Abstract
Carignan is a black grape cultivar widely planted throughout the western Mediterranean Basin. The grape faces significant viticultural hazards such as soil salinization, which affects about 6% of the world’s total land area. The search for salt tolerance genotypes to be introduced in crossbreeding programs and obtaining new cultivars is a key factor. The seed germination and salt tolerance of Carignan were studied from different coastal vineyards across the Mediterranean Basin, and as well as whether the distance from the sea affected germination and salt tolerance. Carignan seeds, independently of the temperature and distance from the sea, germinated more than 50% under 125 mM NaCl concentrations. Seed recovery was elevated, including the capacity of gemination after high salt exposure (500 mM NaCl). The results on germination behavior related to the distance from the sea showed that all tested vineyards, except for the one farthest from the sea, had similar germination responses. The optimum germination condition to select salt-tolerant accessions is alternating temperatures 25/10 °C and 125 mM NaCl. Thanks to the ability of the Carignan to germinate in a saline substrate and their capacity for recovery, it could be useful to crossbreeding programs, for integrating as rootstock selection or for the improvement of cultivars through sexual reproduction.
1. Introduction
The grape faces significant viticultural hazards such as soil salinization, which is a major factor contributing to the loss of the productivity of soils [1]. Recently, FAO [2] reported that worldwide, over 20% of the total irrigated areas are negatively affected by soil salinization problems. Moreover, if left unattended, this problem could expand to over 50% of these areas by 2050 [3]. Globally, more than 800 million hectares of agricultural land have been subjected to soil salinity [4]. This phenomenon widely occurs in the arid and semi-arid areas, which are important grape-producing regions [5,6]. The amount of European Union agricultural land destroyed by salt accumulation each year is estimated to be one million hectares, mainly in the Mediterranean countries, and is a major cause of desertification [7].
In the Mediterranean Basin, land degradation associated with soil alkalization may worsen at increasing rates in the coming decades because of several factors, such as the use of contaminated water for irrigation, intensive farming, poor drainage, and climate change [1,4]. To face this situation, the search for salt tolerance cultivar genotypes to be introduced in crossbreeding programs and obtaining new cultivars is a key factor for crop improvement and increased resilience to face climate change and its consequences [8,9]. Both the salt tolerance and sensitivity of a specific crop depend on its ability to extract water and nutrients from saline soils and to avoid the excessive tissue accumulation of salt ions [10]. The salt sensitivity changes during the various plant growth stages. Often, plants at early growth stages (seedling and establishment) are more sensitive to salt stress than plants at later stages [9,11]. For example, many studies [12,13,14,15] have reported the effect of salinity in inhibiting seed germination, reducing growth and photosynthesis, and altering physiological metabolism, eventually resulting in decreased berry yield and grape plant quality. On the other hand, to mitigate these negative effects, it is necessary to design and identify viable options that are environmentally and economically applicable for sustainable agriculture.
Many studies have focused on selecting salt-tolerant genotypes and varieties [16,17,18,19] as a promising method to benefit from poor water quality and salinized lands. However, choosing suitable selection criteria depending on complex tolerance mechanisms and the diverse plant responses exhibited at various stages of development can promote the identification of useful genotypes [20]. Germination, which determines seedling vigour and plant development, is the most important growth stage but is sensitive to salinity stress [21].
This study was based on the Carignan cultivar, given its important geographical distribution in the Mediterranean Basin; it is traditionally planted across the coastal areas and, in some cases, by small winegrowers as ungrafted plants. Carignan, a black berry grape (Vitis vinifera L. subsp. vinifera), is a late budding and ripening grape that requires a warm climate to achieve full physiological ripeness [22]. Despite its Spanish origin, it is widely planted throughout the Western Mediterranean Basin and around the world [22]. Carignan is mostly planted in France, followed by Spain and Italy, where it is an allowed grape variety in several Appellation d’Origine Contrôlée (AOC), Denominación de Origen Controlada (DOC) and Denominazione di Origine Controllata (DOC) regions, respectively. Elsewhere in Europe and the Middle East, plantings of this cultivar can be found in Croatia, Cyprus, Malta, Turkey, and Israel [20]. In Africa, plantings of Carignan are also found in the North African wine-producing areas of Morocco and Tunisia as well as in South Africa [23].
The aims of this work were to study the seed germination and salt tolerance of the Carignan grape cultivar from different coastal vineyards across the Mediterranean Basin and to evaluate the effect of the distance from the sea on its germination and salt tolerance.
2. Materials and Methods
2.1. Samples Seeds and Vineyards Characteristics
This study was carried out on ripe Carignan seeds of three different areas across the Mediterranean Basin (Italy, France, and Tunisia; see Table 1 for further details). From each country, three vineyards were chosen, one close to the sea (0–5 Km) with high salt input, one intermediate (6–15 Km) with medium salt input, and one far (16–30 Km) with no salt input, except for Tunisia, where this distance was not found because Carignan is only planted close to the sea. In addition, vineyards of different ages and rootstocks were chosen (Table 1).

Table 1.
Sites, collection data, and seed lot details for the growing sites of Carignan vineyards under study. N/A indicates unavailable data.
The berries of Carignan were collected at the time of natural ripening from more than 30 individuals of each vineyard, as representatives for the entire vineyard. The seeds were manually cleaned and were stored under controlled conditions (20 °C and 40% of relative humidity) for two weeks at the Sardinian Germplasm Bank (hereafter BG-SAR) of the University of Cagliari until the germination tests began. In Italy, the Carignan cultivar is grown mostly ungrafted on 2000 hectares of land in the South-Western part of Sardinia, which is mainly characterized by sandy soil [24]. In Tunisia, the main area for viticulture is situated in the North-East region Cap-Bon, which is the main grape-producing area in the country, accounting for 60% of grape production [25]. In France, Carignan is widely found in the south, particularly in the Languedoc wine regions of Aude, Gard, and Hérault. Plantings of Carignan are mostly limited to the warm Mediterranean climates of Southern France given the grape’s inability to sufficiently ripen much further north in the continental climate of Central France or in the damp maritime climate of Southwest France [23].
In five of the selected vineyards, the Carignan plants were grafted onto 110 Richter (V. berlandieri × V. rupestris) rootstocks in France and onto 1103 Paulsen (V. berlandieri × V. rupestris) rootstocks in Italy. In the other vineyards, Carignan was from ungrafted plants.
2.2. Germination Tests
The seeds of Vitis ssp. are usually physiologically dormant and may require considerable periods of cold stratification before they will germinate e.g., [26,27,28]. Investigations on the wild grape (V. vinifera subsp. sylvestris (C.C.Gmel.) Hegi) have shown that germination necessarily takes place after a cold stratification period of 60/90 days [28]. To conduct all the tests, three replicates of 25 seeds for each temperature condition and for different pre-treatments were sown on 1% water agar substrate in plastic Petri dishes of 90 mm diameter under a laminar flow hood (Faster KB).
To break grape seed dormancy and obtain an optimal germination process as well as carry out salt tolerance experiments of the Carignan seeds, the following treatments and pre-treatments were applied: (1) control, without any pre-treatment, incubated directly in germination conditions (see below for further details). (2) Pre-chilling or cold stratification period at 5 °C for three months in a growth chamber (MLR-351, SANYO, Moriguchi, Japan). (3) Acid scarification followed by cold stratification; here, the seeds were exposed to a 37% HCl and water solution (1:1) and, after 10 min, washed with sterile water and placed in the agar substrate. With this pre-treatment, we tested whether the interaction with the digestive tract of animals (mainly birds) increased the final germination, via the simulation of the passage through the digestive tract with the acid exposition, this following a cold period of stratification at 5 °C for three months.
After the cold stratification period, additional three replicates of 25 seeds were sown at different NaCl concentrations (0, 125, 250, 500 mM, these NaCl concentrations were selected in order to simulate seawater at 25%, 50%, and 100%), following the experimental design of [14] for the wild grape and incubated at different temperatures (25 °C, 25/10 °C, 30 °C) in 12 h of light. At the 25/10 °C temperature regime, the higher temperature coincided with the light period. The criterion for germination was the visible radicle protrusion (≥1 mm). The seeds were scored twice by week. After two consecutive weeks without additional germination under control conditions (0 mM NaCl), the non-germinated seeds were washed with distilled water and then sown in new Petri dishes containing 1% water agar substrate for an additional 30 days (recovery phase) at the same incubation temperatures, to evaluate the recovery percentages (hereafter RP). At the end of the tests (for a minimum of 90 days), the viability of each remaining seed was checked by a cut test with a scalpel and subsequent observation of the seed embryo under a binocular microscope [29].
2.3. Data Analysis
The final germination percentages (FGPs) were calculated as the mean of three replicates (±SD), and the RP was calculated with the number of seeds germinated after they were washed with distilled water, according to the following equation: RP = {[(a − b)/(c − b)] × 100}, where a is the total number of seeds germinated in salt solutions plus those that recovered to germination in fresh water, b is the total number of seeds germinated in saline solutions, and c is the total number of seeds.
Generalized linear models (GLMs) were used to evaluate the effect of pre-treatments, distance from the sea, incubation temperatures, rootstock type, and vineyard year on the FGP and RP. A log link function and quasibinomial error structure were used for analyzing the FGP and RP results. Significant differences highlighted by the GLMs were then analyzed by a post hoc pairwise comparison t-test (with Bonferroni adjustment). All the statistical analyses were carried out using R v. 3.1.3 [30].
3. Results
3.1. Seed Germination and Salt Tolerance
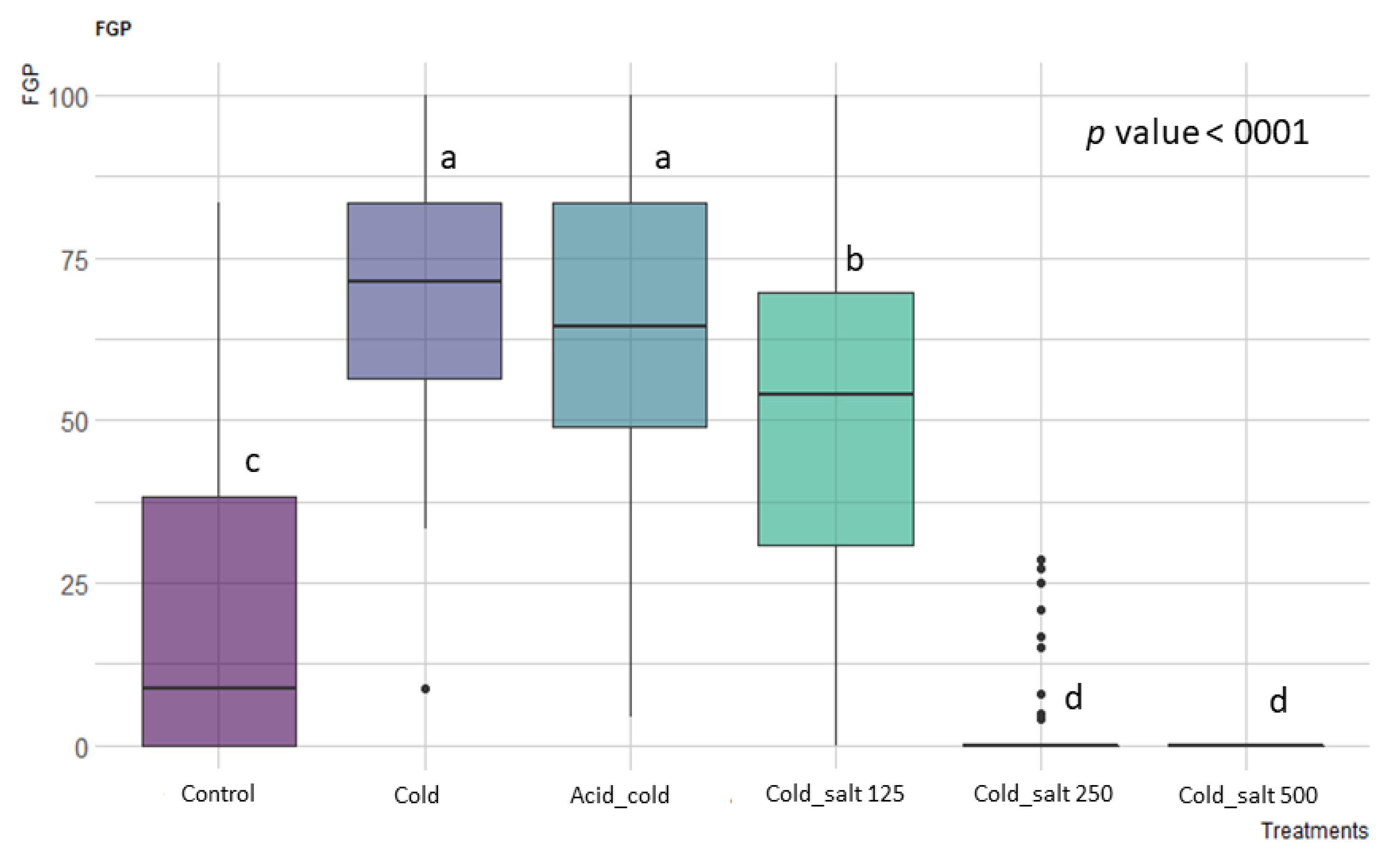
The different pre-treatments and the salinity of the growing substrate showed a significant effect (p < 0.001, Table S1). For all the eight vineyards tested, independently of the tested temperature, the highest germination percentage was recorded under the non-saline substrate and after a cold stratification period of three months at 5 °C (see Figure 1). Subsequent pairwise comparisons using t-tests showed no statistically significant differences among the combinations of pre-treatments, e.g., acid scarification followed by three months of a cold period and only cold stratification (Figure 1). This implies that Carignan cultivar seeds are physiologically dormant, as already described for other species of this genus [27,28], and the pre-treatment with the chloridic acid did not increase the FGP (p > 0.05, Figure 1).
Figure 1.
Effect of treatment and pre-treatments on the final germination percentage (FGP), considering all temperatures tested. Post hoc pairwise t-test comparisons were carried out for each condition and bars with different letters indicate significant differences (p < 0.05).
Germination on the saline substrate with 125 mM after a period of cold stratification showed a higher FGP than the control (p < 0.05, Figure 1), indicating salt tolerance behavior at low salinity levels. On the contrary, the Carignan seeds did not tolerate the salt substrate with concentrations higher than 250 mM (p < 0.001). The incubation temperature also had a significant effect (p = 0.0067, Table S1) on the seed germination of Carignan cultivar accessions. The best temperature condition was the alternate 25/10 °C (p = 0.0357, post hoc pairwise t-test comparison).
In addition, the vineyard years or the rootstock type did not affect the germination of Carignan (p = 0.961 and p = 0.394, respectively).
3.2. Seed Recovery Ability after Salt Exposure
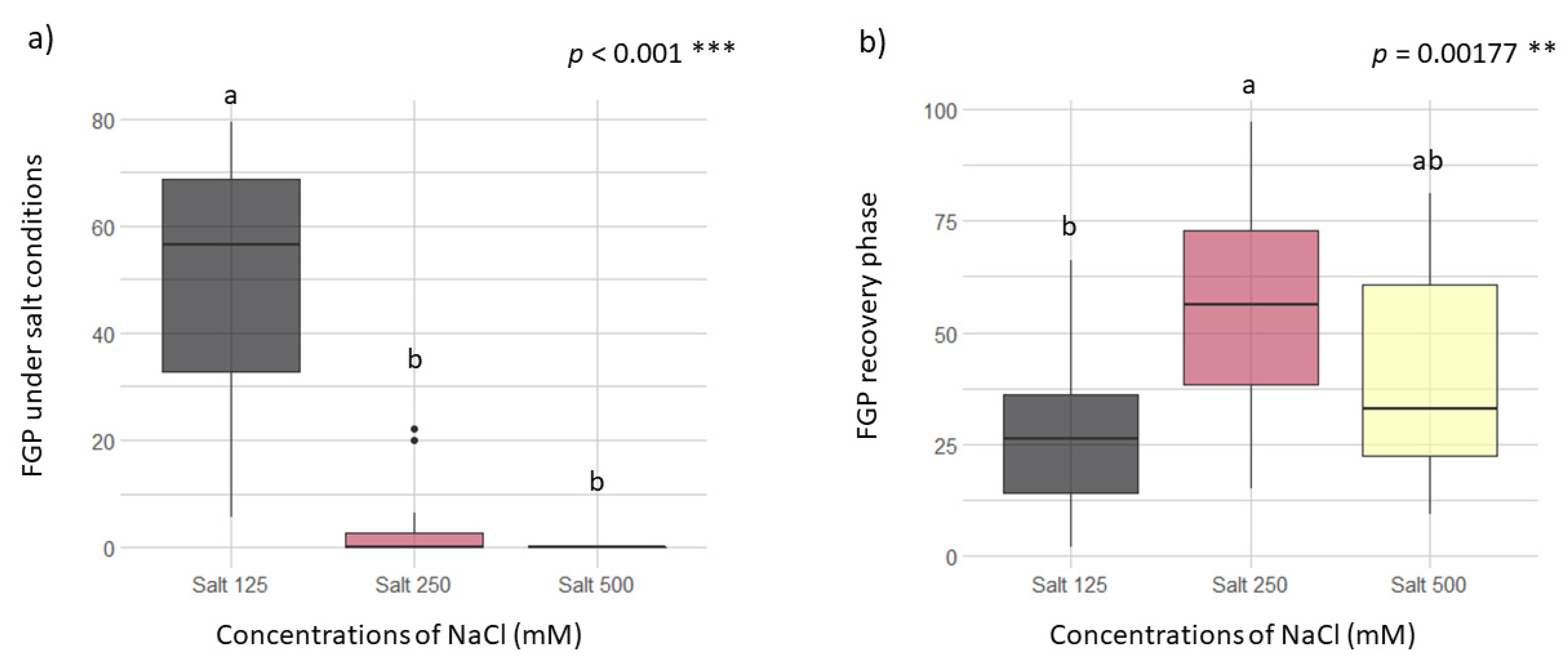
Concentrations of NaCl of 125 mM on the incubation substrate are the maximum salinity level (within tested conditions) to which the seeds were able to germinate (Figure 2a). No significant differences between the NaCl concentrations of 250 and 500 mM were found (p > 0.05 Figure 2a). At these concentrations, seed recovery capacity is influenced by the distance from the sea (p = 0.0344, Table S1) but not for the origin of the vineyards (p > 0.05). Taking into consideration the capacity of recovery after the salt exposition of 250 mM did not show significant differences with the recovery after the salt exposition of 500 mM (p > 0.05, Figure 2b). The highest FGP in the RP after 500 mM salt exposition was 25/10 °C in all the vineyards tested (Figure 3). In general, the highest salinity concentration (500 mM) allowed the recovery of more than ca. 60% (at 25/10 °C) (Figure 2b and Figure 3). Examining the recovery ability with the vineyard years, this did not present a significant effect (p = 0.154, Table S1). However, the rootstock type in this case had a weak effect (p = 0.0731, Table S1) and the best model was performed by the ungrafted type.
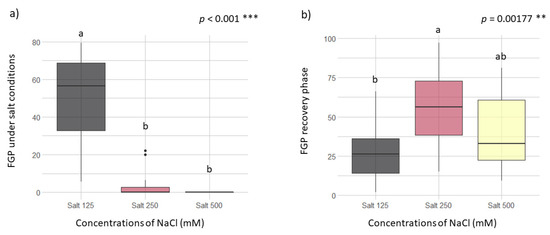
Figure 2.
Effect of different concentrations of NaCl on the final germination percentage (FGP) during the germination test (a) and the recovery phase (b) considering all temperature tested. Post hoc pairwise t-test comparisons were carried out for each condition and bars with different letters indicate significant differences (p < 0.05). Significance of p values codes: *** p < 0.001 and ** p < 0.01.
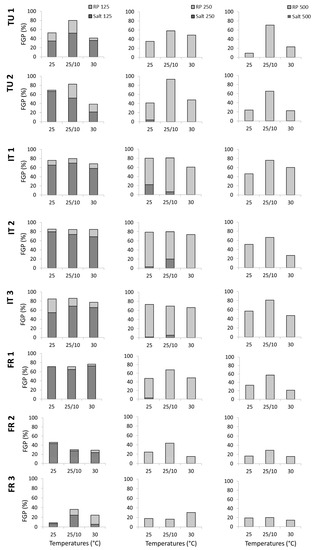
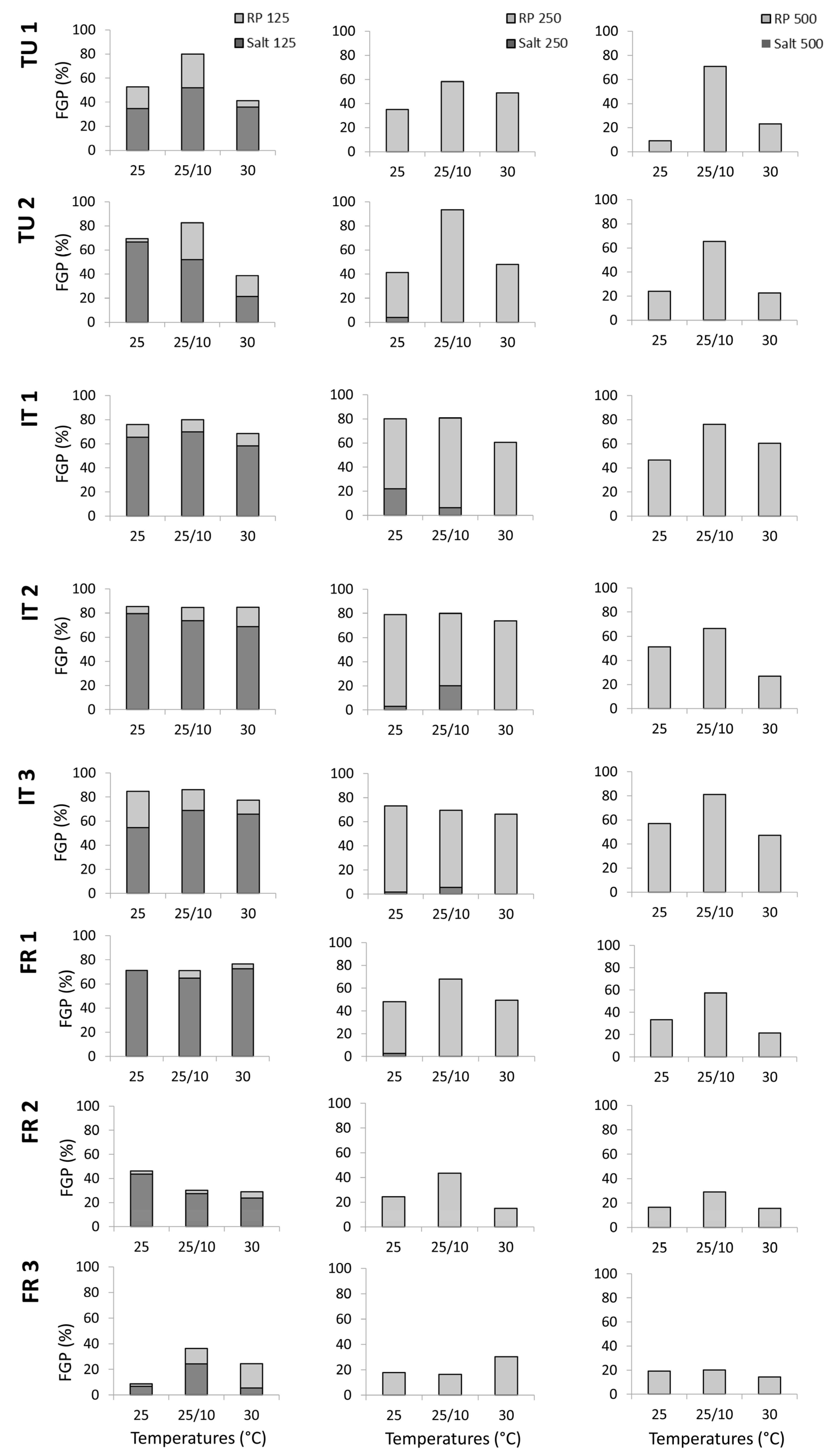
Figure 3.
Detailed information of the final germination percentages (FGPs) during the germination test and during the recovery phase by countries, distance from the sea, and temperatures tested (°C). FR_1 (France, vineyard close from the sea), FR_2 (France, vineyard intermediate from the sea), FR_3 (France, vineyard far from the sea), IT_1 (Italy, vineyard close from the sea), IT_2 (Italy, vineyard intermediate from the sea), IT_3 (Italy, vineyard far from the sea), TU_1 (Tunisia, vineyard close from the sea) and TU_2 (Tunisia, vineyard intermediate from the sea).
3.3. Effect of the Distance from the Sea in the Germination and Salt Tolerance
The effect of the distance from the sea on the FGP showed a significant difference (p = 0.0083, see Table S1) and presented lower influence considering the RP (p = 0.0916, Table S1). The GLM results indicated that the interaction between pre-treatments and distance factors had a statistically significant effect on germination (FGP, Table S1), and also in this case, the interaction factors were less significant considering the RP (Table S1).
By country, in France, the germination trend decreased with the distance from the sea, whereas vineyards close to the sea in Italy and Tunisia showed increased germination.
4. Discussion
Carignan seed accessions, independently of the temperature and distance from the sea, germinated better in the absence of NaCl in the substrate; this is consistent with the behavior of the wild grape that showed the highest germination when salts were absent, both in the germination substrate and in the soil [15]. The same authors reported that the germination percentages of wild grapes decreased with increasing salinity, confirming the pattern observed in this work with the Carignan. In accordance with these results, we can argue that Carignan cultivars have not lost their ancestral characteristics in some aspects of their reproductive biology, concretely in the ecophysiology of germination, although so many cross-practices and modifications have been developed during the domestication processes.
The results obtained in this study showed that the optimum germination process to select salt-tolerant accessions might be the alternating temperature regimen 25/10 °C and 125 mM NaCl. Several studies consider the grape as vulnerable or sensitive to salt stress [31,32,33,34]. In accordance with them, the seeds of the Carignan cultivar presented a considerable reduction of their final germination starting from NaCl concentrations higher than 125 mM, independently of the origin of the tested vineyard and distance from the sea. However, the capacity of recovery in Carignan seeds is high, including the capacity of those germinated after high salt exposures.
In addition, Baskin and Baskin [35] demonstrated in previous studies that at high temperatures, salinity exposure could result in a loss of seed viability and, therefore, poor recovery response. In our case and in the case of the wild grape [15], the seeds showed the inverse pattern, being facilitated by the highest temperatures (alternating regimen 25/10 °C), at least at 125 mM (the salinity at which the wild grape and Carignan cultivar still had satisfactory ability to germinate). This pattern is also in accordance with those detected by Orrù et al. [28], who observed that wild grape seed germination was higher at high temperatures (>20 °C), and that optimal germination for this species occurred under the tested fluctuating regimen (25/10 °C).
The results on salt tolerance of Carignan accessions related to the distance from the sea showed significant differences among them, indicating the wide range of adaptability of this cultivar. These results are in accordance with those described by Gutiérrez-Gamboa et al. [36], which indicated that Carignan usually tolerates warm climates, long, dry summers, and soils with moderate-to-low fertility. Additionally, this cultivar adapts very well to windy conditions (normally conditions near the sea), and its shoots lignify early in the season and mature well. In this sense, Carignan seeds that have germinated under salt stress in such a way that the adult plants tolerate salinity during their growth could be worth investigating.
Thanks to the ability of Carignan to germinate in the saline substrate and its capacity for recovery, including in high concentrations of salt, this cultivar could be useful for crossbreeding programs or better cisgenic programs, which help to create new versions of cultivars that maintain their desirable traits [37]. The breeding efforts for salt-tolerant crop species demonstrate that it is advantageous to introduce salt-tolerant cultivars or landraces that are traditionally used by local farmers in coastal saline areas into breeding programs as they enrich the genetic diversity of our modern high-yield crops with novel genes and alleles for salt tolerance [9]. Currently, there is a notable tendency to revalorize and preserve the minority or autochthonous grape cultivars, and we must consider the historical context of traditional coastal vineyards. This selection could be used to produce salt-tolerant rootstocks for the cultivation of grapevines in areas near the coastline (sandy soils permit the cultivation of the ungrafted Carignan), where salinity is certainly more present than in inland areas. Furthermore, the loss of coastal agricultural land can be due to both natural and artificial factors, such as coast erosion and floating or urban expansion. Coastal vineyards have shown a particular biological value, owing to the uniqueness of plant material, and a particular ecological meaning. In this sense, among the landscape systems threatened by the desertification and salinization processes, there are wide portions of the Mediterranean coast, including some of the most important wine production areas [38].
Knowledge about the ecophysiology, biology, and ecology of the potential of minority or autochthonous cultivars gives us the option to generate useful information devoted to production alternatives to the current wine market. The high quality of grapes and derived enological products as well as the important economic recourses that they offer justify the interest in maintaining such a cultivated environment and the opportunity to restore residual or abandoned vineyards, particularly in those territories where potential lands for agriculture and areas under high urban pressure meet [38]. Lastly, current progress towards the development of sustainable viticulture, including the efficient use of water, opens an important opportunity for this cultivar, which is mostly resistant to pests, salinity, and diseases [39].
5. Conclusions
This work provided new data about the ability of the Carignan cultivar to germinate in a saline substrate and its capacity for recovery, including in high concentrations of salt. This information could be useful for crossbreeding programs or for the improvement of cultivars through sexual reproduction. Furthermore, the case of Carignan is important because in many wine-growing coastal zones of the world, this cultivar can be actively integrated as rootstock selection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/seeds1020012/s1, Table S1: Results from generalized linear models (GLMs) used to evaluate the effect of pre-treatments, distance from the sea, years of the vineyards, rootstock type, and temperatures on the final germination percentage (FGP). Results from GLMs used to evaluate the effect of pre-treatments, distance from the sea, and temperatures on the Recovery phase (RP).
Author Contributions
Conceptualization, A.C.-L., A.L. and G.B.; methodology, A.C.-L. and A.L., formal analysis, A.C.-L.; writing—original draft preparation, A.C.-L., A.L., B.G., F.B. and G.B.; writing—review and editing, A.C.-L., A.L., B.G., F.B. and G.B.; supervision, A.C.-L., A.L. and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been carried out with the financial assistance of the European Union under the ENI CBC Mediterranean Sea Basin Programme in the framework of the BESTMEDGRAPE project. Andrea Lallai grant was funded by Programma Operativo Nazionale Ricerca e Innovazione PON-R&I 2014-2020—Ministero dell’Università e della Ricerca (MUR). Number grant DOT1304527.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors sincerely thank the wineries Domaine Neferis (Tunisia) and Cantina Santadi—Società Cooperativa Agricola (Sardinia), and the INRAE-Institut National de Recherche pour l’Agriculture, l’alimentation et l’environnement (France) for providing the seed material necessary to develop these experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- FAO Management of Salt Affected Soils: ‘Soil Management’ under ‘FAO SOILS PORTAL’ Food and Agriculture Organization of the ‘United Nations’. 2020 Rome. Available online: http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-affected-soils/en/ (accessed on 29 April 2022).
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Fu, Q.Q.; Tan, Y.Z.; Zhai, H.; Du, Y.P. Evaluation of salt resistance mechanisms of grapevine hybrid rootstocks. Sci. Hortic. 2019, 243, 148–158. [Google Scholar] [CrossRef]
- Haider, M.S.; Jogaiah, S.; Pervaiz, T.; Zhao, Y.X.; Khan, N.; Fang, J.G. Physiological and transcriptional variations inducing complex adaptive mechanisms in grapevine by salt stress. Environ. Exp. Bot. 2019, 162, 455–467. [Google Scholar] [CrossRef]
- Stolte, J.; Tesfai, M.; Øygarden, L.; Kværnø, S.; Keizer, J.; Verheijen, F.; Panagos, P.; Ballabio, C.; Hessel, R. Soil Threats in Europe: Status, Methods, Drivers and Effects on Ecosystem Services; A Review Report, Deliverable 2.1 of the RECARE Project; Office for Official Publications of the European Community: Luxembourg, 2015; Volume EUR 27607, p. 105. Available online: https://data.europa.eu/doi/10.2788/828742 (accessed on 29 April 2022).
- Isayenkov, S.V. Genetic sources for the development of salt tolerance in crops. Plant Growth Regul. 2019, 89, 1–17. [Google Scholar] [CrossRef]
- Ismail, A.M.; Horie, T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, R.; Jamil, S.; Shahzad, M.; Zörb, C.; Irshad, U.; Khan, N.; Younas, M.; Khan, S.A. Metabolic profiling to elucidate genetic elements due to salt stress. Clean 2017, 45, 1600574. [Google Scholar] [CrossRef]
- Moud, A.M.; Maghsoudi, K. Salt stress effects on respiration and growth of germinated seeds of different wheat (Triticum aestivum L.) cultivars. World J. Agric. Sci. 2008, 4, 351–358. [Google Scholar]
- Cramer, G.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.H.; Bi, W.L.; Chen, P.; Xu, Y.; Wang, Q.C. Abiotic stress improves in vitro biological indexing of Grapevine leafroll-associated virus-3 in red grapevine cultivars. Aust. J. Grape Wine Res. 2015, 21, 490–495. [Google Scholar] [CrossRef]
- Sohrabi, S.; Ebadi, A.; Jalali, S.; Salami, S.A. Enhanced values of various physiological traits and VvNAC1 gene expression showing better salinity stress tolerance in some grapevine cultivars as well as rootstocks. Sci. Hortic. 2017, 225, 317–326. [Google Scholar] [CrossRef]
- Santo, A.; Orrù, M.; Sarigu, M.; Ucchesu, M.; Sau, S.; Lallai, A.; D’hallewin, G.; Bacchetta, G. Salt tolerance of wild grapevine seeds during the germination phase. Sci. Hortic. 2019, 255, 115–120. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Ibrahim, A.; Ghazy, A.; Attia, K.; Al-Ghamdi, A.A.; Al-Dosary, M.A. Assessing the correlations and selection criteria between different traits in wheat salt-tolerant genotypes. Saudi J. Biol. Sci. 2021, 28, 5414–5427. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, B.K.; Yoon, I.S.; Kim, K.H.; Kwon, T.R. Salt tolerance in rice: Focus on mechanisms and approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Heidari, R.; Abbaspour, N.; Rahmani, F. Evaluation of salinity effects on ionic balance and compatible solute contents in nine grape (Vitis L.) genotypes. J. Plant Nutr. 2014, 37, 1817–1836. [Google Scholar] [CrossRef]
- Jogaiah, S.; Ramteke, S.D.; Sharma, J.; Upadhyay, A.K. Moisture and salinity stress induced changes in biochemical constituents and water relations of different grape rootstock cultivars. Int. J. Agron. 2014, 2014, 789087. [Google Scholar] [CrossRef] [Green Version]
- Mbarki, S.; Skalicky, M.; Vachova, P.; Hajihashemi, S.; Jouini, L.; Zivcak, M.; Tlustos, P.; Brestic, M.; Hejnak, V.; Zoghlami Khelil, A. Comparing salt tolerance at seedling and germination stages in local populations of Medicago ciliaris L. to Medicago intertexta L. and Medicago scutellata L. Plants 2020, 94, 526. [Google Scholar] [CrossRef] [Green Version]
- Abiala, M.A.; Abdelrahman, M.; Burritt, D.J.; Tran, L.S.P. Salt stress tolerance mechanisms and potential applications of legumes for sustainable reclamation of salt-degraded soils. Land Degrad. Dev. 2018, 29, 3812–3822. [Google Scholar] [CrossRef]
- Clarke, O.; Rand, M. Oz Clarke’s Encyclopedia of Grapes; Harcourt Books: Boston, MA, USA, 2001; p. 58. ISBN 978-0-1510-0714-1. [Google Scholar]
- Hudin, M. The Grand Carignan Tasting. Wine on VI. Available online: https://www.hudin.com/the-grand-carignan-tasting/ (accessed on 11 April 2021).
- Mercenaro, L.; Nieddu, G.; Porceddu, A.; Pezzotti, M.; Camiolo, S. Sequence polymorphisms and structural variations among four grapevine (Vitis vinifera L.) cultivars representing Sardinian agriculture. Front. Plant Sci. 2017, 8, 1279. [Google Scholar] [CrossRef] [Green Version]
- Lasram, S.; Bellí, N.; Chebil, S.; Nahla, Z.; Ahmed, M.; Sanchis, V.; Ghorbel, A. Occurrence of ochratoxigenic fungi and ochratoxin A in grapes from a Tunisian vineyard. Int. J. Food Microbiol. 2007, 114, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Conner, P.J. Effects of stratification, germination, temperature, pre-treatment with gibberellic acid and hydrogen peroxide on germination of ‘Fry’ Muscardine (Vitis rotundifolia) seed. HortScience 2008, 43, 853–856. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.Q.; Song, S.Q.; Li, S.H.; Gan, Y.Y.; Wu, J.H.; Cheng, H.Y. Seed dormancy and germination in Vitis amurensis and its variation. Seed Sci. Res. 2011, 21, 255–265. [Google Scholar] [CrossRef]
- Orrù, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Thermal thresholds as predictors of seed dormancy release and germination timing: Altitude-related risks from climate warming for the wild grapevine Vitis vinifera subsp. sylvestris. Ann. Bot. 2012, 110, 1651–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISTA. International Rules for Seed Testing; The International Seed Testing Association: Bassersdorf, Germany, 2008; Chapter 5; p. 8. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Maas, E.V.; Hoffman, G.J. Crop Salt Tolerance. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Hawker, J.S.; Walker, R.R. The effect of sodium chloride on the growth and fruiting of Cabernet Sauvignon vines. Am. J. Enol. Vitic. 1978, 29, 172–176. [Google Scholar]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Correll, R.L. Rootstock effects of salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. cv. Sultana) I. Yield and vigour inter-relationships. Aust. J. Grape Wine Res. 2002, 8, 3–14. [Google Scholar] [CrossRef]
- Shani, U.; Waisel, Y.; Eshel, A.; Xue, S.; Ziv, G. Responses to salinity of grapevine plants with split root systems. New Phytol. 1993, 124, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; ISBN 978-0-1241-6677-6. [Google Scholar]
- Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y. Terroir and typicity of Carignan from Maule Valley (Chile): The resurgence of a minority variety. OENO One 2019, 53, 75–93. [Google Scholar] [CrossRef] [Green Version]
- Gray, D.J.; Zhijian, T.L.; Sadanand, A.D. Precision breeding of grapevine (Vitis vinifera L.) for improved traits. Plant Sci. 2014, 228, 3–10. [Google Scholar] [CrossRef]
- Biasi, R.; Barbera, G.; Marino, E.; Brunori, E.; Nieddu, G. Viticulture as crucial cropping system for counteracting the desertification of coastal land. Acta Hortic. 2010, 931, 71–77. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Liu, S.Y.; Pszczólkowski, P. Resurgence of minority and autochthonous grapevine varieties in South America: A review of their oenological potential. J. Sci. Food Agric. 2020, 100, 465–482. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).