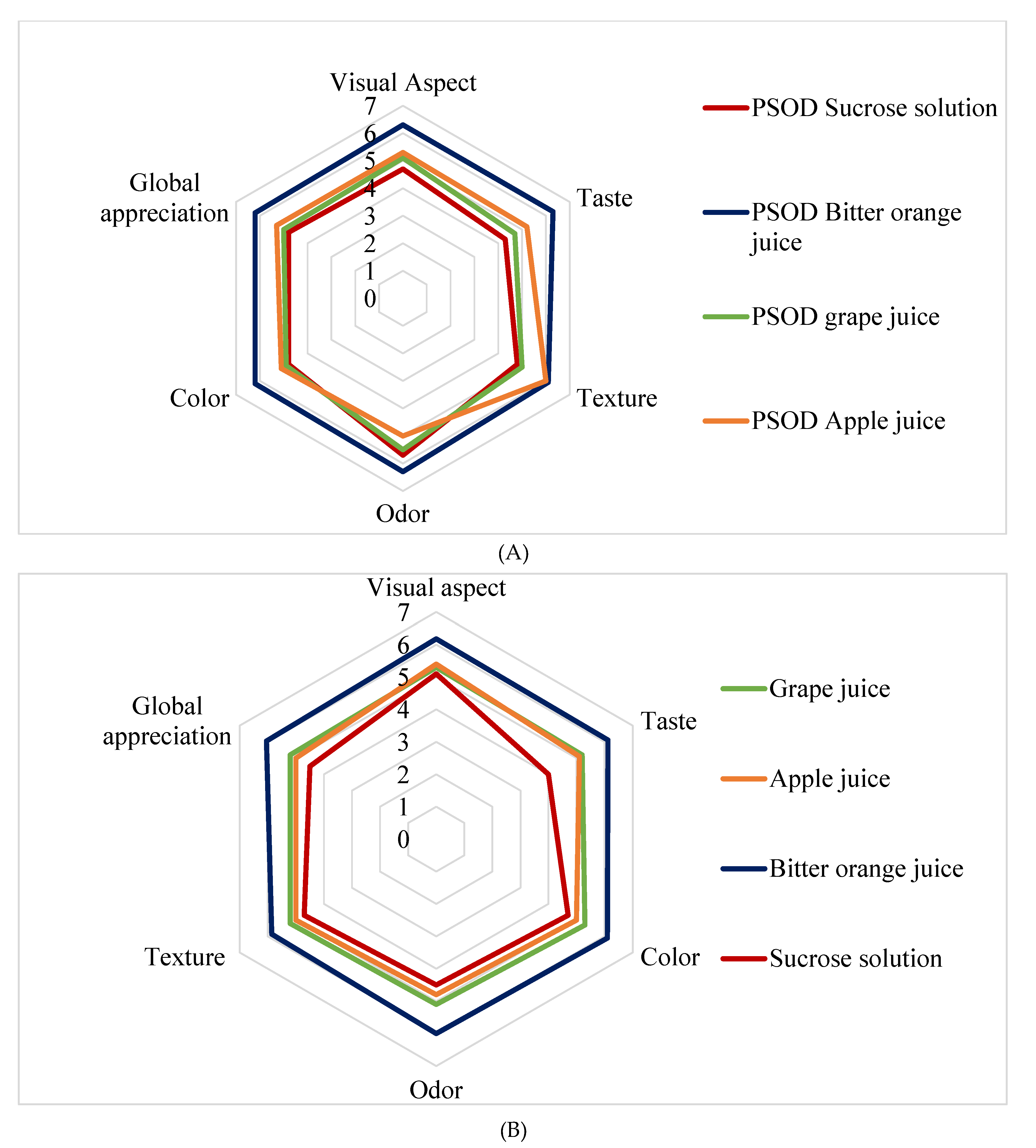
3.6. Hedonic Evaluation of PSs and OSs
3.6.1. Pomegranate Seeds
3.6.2. Osmotic Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amami, E. Amélioration de la Déshydratation Osmotique des Produits Végétaux par Champ Électrique Pulsé. Available online: https://bibliotheque.utc.fr/Default/doc/SYRACUSE/199893/amelioration-de-la-deshydratation-osmotique-des-produits-vegetaux-par-champ-electrique-pulse (accessed on 9 June 2022).
- Masztalerz, K.; Łyczko, J.; Lech, K. Effect of Filtrated Osmotic Solution Based on Concentrated Chokeberry Juice and Mint Extract on the Drying Kinetics, Energy Consumption and Physicochemical Properties of Dried Apples. Molecules 2021, 26, 3274. [Google Scholar] [CrossRef] [PubMed]
- Bchir, B.; Rabetafika, H.N.; Paquot, M.; Blecker, C. Effect of pear, apple and date fibres from cooked fruit by-products on dough performance and bread quality. Food Bioprocess Technol. 2014, 7, 1114–1127. [Google Scholar] [CrossRef]
- Nono, Y.J.; Nuadje, G.B.; Raoult-Wack, A.-L.; Giroux, F. Comportement de certains fruits tropicaux traités par déshydratation-imprégnation par immersion dans une solution de saccharose. Fruits 2001, 56, 75–83. [Google Scholar] [CrossRef]
- Nono, Y.J.; Reynes, M.; Zakhia, N.; Raoult-Wack, A.-L.; Giroux, F. Mise au point d’un procédé combiné de déshydratation imprégnation par immersion et séchage de bananes (Musa acuminata groupe Cavendish). J. Food Eng. 2002, 55, 231–236. [Google Scholar] [CrossRef]
- Kowalska, H.; Lenart, A.; Leszczyk, D. The effect of blanching and freezing on osmotic dehydration of pumpkin. J. Food Eng. 2008, 86, 30–38. [Google Scholar] [CrossRef]
- Bchir, B.; Besbes, S.; Attia, H.; Blecker, C. Osmotic dehydration of pomegranate seeds: Mass transfer kinetics and differential scanning calorimetry characterization. Int. J. Food Sci. Technol. 2009, 44, 2208–2217. [Google Scholar] [CrossRef]
- Besbes, S.; Drira, L.; Blecker, C.; Deroanne, C.; Attia, H. Adding value to hard date (Phoenix dactylifera L.): Compositional, functional and sensory characteristics of date jam. Food Chem. 2009, 112, 406–411. [Google Scholar] [CrossRef]
- Sarawong, C.; Schoenlechner, R.; Sekiguchi, K.; Berghofer, E.; Ng, P.K. Effect of extrusion cooking on the physicochemical properties, resistant starch, phenolic content and antioxidant capacities of green banana flour. Food Chem. 2014, 143, 33–39. [Google Scholar] [CrossRef]
- Bchir, B.; Besbes, S.; Karoui, R.; Paquot, M.; Attia, H.; Blecker, C. Osmotic dehydration kinetics of pomegranate seeds using date juice as an immersion solution base. Food Bioprocess Technol. 2012, 5, 999–1009. [Google Scholar] [CrossRef]
- Sharif, N.; Pirouzifard, M.; Alizadeh, M.; Esmaiili, M. Concentrated fruit juice as an osmotic solution in production of candied apple. Agro Food Ind. Hi Tech. 2013, 24, 73–76. [Google Scholar]
- Samborska, K.; Eliasson, L.; Marzec, A.; Kowalska, J.; Piotrowski, D.; Lenart, A.; Kowalska, H. The effect of adding berry fruit juice concentrates and by-product extract to sugar solution on osmotic dehydration and sensory properties of apples. J. Food Sci. Technol. 2019, 56, 1927–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bchir, B.; Besbes, S.; Karoui, R.; Attia, H.; Paquot, M.; Blecker, C. Effect of air-drying conditions on physico-chemical properties of osmotically pre-treated pomegranate seeds. Food Bioprocess Technol. 2012, 5, 1840–1852. [Google Scholar] [CrossRef]
- Bchir, B.; Besbes, S.; Giet, J.-M.; Attia, H.; Blecker, C. Synthèse des connaissances sur la déshydratation osmotique. BASE 2011, 15, 129–142. [Google Scholar]
- Akdowa, E.P. Optimisation de l’Utilisation du Taro (Colocasia esculenta) Variété Lamba en Panification par l’Usage de la Gomme Grewia mollis. Juss (Famille Tiliaceae). Ph.D. Thesis, Université de Lorraine, Nancy, France, 2014. [Google Scholar]
- Kchaou, W.; Abbès, F.; Blecker, C.; Attia, H.; Besbes, S. Effects of extraction solvents on phenolic contents and antioxidant activities of Tunisian date varieties (Phoenix dactylifera L.). Ind. Crops Prod. 2013, 45, 262–269. [Google Scholar] [CrossRef]
- Serea, C.; Barna, O. Phenolic content and antioxidant activity in oat. Food Sci. Technol. 2011, 12, 164–168. [Google Scholar]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Mechraoui, N.; Belkhadem, S. Essai d’Incorporation de la Farine de Dattes Variétés «Mech-Degla» en Biscuiterie. Master’s Thesis, Université Echahid Hamma Lakhdar, El-oued, Algeria, 2009. [Google Scholar]
- Masmoudi, M.; Besbes, S.; Blecker, C.; Attia, H. Preparation and characterization of osmodehydrated fruits from lemon and date by-products. Food Sci. Technol. Int. 2007, 13, 405–412. [Google Scholar] [CrossRef]
- Briois, E.; Dusautois, C.; Heno, P. Applications alimentaires des sirops de glucose à haute teneur en maltose. Ind. Aliment. Et Agric. 1998, 115, 79–81. [Google Scholar]
- Žilić, S.; Vančetović, J.; Janković, M.; Maksimović, V. Chemical composition, bioactive compounds, antioxidant capacity and stability of floral maize (Zea mays L.) pollen. J. Funct. Foods 2014, 10, 65–74. [Google Scholar] [CrossRef]
- Macheix, J.-J.; Fleuriet, A.; Jay-Allemand, C. Les Composés Phénoliques Des Végétaux: Un Exemple de Métabolites Secondaires D’importance Économique; PPUR Presses Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 2005. [Google Scholar]
- Delgado, A.; Rubiolo, A.C. Microstructural changes in strawberry after freezing and thawing processes. LWT Food Sci. Technol. 2005, 38, 135–142. [Google Scholar] [CrossRef]
- Fernandes, F.A.; Oliveira, F.I.; Rodrigues, S. Use of ultrasound for dehydration of papayas. Food Bioprocess Technol. 2008, 1, 339–345. [Google Scholar] [CrossRef]
- Azuara, E.; Flores, E.; Beristain, C.I. Water diffusion and concentration profiles during osmodehydration and storage of apple tissue. Food Bioprocess Technol. 2009, 2, 361–367. [Google Scholar] [CrossRef]

| Parameter | PSs Before OD | PSs After OD | ||||
|---|---|---|---|---|---|---|
| Sucrose Solution | Bitter-Orange Juice | Apple Juice | Grape Juice | |||
| Dry matter | 0.19 ± 0.01 a | 0.32 ± 0.01 b | 0.34 ± 0.04 c | 0.40 ± 0.73 d | 0.40 ± 1.39 d | |
| Brix | 15.16 ± 0.28 a | 29.05 ± 0.90 b | 33.16 ± 1.06 c | 36.23 ± 1.55 d | 39.13 ± 0.92 e | |
| aw | 0.948 ± 0.001 d | 0.911 ± 0.002 b | 0.906 ± 0.008 a | 0.932 ± 0.005 c | 0.956 ± 0.001 e | |
| CIE lab parameters | L* | 29.12 ± 0.58 b | 31.92 ± 0.91 d | 32.30 ± 1.19 e | 27.82 ± 0.79 a | 30.06 ± 0.10 c |
| a* | 10.39 ± 0.79 c | 8.69 ± 0.42 a | 11.07 ± 1.86 d | 9.68 ± 0.17 b | 8.76 ± 0.14 a | |
| b* | 11.54 ± 0.21 c | 12.69 ± 0.24 d | 8.53 ± 0.48 a | 12.00 ± 0.14 d | 9.40 ± 0.34 b | |
| C* | 15.34 ± 0.18 c | 15.77 ± 0.14 c | 13.99 ± 1.78 b | 15.05 ± 0.27 c | 12.85 ± 0.34 a | |
| h° | 44.84 ± 3.45 b | 55.78 ± 1.61 e | 38.07 ± 2.62 a | 50.68 ± 0.39 d | 47.00 ± 1.18 c | |
| Browning index | 0.050 ± 0.002 a | 0.090 ± 0.007 d | 0.062 ± 0.004 c | 0.054 ± 0.002 b | 0.049 ± 0.009 a | |
| Total phenols (meqGA/100 g) | 384.22 ± 25.65 e | 341.31 ± 21.13 c | 376.49 ± 17.61 d | 256.03 ± 17.69 a | 314.10 ± 21.48 b | |
| IC50 (mg/mL) | 57.43 ± 1.23 d | 59.55 ± 0.45 e | 52.78 ± 0.98 c | 49.80 ± 1.09 b | 49.25 ± 0.78 a | |
| Hardness (N) | 0.66 ± 0.09 e | 0.48 ± 0.08 b | 0.36 ± 0.06 a | 0.55 ± 0.12 d | 0.53 ± 0.09 c | |
| Parameters | Sucrose Solution | Bitter-Orange Juice | Apple Juice | Grape Juice | |||||
|---|---|---|---|---|---|---|---|---|---|
| Before OD | After OD | Before OD | After OD | Before OD | After OD | Before OD | After OD | ||
| Dry matter (%) | 0.55 ± 0.09 b | 0.45 ± 0.07 a | 0.51 ± 0.08 b | 0.43 ± 0.06 a | 0.57 ± 0.10 b | 0.45 ± 0.04 a | 0.53 ± 0.09 b | 0.46 ± 0.03 a | |
| Brix | 55.00 ± 0.00 b | 50.69 ± 0.41 a | 55.00 ± 0.00 b | 49.66 ± 0.76 a | 55.00 ± 0.00 b | 49.23 ± 0.05 a | 55.00 ± 0.00 b | 49.63 ± 0.11 a | |
| pH | 6.41 ± 0.22 b | 5.56 ± 0.21 a | 2.67 ± 0.01 a | 2.71± 0.02 a | 4.42 ± 0.13 b | 4.37 ± 0.05 a | 3.90 ± 0.04 b | 3.81 ± 0.02 a | |
| CIE lab parameters | L* | 13.84 ± 1.49 a | 14.55 ± 1.45 b | 23.02 ± 0.88 b | 22.25 ± 0.62 a | 21.27 ± 0.13 a | 21.74 ± 0.07 a | 29.30 ± 1.00 a | 28.90 ± 0.97 a |
| a* | 0.15 ± 0.01 b | −0.06 ± 0 a | −1.07 ± 0.37 a | 1.77 ± 0.77 b | 5.54 ± 0.34 a | 6.13 ± 1.06 b | 4.22 ± 0.24 a | 4.47 ± 0.28 b | |
| b* | 0.12 ± 0.01 a | 0.44 ± 0.02 b | 9.58 ± 0.62 b | 8.39 ± 0.52 a | 11.32 ± 0.24 a | 12.11 ± 0.59 b | 12.29 ± 1.22 b | 11.67 ± 1.25 a | |
| C* | 0.19 ± 0.01 a | 0.44 ± 0.04 b | 9.65 ± 0.57 b | 8.59 ± 0.65 a | 12.69 ± 0.04 a | 13.10 ± 0.24 b | 12.99 ± 0.65 a | 12.50 ± 0.68 a | |
| h° | 266.26 ± 2.48 b | 149.41 ± 6.40 a | 96.47 ± 2.54 b | 78.29 ± 4.53 a | 62.47 ± 2.89 a | 67.93 ± 4.97 b | 71.09 ± 0.26 b | 69.08 ± 0.38 a | |
| Total phenols (meqGA/100 g) | 0.88 ± 0.07 a | 6.19 ± 0.56 b | 31.03 ± 1.32 a | 60.17 ± 2.33 b | 14.31 ± 0.54 a | 32.06 ± 0.92 b | 54.13 ± 2.12 a | 79.82 ± 3.16 b | |
| IC50 (mg/mL) | ND | 2.41 ± 0.45 | 0.26 ± 0.09 a | 0.50 ± 0.07 b | 0.18 ± 0.01 a | 0.40 ± 0.01 b | 0.28 ± 0.01 a | 0.35 ± 0.01 b | |
| Viscosity (mPa·s) | 72 ± 2 | 57 ± 4 | 80 ± 5 | 59 ± 3 | 80 ± 2 | 55 ± 1 | 80 ± 2 | 62 ± 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebii, H.; Bouaziz, M.A.; Sghaier, K.; Danthine, S.; Blecker, C.; Besbes, S.; Attia, H.; Bchir, B. The Effect of Selected Fruit (Apple, Bitter Orange and Grape) Juice Concentrates Used as Osmotic Agents on the Osmotic-Dehydration Kinetics and Physico-Chemical Properties of Pomegranate Seeds. Seeds 2022, 1, 198-209. https://doi.org/10.3390/seeds1030017
Sebii H, Bouaziz MA, Sghaier K, Danthine S, Blecker C, Besbes S, Attia H, Bchir B. The Effect of Selected Fruit (Apple, Bitter Orange and Grape) Juice Concentrates Used as Osmotic Agents on the Osmotic-Dehydration Kinetics and Physico-Chemical Properties of Pomegranate Seeds. Seeds. 2022; 1(3):198-209. https://doi.org/10.3390/seeds1030017
Chicago/Turabian StyleSebii, Haifa, Mohamed Ali Bouaziz, Khadija Sghaier, Sabine Danthine, Christophe Blecker, Souhail Besbes, Hamadi Attia, and Brahim Bchir. 2022. "The Effect of Selected Fruit (Apple, Bitter Orange and Grape) Juice Concentrates Used as Osmotic Agents on the Osmotic-Dehydration Kinetics and Physico-Chemical Properties of Pomegranate Seeds" Seeds 1, no. 3: 198-209. https://doi.org/10.3390/seeds1030017
APA StyleSebii, H., Bouaziz, M. A., Sghaier, K., Danthine, S., Blecker, C., Besbes, S., Attia, H., & Bchir, B. (2022). The Effect of Selected Fruit (Apple, Bitter Orange and Grape) Juice Concentrates Used as Osmotic Agents on the Osmotic-Dehydration Kinetics and Physico-Chemical Properties of Pomegranate Seeds. Seeds, 1(3), 198-209. https://doi.org/10.3390/seeds1030017









