Differential Seed Germination Responses of Tomato Landraces to Temperature under Climate Change Scenarios
Abstract
:1. Introduction
2. Materials and methods
2.1. Seed Collection
2.2. Study Area
2.3. Germination Tests
FGP = (Tc − T)/(Tc − To2) if To2 < T < Tc
FGP = 1 if To1 ≤ T ≤ To2
FGP = 0 if T ≤ Tb or T ≥ Tc
R50 = (Tc − T)/(Tc − To) if To ≤ T < Tc
R50 = 0 if T ≤ Tb or T ≥ Tc
2.4. Future Climate Projection
2.5. Assessing the Current and Future Temperature on Germination
3. Results
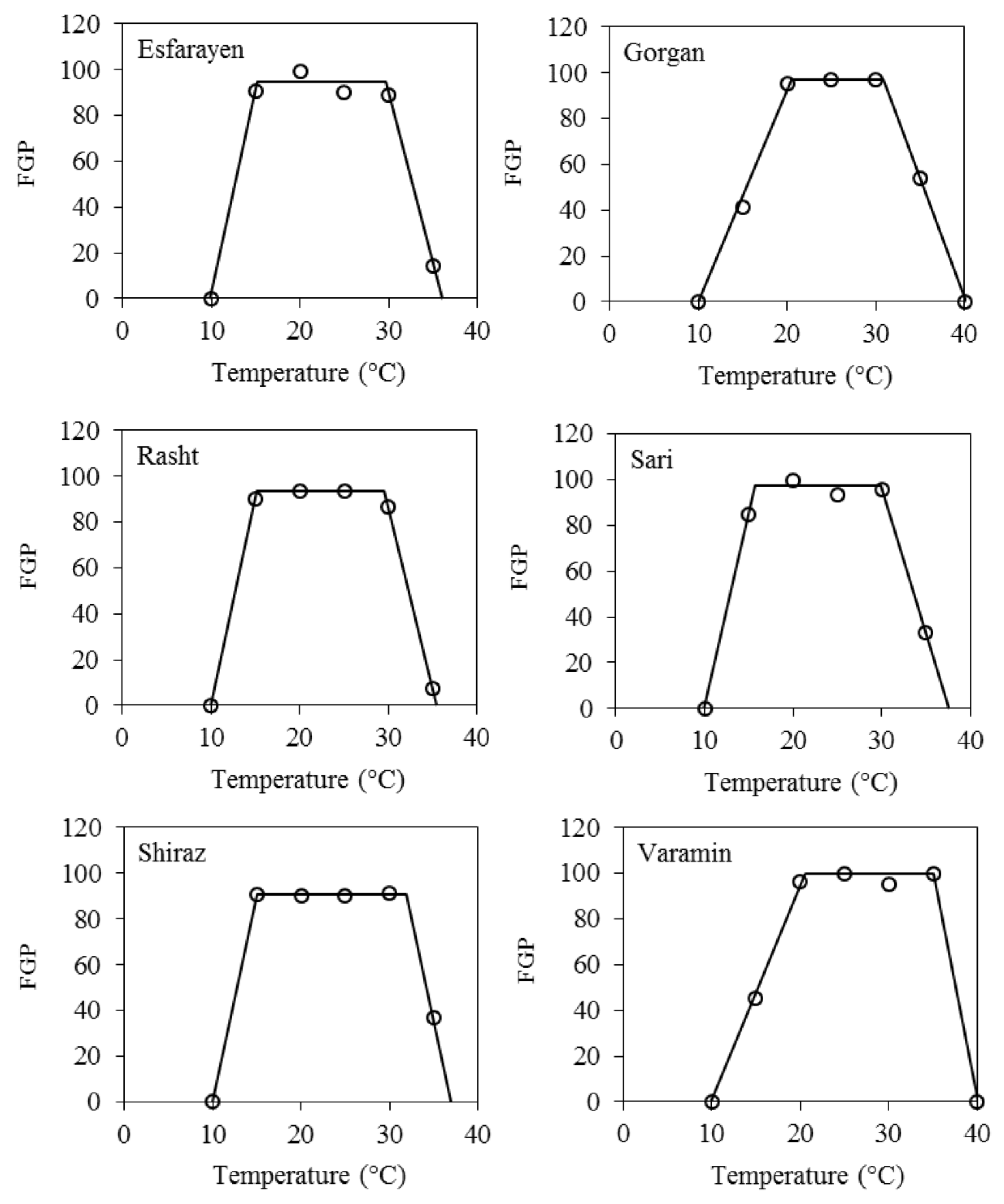
3.1. Final Germination Percentage
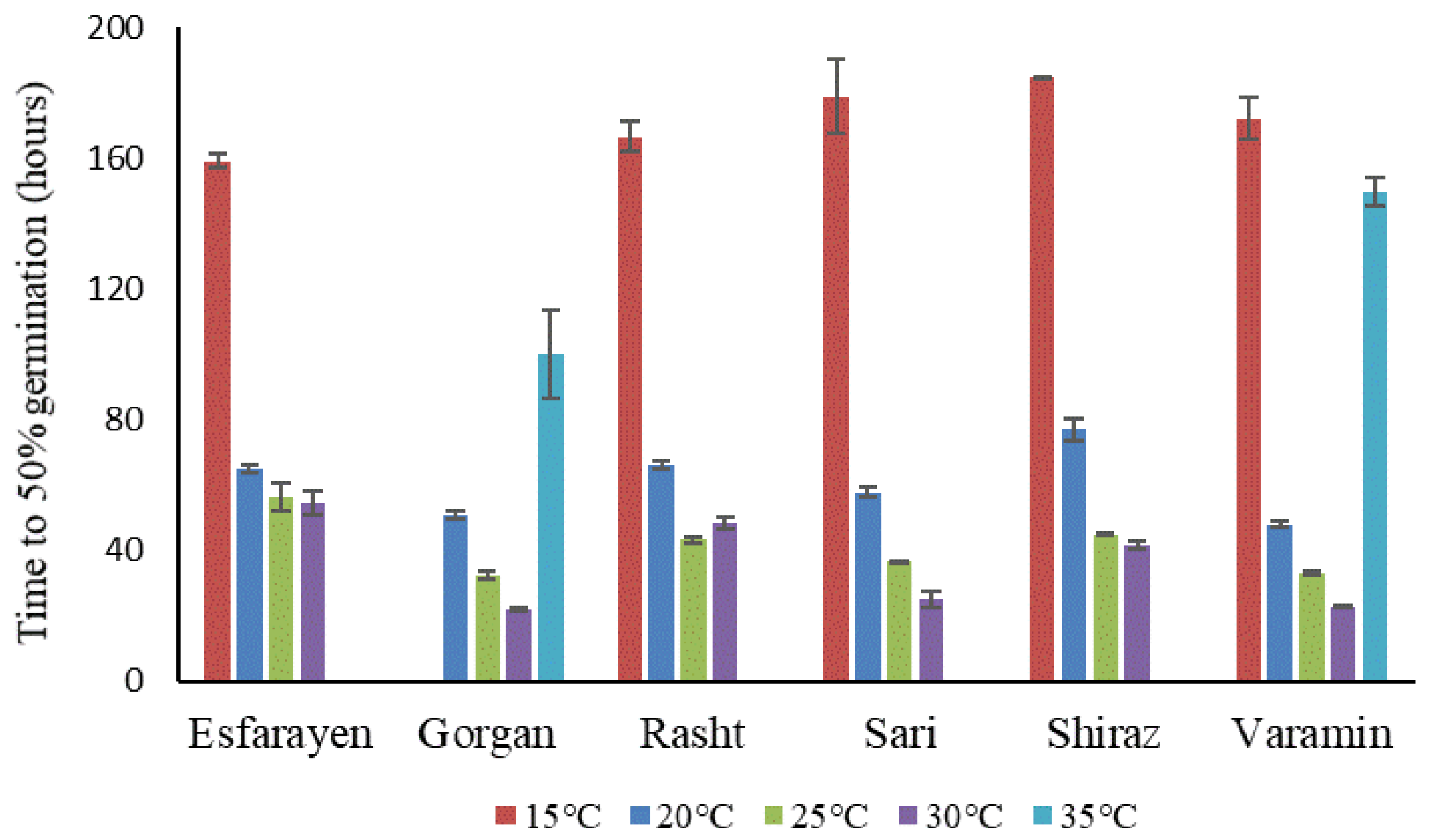
3.2. Germination Time
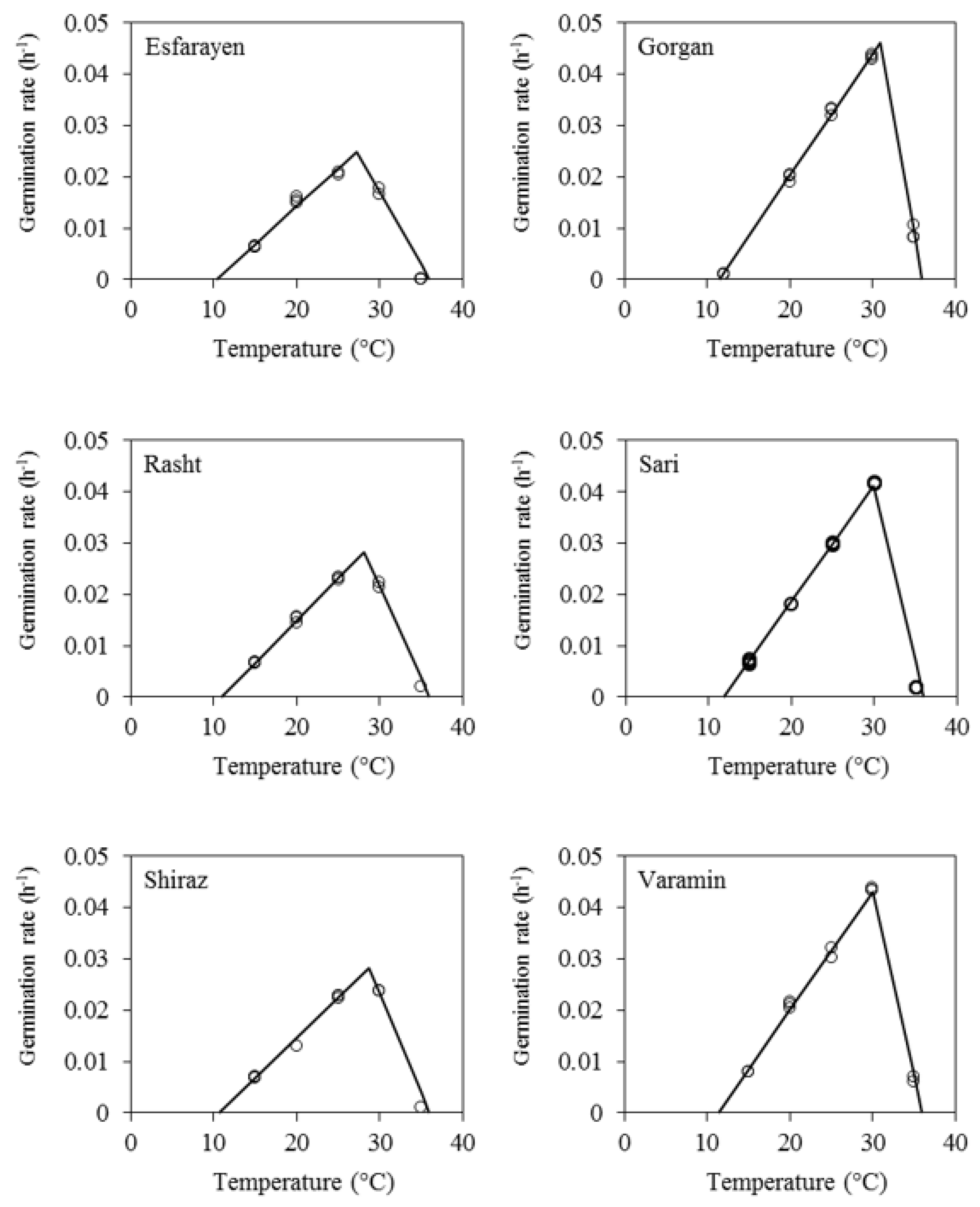
3.3. Germination Rate
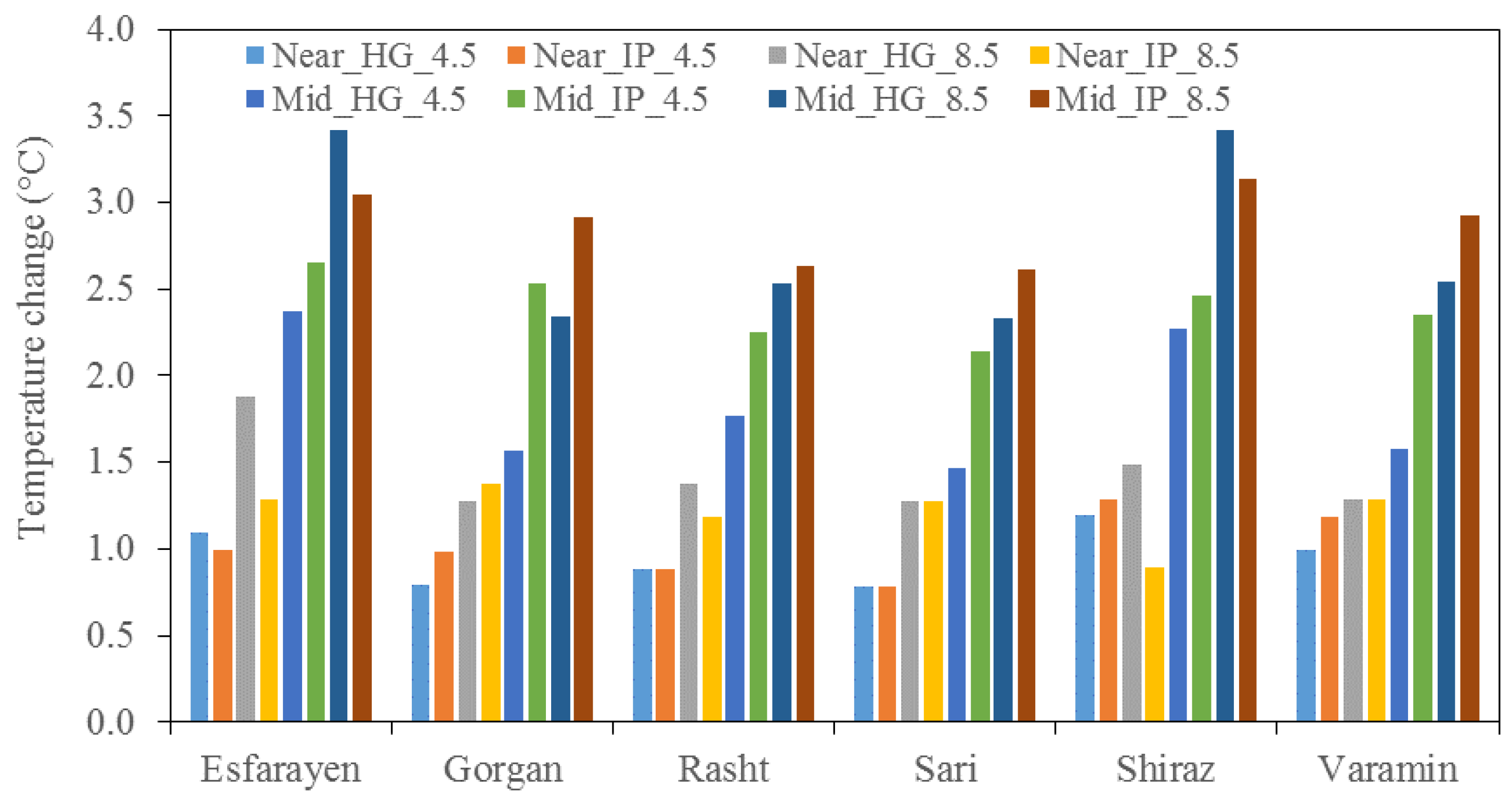
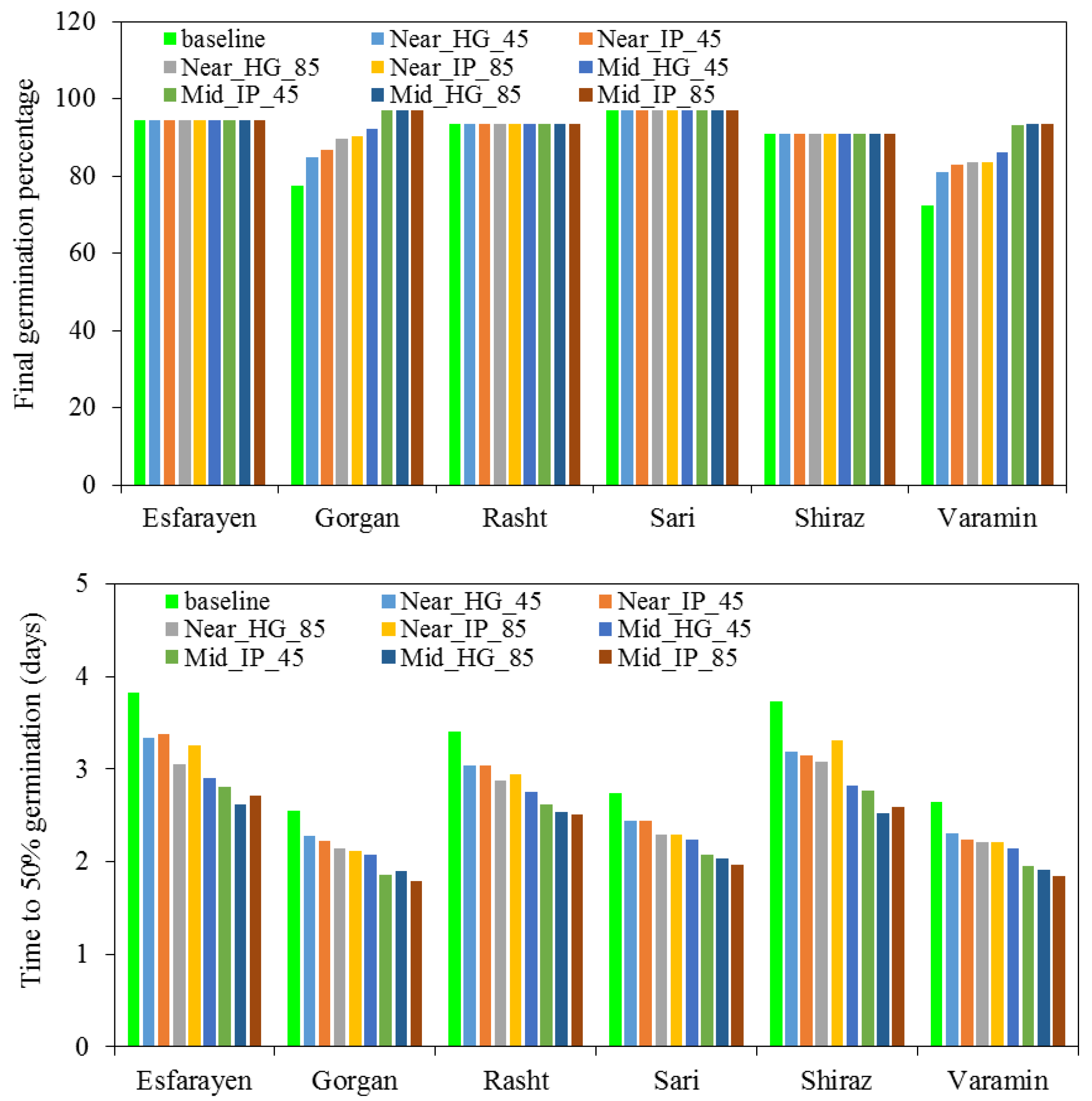
3.4. Climate Change Scenarios
3.5. Effect of Future Climate on Germination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, G.C.; Bartsch, M.; Barua, D.; Nakabayashi, K.; Debieu, M.; Kronholm, I.; Koornneef, M.; Soppe, W.J.; Donohue, K.; de Meaux, J. DOG1 expression is predicted by the seed-maturation environment and contributes to geographical variation in germination in Arabidopsis thaliana. Mol. Ecol. 2011, 20, 3336–3349. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, A.G.; Knutti, R.; Lehner, F.; Deser, C.; Sanderson, B.M. Precipitation variability increases in a warmer climate. Sci. Rep. 2017, 7, 17966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjellström, E.; Nikulin, G.; Strandberg, G.; Christensen, O.B.; Jacob, D.; Keuler, K.; Lenderink, G.; van Meijgaard, E.; Schär, C.; Somot, S.; et al. European climate change at global mean temperature increases of 1.5 and 2 °C above pre-industrial conditions as simulated by the EURO-CORDEX regional climate models. Earth Syst. Dyn. Discuss. 2018, 9, 459–478. [Google Scholar] [CrossRef] [Green Version]
- Piñar Fuentes, J.C.; Cano-Ortiz, A.; Musarella, C.M.; Quinto Canas, R.; Pinto Gomes, C.J.; Spampinato, G.; del Río, S.; Cano, E. Bioclimatology, structure, and conservation perspectives of Quercus pyrenaica, Acer opalus subsp. Granatensis, and Corylus avellana deciduous forests on Mediterranean bioclimate in the south-central part of the Iberian Peninsula. Sustainability 2019, 11, 6500. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.A.; de Lemos Filho, J.P.; Marques, A.R. Seed germination of bromeliad species from the campo rupestre: Thermal time requirements and response under predicted climate-change scenarios. Flora 2018, 238, 119–128. [Google Scholar] [CrossRef]
- Zogas, A.; Kosman, E.; Sternberg, M. Germination strategies under climate change scenarios along an aridity gradient. J. Plant Ecol. 2020, 13, 470–477. [Google Scholar] [CrossRef]
- Frate, L.; Carranza, M.L.; Evangelista, A.; Stinca, A.; Joop, H.J.; Schaminée, J.H.J.; Stanisci, A. Climate and land use change impacts on Mediterranean high-mountain vegetation in the Apennines since the 1950s. Plant Ecol. Plant Ecol. Divers. 2018, 11, 85–96. [Google Scholar] [CrossRef]
- Jiménez, S.; Fattahi, M.; Bedis, K.; Nasrolahpour-moghadam, S.; Irigoyen, J.J.; Gogorcena, Y. Interactional effects of climate change factors on the water status, photosynthetic rate, and metabolic regulation in peach. Front. Plant Sci. 2020, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Dantas, B.F.; Moura, M.S.; Pelacani, C.R.; Angelotti, F.; Taura, T.A.; Oliveira, G.M.; Bispo, J.S.; Matias, J.R.; Silva, F.F.; Pritchard, H.W.; et al. Rainfall, not soil temperature, will limit the seed germination of dry forest species with climate change. Oecologia 2020, 192, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, A.; Campbell, R.; Díaz, R.; Martínez, J.; Escalante, D.; Martínez-Montero, M.E.; Quintana, N.; Yabor, L.; Höfer, M.; Lorenzo, J.C. Chickpea seed cryostorage alters germinant but not adult plant growth. Biologia 2020, 76, 55–61. [Google Scholar] [CrossRef]
- Mahmood, A.H.; Florentine, S.K.; Chauhan, B.S.; McLaren, D.A.; Palmer, G.C.; Wright, W. Influence of various environmental factors on seed germination and seedling emergence of a noxious environmental weed: Green galenia (Galenia pubescens). Weed Sci. 2016, 64, 486–494. [Google Scholar] [CrossRef]
- Torabi, B.; Attarzadeh, M.; Soltani, A. Germination response to temperature in different safflower (Carthamus tinctorius) cultivars. Seed Technol. 2013, 35, 47–59. Available online: https://www.jstor.org/stable/24642240 (accessed on 6 January 2022).
- Soltani, E.; Baskin, C.C.; Baskin, J.M. A graphical method for identifying the six types of non-deep physiological dormancy in seeds. Plant Biol. 2017, 19, 673–682. [Google Scholar] [CrossRef]
- Moghaddam, P.R.; Feizi, H.; Mondani, F. Evaluation of tomato production systems in terms of energy use efficiency and economical analysis in Iran. Not. Sci. Biol. 2011, 3, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Sacco, A.; Ruggieri, V.; Parisi, M.; Festa, G.; Rigano, M.M.; Picarella, M.E.; Mazzucato, A.; Barone, A. Exploring a tomato landraces collection for fruit-related traits by the aid of a high-throughput genomic platform. PLoS ONE 2015, 10, e0137139. [Google Scholar] [CrossRef] [Green Version]
- Torabi, B.; Archontoulis, S.V.; Hoogenboom, G. A new function for prediction of biological processes response to temperature. Int. J. Plant Prod. 2020, 14, 9–22. [Google Scholar] [CrossRef]
- Zaferanieh, M.; Mahdavi, B.; Torabi, B. Effect of temperature and water potential on Alyssum homolocarpum seed germination: Quantification of the cardinal temperatures and using hydrothermal time. S. Afr. J. Bot. 2020, 131, 259–266. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Schaum, C.E.; Trimmer, M. The temperature dependence of phytoplankton stoichiometry: Investigating the roles of species sorting and local adaptation. Front. Microbiol. 2017, 8, 2003. [Google Scholar] [CrossRef]
- Aguirre-Liguori, J.A.; Gaut, B.S.; Jaramillo-Correa, J.P.; Tenaillon, M.I.; Montes-Hernández, S.; García-Oliva, F.; Hearne, S.J.; Eguiarte, L.E. Divergence with gene flow is driven by local adaptation to temperature and soil phosphorus concentration in teosinte subspecies (Zea mays parviglumis and Zea mays mexicana). Mol. Ecol. 2019, 28, 2814–2830. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pascual, E.; Jiménez-Alfaro, B. Phenotypic plasticity in seed germination relates differentially to overwintering and flowering temperatures. Seed Sci. Res. 2014, 24, 273. [Google Scholar] [CrossRef]
- Noble, D.W.; Stenhouse, V.; Schwanz, L.E. Developmental temperatures and phenotypic plasticity in reptiles: A systematic review and meta-analysis. Biol. Rev. 2018, 93, 72–97. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.A.; Nicotra, A.B.; Kruuk, L.E. Sparse evidence for selection on phenotypic plasticity in response to temperature. Philos. Trans. R. Soc. B 2019, 374, 20180185. [Google Scholar] [CrossRef] [Green Version]
- Fasih, M.; Afshari, R.T. The morphophysiological dormancy of Ferula ovina seeds is alleviated by low temperature and hydrogen peroxide. Seed Sci. Res. 2018, 28, 52. [Google Scholar] [CrossRef]
- Bafoil, M.; Jemmat, A.; Martinez, Y.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. Effects of low temperature plasmas and plasma activated waters on Arabidopsis thaliana germination and growth. PLoS ONE 2018, 13, e0195512. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop wild relatives (CWR) priority in Italy: Distribution, ecology, in situ and ex situ conservation and expected actions. Sustainability 2021, 13, 1682. [Google Scholar] [CrossRef]
- Donohue, K.; Dorn, L.; Griffith, C.; Kim, E.; Aguilera, A.; Polisetty, C.R.; Schmitt, J. The evolutionary ecology of seed germination of Arabidopsis thaliana: Variable natural selection on germination timing. Evolution 2005, 59, 758–770. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Yang, Z.; Zhang, N. Geographic variations in seed germination of Dalbergia odorifera T. Chen in response to temperature. Ind. Crops Prod. 2017, 102, 45–50. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, B. Seed germination response to high temperature and water stress in three invasive Asteraceae weeds from Xishuangbanna, SW China. PLoS ONE 2018, 13, e0191710. [Google Scholar] [CrossRef] [Green Version]
- Mobli, A.; Mijani, S.; Ghanbari, A.; Rastgoo, M. Seed germination and emergence of two flax-leaf alyssum (Alyssum linifolium Steph. ex. Willd.) populations in response to environmental factors. Crop Pasture Sci. 2019, 70, 807–813. [Google Scholar] [CrossRef]
- Bidgoly, R.O.; Balouchi, H.; Soltani, E.; Moradi, A. Effect of temperature and water potential on Carthamus tinctorius L. seed germination: Quantification of the cardinal temperatures and modeling using hydrothermal time. Ind. Crops Prod. 2018, 113, 21–127. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Porter, C.H.; Shelia, V.; Boote, K.J.; Singh, U.; White, J.W.; Hunt, L.A.; Ogoshi, R.; Lizaso, J.I.; Koo, J.; et al. Decision Support System for Agrotechnology Transfer (DSSAT); Version 4.7.5.; DSSAT Foundation: Gainesville, FL, USA, 2019; Available online: https://DSSAT.net (accessed on 6 January 2022).
- Gudmundsson, S.V.; Anger, A. Global carbon dioxide emissions scenarios for aviation derived from IPCC storylines: A meta-analysis. Transp. Res. D Transp. Environ. 2012, 17, 61–65. [Google Scholar] [CrossRef]
- Hawkins, E.; Ortega, P.; Suckling, E.; Schurer, A.; Hegerl, G.; Jones, P.; Joshi, M.; Osborn, T.J.; Masson-Delmotte, V.; Mignot, J.; et al. Estimating changes in global temperature since the preindustrial period. Bull. Am. Meteorol. Soc. 2017, 98, 1841–1856. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.A.; Kainuma, M.; Riahi, K.; Weyant, J. A special issue on the RCPs. Clim. Change 2011, 109, 1. [Google Scholar] [CrossRef] [Green Version]
- Thomson, A.M.; Calvin, K.V.; Smith, S.J.; Kyle, G.P.; Volke, A.; Patel, P.; Delgado-Arias, S.; Bond-Lamberty, B.; Wise, M.A.; Clarke, L.E.; et al. RCP4.5: A pathway for stabilization of radiative forcing by 2100. Clim. Change 2011, 109, 77. [Google Scholar] [CrossRef] [Green Version]
- Riahi, K.; Rao, S.; Krey, V.; Cho, C.; Chirkov, V.; Fischer, G.; Kindermann, G.; Nakicenovic, N.; Rafaj, P. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Clim. Change 2011, 109, 33. [Google Scholar] [CrossRef] [Green Version]
- Hudson, N.; Ruane, A.C. Guide for Running AgMIP Climate Scenario Generation Tools with R in Windows, Version 2.3. Appendix 2. 2013. Available online: https://ntrs.nasa.gov/citations/20150007687 (accessed on 6 January 2022).
- Rosenzweig, C.; Jones, J.W.; Hatfield, J.L.; Ruane, A.C.; Boote, K.J.; Thorburn, P.; Antle, J.M.; Nelson, G.C.; Porter, C.; Janssen, S.; et al. The agricultural model intercomparison and improvement project (AgMIP): Protocols and pilot studies. Agric. Meteorol. 2013, 170, 166–182. [Google Scholar] [CrossRef] [Green Version]
- Rosenzweig, C.; Hillel, D. Handbook of Climate Change and Agroecosystems: The Agricultural Model Intercomparison and Improvement Project (AgMIP) Integrated Crop and Economic Assessments—Joint Publication with American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America (In 2 Parts); Imperial College Press: London, UK, 2015. [Google Scholar]
- Hatami, E.; Raeini Sarjaz, M.; Chalavi, V. Evaluation of the thermal and humidity relations and cucumber yield within plastic tunnel microclimates. J. Agric. Meteorol. 2013, 1, 26–36. [Google Scholar]
- Calzada López, S.G.; Shibata, J.K.; Uscanga Mortera, E.; García Esteva, A.; Yáñez Jiménez, P. Cardinal temperatures and germination rate in husk tomato cultivars. Rev. Mex. De Cienc. Agrícolas 2014, 8, 1451–1458. [Google Scholar]
- Saberali, S.F.; Shirmohamadi-Aliakbarkhani, Z. Quantifying seed germination response of melon (Cucumis melo L.) to temperature and water potential: Thermal time, hydrotime and hydrothermal time models. S. Afr. J. Bot. 2020, 130, 240–249. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Bradford, K.J. Hydrothermal time analysis of tomato seed germination responses to priming treatments. J. Exp. Bot. 1999, 50, 89–99. [Google Scholar] [CrossRef]
- Jones, J.B. Instructions for Growing Tomatoes in the Garden and Green-House; GroSystems: Anderson, SC, USA, 2013. [Google Scholar]
- Sousaraei, N.; Torabi, B.; Mashaiekhi, K.; Soltani, E.; Mousavizadeh, S.J. Variation of seed germination response to temperature in tomato landraces: An adaptation strategy to environmental conditions. Sci. Hort. 2021, 281, 109987. [Google Scholar] [CrossRef]
- Labouriau, L.G.; Osborn, J.H. Temperature dependence of the germination of tomato seeds. J. Therm. Biol. 1984, 9, 285–294. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [Green Version]
- Brzezinski, C.R.; Abati, J.; Henning, F.A.; Henning, A.A.; França Neto, J.D.B.; Krzyzanowski, F.C.; Zucareli, C. Spray volumes in the industrial treatment on the physiological quality of soybean seeds with different levels of vigor. Res. J. Seed Sci. 2017, 39, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.S.; Schmid, L.P.; Mielezrski, F.; Pavan, B.E. Physiological quality of rice seeds stored in different environments and packages. J. Exp. Agric. Int. 2018, 23, 1–9. [Google Scholar] [CrossRef]
- Torabi, B.; Soltani, E.; Archontoulis, S.V.; Rabii, A. Temperature and water potential effects on Carthamus tinctorius L. seed germination: Measurements and modeling using hydrothermal and multiplicative approaches. Rev. Bras. Bot. 2016, 39, 427–436. [Google Scholar] [CrossRef]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N.; et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef] [Green Version]
- Essemine, J.; Ammar, S.; Bouzid, S. Impact of heat stress on germination and growth in higher plants: Physiological, biochemical and molecular repercussions and mechanisms of defense. J. Biol. Sci. 2010, 10, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zou, Y.; Ding, S.; Zhang, J.; Yu, X.; Cao, J.; Lu, G. Polyamine accumulation in transgenic tomato enhances the tolerance to high temperatures stress. J. Integr. Plant Biol. 2009, 51, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Graae, B.J.; Alsos, I.G.; Ejrnaes, R. The impact of temperature regimes on development, dormancy breaking and germination of dwarf shrub seeds from arctic, alpine and boreal sites. Plant Ecol. 2008, 198, 275–284. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Dixon, K.W.; Silveira, F.A.; Chapman, T.; Di Sacco, A.; Mondoni, A.; Turner, S.R.; Cross, A.T. Dormancy and germination: Making every seed count in restoration. Restor. Ecol. 2020, 28, S256–S265. [Google Scholar] [CrossRef]
- Milbau, A.; Graae, B.J.; Shevtsova, A.; Nijs, I. Effects of a warmer climate on seed germination in the subarctic. Ann. Bot. 2009, 104, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Chhetri, S.B.; Rawal, D.S. Germination phenological response identifies flora risk to climate change. Climate 2017, 5, 73. [Google Scholar] [CrossRef] [Green Version]
- Fennessey, N.M.; Kirshen, P.H. Evaporation and evapo-transpiration under climate change in New England. J. Water Res. Plan. Manag. 1994, 120, 48–69. [Google Scholar] [CrossRef]
- Falloon, P.; Betts, R. Climate impacts on European agriculture and water management in the context of adaptation and mitigation—The importance of an integrated approach. Sci. Total Environ. 2010, 408, 5667–5687. [Google Scholar] [CrossRef]
- Abe, T.; Matsunaga, M. Geographic variation in germination traits in Melia azedarach and Rhaphiolepis umbellata. Am. J. Plant Sci. 2011, 2, 52–55. [Google Scholar] [CrossRef] [Green Version]
- White, J.W.; Hoogenboom, G.; Kimball, B.A.; Wall, G.W. Methodologies for simulating impacts of climate change on crop production. Field Crop Res. 2011, 124, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Van Ittersum, M.K.; Rabbinge, R. Concepts in production ecology for analysis and quantification of agricultural input-output combinations. Field Crop Res. 1997, 52, 197–208. [Google Scholar] [CrossRef]

| Location | Latitude and Longitude | Average Annual Temperature (°C) | Average Annual Rainfall (mm) | Above Sea Level (m) |
|---|---|---|---|---|
| Rasht | 37.16° N, 49.36° E | 16.5 | 1359 | 5 |
| Gorgan | 36.83° N, 54.48° E | 18.4 | 675 | 155 |
| Esfarayen | 37.73° N, 57.5° E | 16.5 | 212 | 1249 |
| Sari | 36.4° N, 53.5° E | 18.2 | 789 | 32 |
| Varamin | 35.32° N, 51.64° E | 18 | 156 | 918 |
| Shiraz | 29.61° N, 52.54° E | 18.4 | 337 | 1486 |
| Landrace | Tb ± SE | To1 ± SE | To2 ± SE | Tc ± SE |
|---|---|---|---|---|
| Esfarayen | 9.9 ± 0.21 | 15.2 ± 0.22 | 29.6 ± 0.27 | 36 ± 0.20 |
| Gorgan | 10 ± 0.34 | 20.5 ± 0.37 | 30.8 ± 0.68 | 40.2 ± 0.35 |
| Rasht | 10± 0.06 | 15.2 ± 0.08 | 29.5 ± 0.09 | 35.5 ± 0.06 |
| Sari | 9.9 ± 0.05 | 15.7 ± 0.05 | 29.9 ± 0.05 | 37.6 ± 0.10 |
| Shiraz | 10 ± 0.08 | 15 ± 0.10 | 31.9 ± 0.31 | 37 ± 0.09 |
| Varamin | 9.9 ± 0.33 | 20.5 ± 0.35 | 35 ± 0.17 | 40.1 ± 0.18 |
| Landrace | Tb ± SE | To ± SE | Tc | Rmax | Dmin | TT |
|---|---|---|---|---|---|---|
| Esfarayen | 10.3 ± 0.99 | 27.2 ± 0.49 | 36 | 0.0248 | 40.3 | 35.7 |
| Gorgan | 11.5 ± 0.20 | 30.9 ± 0.23 | 36 | 0.0461 | 21.7 | 15.7 |
| Rasht | 10.9 ± 0.34 | 28.1 ± 0.15 | 36 | 0.0284 | 35.2 | 24.3 |
| Sari | 11.9 ± 0.89 | 29.9 ± 0.31 | 36 | 0.0411 | 24.3 | 24.3 |
| Shiraz | 10.6 ± 0.67 | 28.7 ± 0.26 | 36 | 0.0282 | 35.5 | 24.7 |
| Varamin | 11.3 ± 0.39 | 30.0 ± 0.12 | 36 | 0.0437 | 23.0 | 16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousaraei, N.; Torabi, B.; Soltani, E.; Mashayekhi, K.; Medina, J. Differential Seed Germination Responses of Tomato Landraces to Temperature under Climate Change Scenarios. Seeds 2022, 1, 36-48. https://doi.org/10.3390/seeds1010005
Sousaraei N, Torabi B, Soltani E, Mashayekhi K, Medina J. Differential Seed Germination Responses of Tomato Landraces to Temperature under Climate Change Scenarios. Seeds. 2022; 1(1):36-48. https://doi.org/10.3390/seeds1010005
Chicago/Turabian StyleSousaraei, Naeimeh, Benjamin Torabi, Elias Soltani, Kambiz Mashayekhi, and Joaquín Medina. 2022. "Differential Seed Germination Responses of Tomato Landraces to Temperature under Climate Change Scenarios" Seeds 1, no. 1: 36-48. https://doi.org/10.3390/seeds1010005
APA StyleSousaraei, N., Torabi, B., Soltani, E., Mashayekhi, K., & Medina, J. (2022). Differential Seed Germination Responses of Tomato Landraces to Temperature under Climate Change Scenarios. Seeds, 1(1), 36-48. https://doi.org/10.3390/seeds1010005







