Biotic and Abiotic Interactions Shape Seed Germination of a Fire-Prone Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Obtaining Seeds
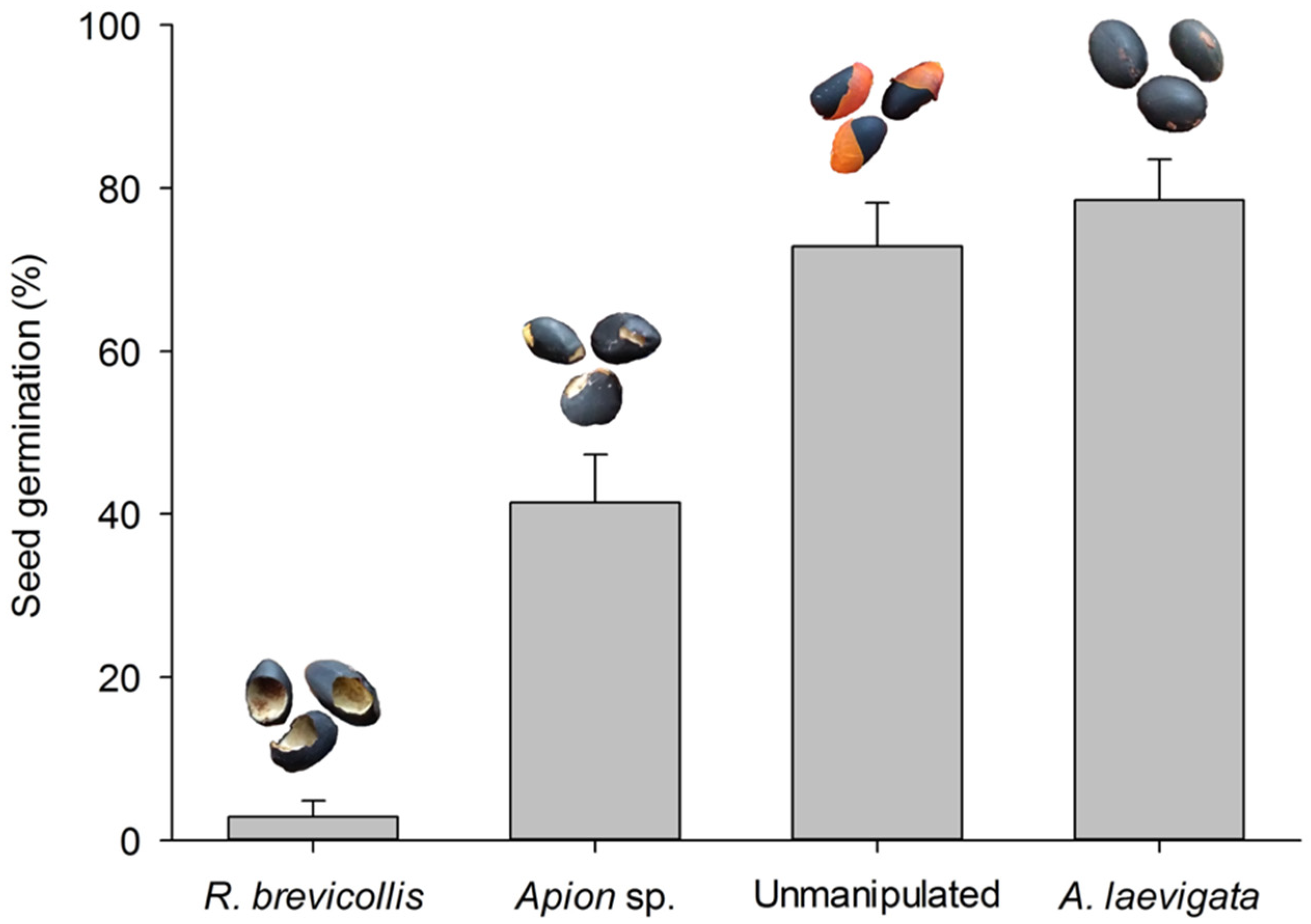
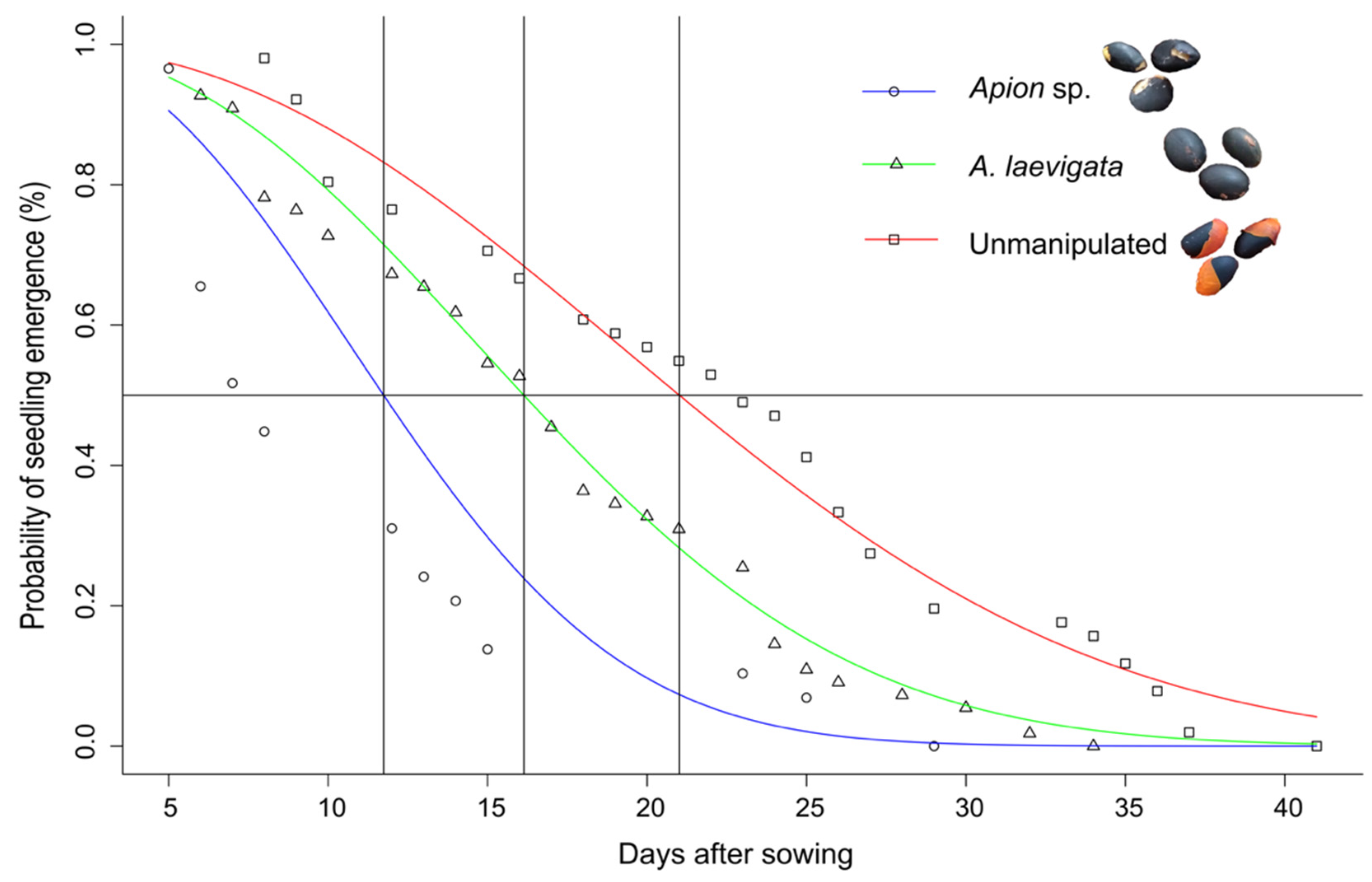
2.3. Bioassay 1: Effects of Predation on Seed Germination
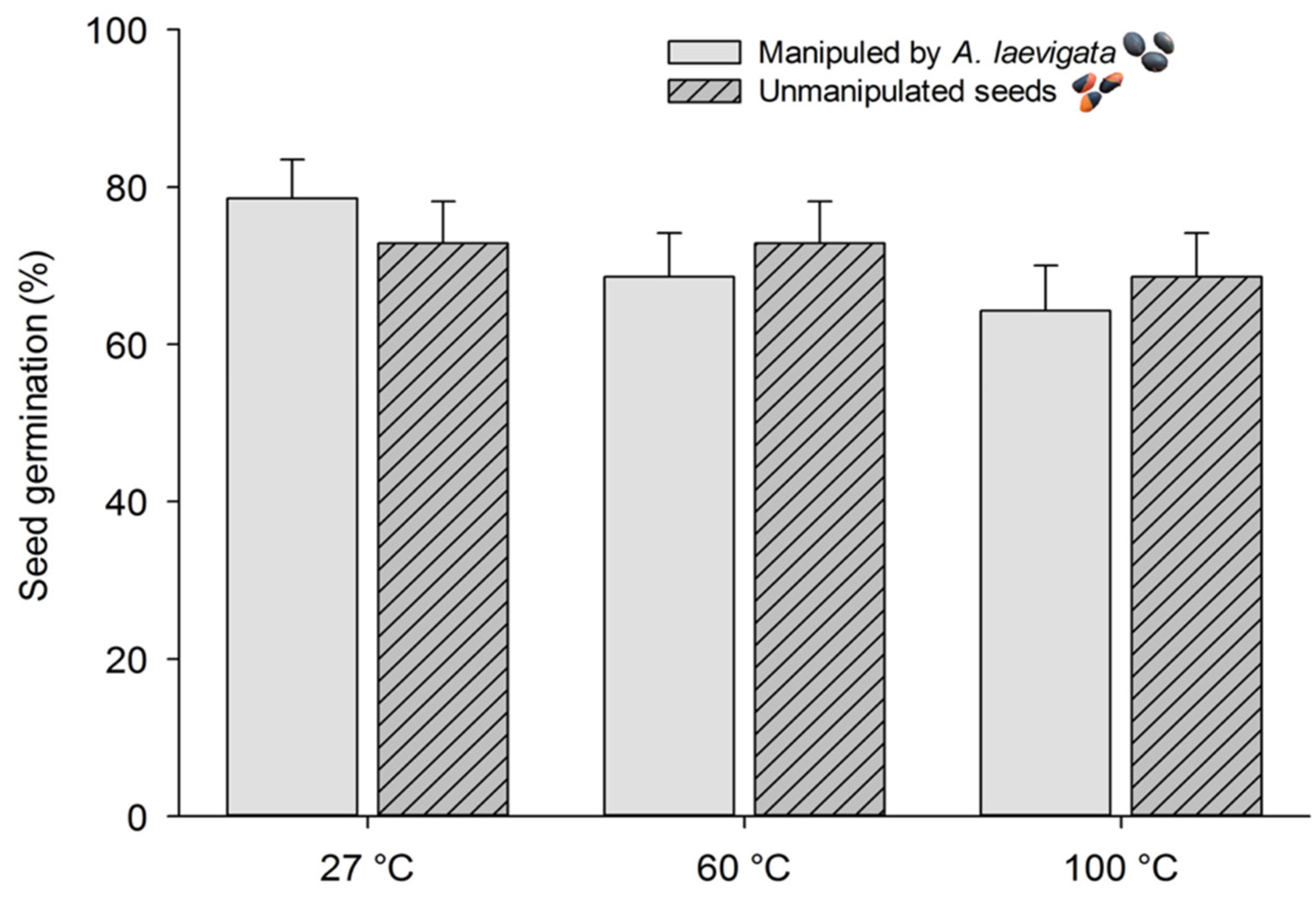
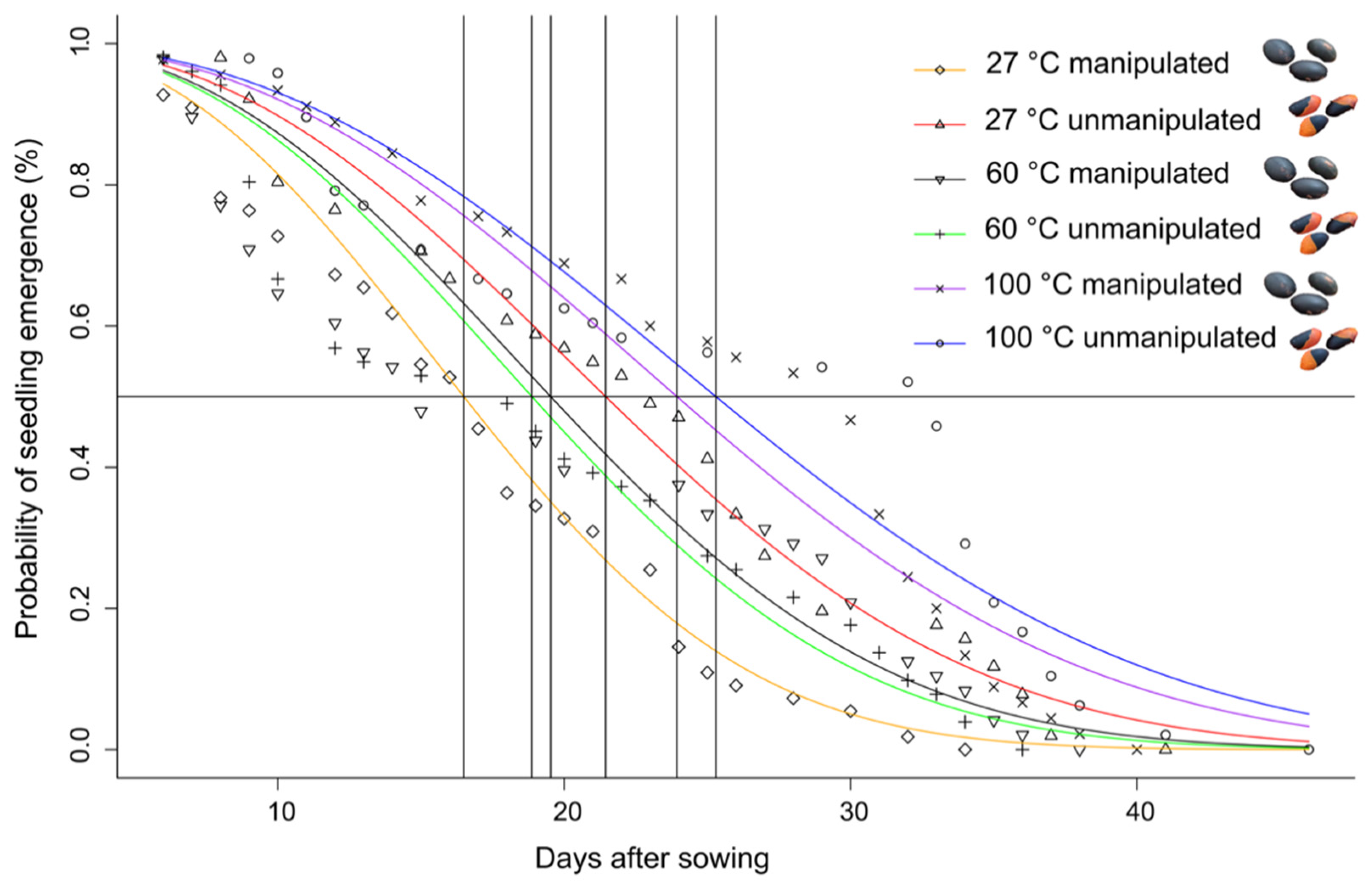
2.4. Bioassay 2: Effects of Heating and Manipulation by Atta Laevigata on Seed Germination
3. Results
3.1. Bioassay 1
3.2. Bioassay 2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, J.A.; Harms, K.E. Seed arrival, ecological filters, and plant species richness: A meta-analysis. Ecol. Lett. 2009, 12, 1250–1260. [Google Scholar] [CrossRef]
- Vellend, M. Conceptual Synthesis in Community Ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef]
- Grman, E.; Bassett, T.; Zirbel, C.R.; Brudvig, L.A. Dispersal and establishment filters influence the assembly of restored prairie plant communities. Restor. Ecol. 2015, 23, 892–899. [Google Scholar] [CrossRef]
- Harms, K.E.; Gagnon, P.R.; Passmore, H.A.; Myers, J.A.; Platt, W.J. Groundcover community assembly in high-diversity pine savannas: Seed arrival and fire-generated environmental filtering. Ecosphere 2017, 8, e01716. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Souza, M.L.; Fagundes, M. Seed Size as Key Factor in Germination and Seedling Development of Copaifera langsdorffii (Fabaceae). Am. J. Plant Sci. 2014, 5, 2566–2573. [Google Scholar] [CrossRef]
- Zida, D.; Sanou, L.; Diawara, S.; Savadogo, P.; Thiombiano, A. Herbaceous seeds dominates the soil seed bank after long-term prescribed fire, grazing and selective tree cutting in savanna-woodlands of West Africa. Acta Oecologica 2020, 108, 103607. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, N.; Traveset, A. Predispersal seed-predation by insects in the Venezuelan Central Plain: Overall patterns and traits that influence its biology and taxonomic groups. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 193–209. [Google Scholar] [CrossRef]
- Fagundes, M.; Maia, M.L.; Queiroz, A.; Fernandes, G.W.; Costa, F.V. Seed predation of Copaifera langsdorffii Desf. (Fabaceae: Caesalpinioideae) by Rhinochenus brevicollis Chevrolat (Coleoptera: Curculionidae) in a brazilian Cerrado fragment. Ecol. Austral 2013, 23, 218–221. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, Z.; Li, D.; Guo, Q. Pre-Dispersal Seed Predation in a Species-Rich Forest Community: Patterns and the Interplay with Determinants. PLoS ONE 2015, 10, e0143040. [Google Scholar] [CrossRef]
- Souza, M.L.; Fagundes, M. Seed predation of Copaifera langsdorffii (Fabaceae): A tropical tree with supra-annual fruiting. Plant Species Biol. 2016, 32, 66–73. [Google Scholar] [CrossRef]
- Han, Y.J.; Baskin, J.M.; Tan, D.Y.; Baskin, C.C.; Wu, M.Y. Effects of predispersal insect seed predation on the early life history stages of a rare cold sand-desert legume. Sci. Rep. 2018, 8, 3240. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Kajimura, H. Effects of insect predation on hypocotyl survival and germination success of mature Quercus variabilis acorns. J. For. Res. 2000, 5, 31–34. [Google Scholar] [CrossRef]
- Blubaugh, C.K.; Kaplan, I. Invertebrate Seed Predators Reduce Weed Emergence Following Seed Rain. Weed Sci. 2016, 64, 80–86. [Google Scholar] [CrossRef]
- De Sousa-Lopes, B.; Alves-Da-Silva, N.; Ribeiro-Costa, C.S.; Del-Claro, K. Temporal distribution, seed damage and notes on the natural history of Acanthoscelides quadridentatus and Acanthoscelides winderi (Coleoptera: Chrysomelidae: Bruchinae) on their host plant, Mimosa setosa var. paludosa (Fabaceae: Mimosoideae), in the Brazilian Cerrado. J. Nat. Hist. 2019, 53, 611–623. [Google Scholar] [CrossRef]
- Vallejo-Marín, M.; Dominguez, C.; Dirzo, R. Simulated seed predation reveals a variety of germination responses of neotropical rain forest species. Am. J. Bot. 2006, 93, 369–376. [Google Scholar] [CrossRef]
- Karban, R.; Lowenberg, G. Feeding by seed bugs and weevils enhances germination of wild Gossypium species. Oecologia 1992, 92, 196–200. [Google Scholar] [CrossRef]
- Silva, A.V.; Rossi, M.N. When a seed-feeding beetle is a predator and also increases the speed of seed germination: An intriguing interaction with an invasive plant. Evol. Ecol. 2019, 33, 211–232. [Google Scholar] [CrossRef]
- Pereira, A.C.F.; Fonseca, F.; Mota, G.R.; Fernandes, A.K.C.; Fagundes, M.; Reis-Júnior, R.; Faria, M.L. Ecological Interactions Shape the Dynamics of Seed Predation in Acrocomia aculeata (Arecaceae). PLoS ONE 2014, 9, e98026. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the Number of Tree Species in Tropical Forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Branco, M.; Branco, C.; Merouani, H.; Almeida, M.H. Germination success, survival and seedling vigour of Quercus suber acorns in relation to insect damage. For. Ecol. Manag. 2002, 166, 159–164. [Google Scholar] [CrossRef]
- Perea, R.; Fernandes, G.W.; Dirzo, R. Embryo size as a tolerance trait against seed predation: Contribution of embryo-damaged seeds to plant regeneration. Perspect. Plant Ecol. Evol. Syst. 2018, 31, 7–16. [Google Scholar] [CrossRef]
- Barroso, A.; Amor, F.; Cerda, X.; Boulay, R.R. Dispersal of non-myrmecochorous plants by a “keystone disperser” ant in a Mediterranean habitat reveals asymmetric interdependence. Insectes Sociaux 2012, 60, 75–86. [Google Scholar] [CrossRef]
- Camargo, P.H.S.A.; Martins, M.M.; Feitosa, R.M.; Christianini, A.V. Bird and ant synergy increases the seed dispersal effectiveness of an ornithochoric shrub. Oecologia 2016, 181, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.H.; Rodrigues, S.B.; Piratelli, A.J.; Oliveira, P.S.; Christianini, A.V. Interhabitat variation in diplochory: Seed dispersal effectiveness by birds and ants differs between tropical forest and savanna. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 48–57. [Google Scholar] [CrossRef]
- Van der Pijl, L. Ecological dispersal classes, established on the basis of the dispersing agents. In Principles of Dispersalin Higher Plants, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 22–90. [Google Scholar]
- Boesewinkel, F.D.; Bouman, F. The seed: Structure. In Embryology of Angiosperms; Johri, B.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 567–610. [Google Scholar]
- Penn, H.J.; Crist, T.O. From dispersal to predation: A global synthesis of ant-seed interactions. Ecol. Evol. 2018, 8, 9122–9138. [Google Scholar] [CrossRef]
- Janzen, D.H. Seed predation by animals. Annu. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Fernandes, T.V.; Paolucci, L.N.; Solar, R.R.C.; Neves, F.S.; Campos, R.I. Ant removal distance, but not seed manipulation and deposition site increases the establishment of a myrmecochorous plant. Oecologia 2019, 192, 133–142. [Google Scholar] [CrossRef]
- Culver, D.C.; Beattie, A.J. Myrmecochory in Viola: Dynamics of Seed-Ant Interactions in Some West Virginia Species. J. Ecol. 1978, 66, 53. [Google Scholar] [CrossRef]
- Zettler, J.A.; Spira, T.P.; Alen, C.R. Ant–seed mutualisms: Can red imported fire ants sour the relationship? Biol. Conserv. 2011, 101, 249–253. [Google Scholar] [CrossRef]
- Dayrell, R.L.C.; Gonçalves-Alvim, S.; Negreiros, D.; Fernandes, G.W.; Silveira, F. Environmental control of seed dormancy and germination of Mimosa calodendron (Fabaceae): Implications for ecological restoration of a highly threatened environment. Braz. J. Bot. 2015, 38, 395–399. [Google Scholar] [CrossRef]
- Leal, I.R.; Wirth, R.; Tabarelli, M. Seed Dispersal by Ants in the Semi-arid Caatinga of North-east Brazil. Ann. Bot. 2007, 99, 885–894. [Google Scholar] [CrossRef]
- Prior, K.M.; Saxena, K.; Frederickson, M. Seed handling behaviours of native and invasive seed-dispersing ants differentially influence seedling emergence in an introduced plant. Ecol. Èntomol. 2013, 39, 66–74. [Google Scholar] [CrossRef]
- Baskin, C.C. Breaking physical dormancy in seeds: Focusing on the lens. New Phytol. 2003, 158, 229–232. [Google Scholar] [CrossRef]
- Ribeiro, L.C.; Borghetti, F. Comparative effects of desiccation, heat shock and high temperatures on seed germination of savanna and forest tree species. Austral Ecol. 2013, 39, 267–278. [Google Scholar] [CrossRef]
- Fichino, B.S.; Dombroski, J.R.G.; Pivello, V.; Fidelis, A. Does Fire Trigger Seed Germination in the Neotropical Savannas? Experimental Tests with Six Cerrado Species. Biotropica 2016, 48, 181–187. [Google Scholar] [CrossRef]
- Wiggers, M.S.; Hiers, J.K.; Barnett, A.; Boyd, R.S.; Kirkman, L.K. Seed heat tolerance and germination of six legume species native to a fire-prone longleaf pine forest. Plant Vecol. 2016, 218, 151–171. [Google Scholar] [CrossRef]
- Moreira, B.; Tormo, J.; Estrelles, E.; Pausas, J.G. Disentangling the role of heat and smoke as germination cues in Mediterranean Basin flora. Ann. Bot. 2010, 105, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.C.; Pedrosa, M.; Borghetti, F. Heat shock effects on seed germination of five Brazilian savanna species. Plant Biol. 2012, 15, 152–157. [Google Scholar] [CrossRef]
- Ooi, M.K.J.; Denham, A.J.; Santana, V.M.; Auld, T.D. Temperature thresholds of physically dormant seeds and plant functional response to fire: Variation among species and relative impact of climate change. Ecol. Evol. 2014, 4, 656–671. [Google Scholar] [CrossRef] [PubMed]
- Zirondi, H.L.; Silveira, F.; Fidelis, A. Fire effects on seed germination: Heat shock and smoke on permeable vs impermeable seed coats. Flora 2019, 253, 98–106. [Google Scholar] [CrossRef]
- Fidelis, A.; Daibes, L.F.; Martins, A.R. To resist or to germinate? The effect of fire on legume seeds in Brazilian subtropical grasslands. Acta Bot. Bras. 2016, 30, 147–151. [Google Scholar] [CrossRef][Green Version]
- Junior, C.H.L.S.; Pessôa, A.C.M.; Carvalho, N.S.; Reis, J.B.C.; Anderson, L.O.; Aragão, L.E.O.C. The Brazilian Amazon deforestation rate in 2020 is the greatest of the decade. Nat. Ecol. Evol. 2020, 5, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Van Schaik, C.P.; Terborgh, J.W.; Wright, S.J. The phenology of tropical forests: Adaptive significance and consequences for primary consumers. Annu. Rev. Ecol. Syst. 1993, 24, 353–377. [Google Scholar] [CrossRef]
- Souza, M.L.; Solar, R.R.; Fagundes, M. Reproductive strategy of Copaifer alangsdorffii (Fabaceae): More seeds or better seeds? Rev. Biol. Trop. 2015, 63, 1161–1167. [Google Scholar]
- Simon, M.; Pennington, T. Evidence for Adaptation to Fire Regimes in the Tropical Savannas of the Brazilian Cerrado. Int. J. Plant Sci. 2012, 173, 711–723. [Google Scholar] [CrossRef]
- Christianini, A.V.; Mayhé-Nunes, A.J.; Oliveira, P.S. The role of ants in the removal of non-myrmecochorous diaspores and seed germination in a neotropical savanna. J. Trop. Ecol. 2007, 23, 343–351. [Google Scholar] [CrossRef]
- Christianini, A.V.; Mayhé-Nunes, A.J.; Oliveira, P.S. Exploitation of fallen diaspores by ants: Are there ant–plant partner choices? Biotropica 2012, 44, 360–367. [Google Scholar] [CrossRef]
- Costa, A.N.; Vasconcelos, H.L.; Vieira-Neto, E.H.; Bruna, E.M. Do herbivores exert top-down effects in Neotropical savannas? Estimates of biomass consumption by leaf-cutter ants. J. Veg. Sci. 2008, 19, 849–854. [Google Scholar] [CrossRef]
- Costa, A.N.; Vasconcelos, H.L.; Bruna, E.M. Biotic drivers of seedling establishment in Neotropical savannas: Selective granivory and seedling herbivory by leaf-cutter ants as an ecological filter. J. Ecol. 2016, 105, 132–141. [Google Scholar] [CrossRef]
- Fernandes, T.V.; Paolucci, L.N.; Carmo, F.M.S.; Sperber, C.F.; Campos, R.I. Seed manipulation by ants: Disentangling the effects of ant behaviours on seed germination. Ecol. Èntomol. 2018, 43, 712–718. [Google Scholar] [CrossRef]
- Veloso, A.; Silva, P.S.; Siqueira, W.K.; Duarte, K.L.; Gomes, I.L.V.; Santos, H.T.; Fagundes, M. Intraspecific variation in seed size and light intensity affect seed germination and initial seedling growth of a tropical shrub. Acta Bot. Bras. 2017, 31, 736–741. [Google Scholar] [CrossRef]
- Fagundes, M.; Cuevas-Reyes, P.; Araujo, W.S.; Faria, M.L.; Valerio, H.M.; Pimenta, M.A. Influence of light availability and seed mass on germinability and initial growth of two congeneric species of Fabaceae. Acta Bot. Mex. 2020, 127, e1638. [Google Scholar]
- Fagundes, M.; Barbosa, E.M.; Oliveira, J.B.B.S.; Brito, B.G.S.; Freitas, K.T.; Freitas, K.F.; Reis-Junior, R. Galling inducing insects associated with a tropical shrub: The role of resource concentration and species interactions. Ecol. Austral 2019, 29, 12–19. [Google Scholar] [CrossRef]
- Ramírez, N. Historia de vida de Copaifera pubiflora Benth. (Fabaceae, Caesalpinioideae) em los Altos Llanos Centrales Venezolanos. Trib. Del Investig. 1994, 1, 69–75. [Google Scholar]
- De Lima, R.M.; Santos, S.B.D.O.; Vaz, S.; Gomes, A.L.S.; De Sousa, W.O. Description of the larva and pupa of Apion brevicorne Gerstaecker, 1854 (Coleoptera: Brentidae: Apioninae) with biological information. Papéis Avulsos De Zool. 2020, 60, e202060. [Google Scholar] [CrossRef]
- Leal, I.; Oliveira, P.S. Interactions between Fungus-Growing Ants (Attini), Fruits and Seeds in Cerrado Vegetation in Southeast Brazil1. Biotropica 1998, 30, 170–178. [Google Scholar] [CrossRef]
- Giladi, I. Choosing benefits or partners: A review of the evidence for the evolution of myrmecochory. Oikos 2006, 112, 481–492. [Google Scholar] [CrossRef]
- INMET, Instituto Nacional de Meteorologia. Available online: http://www.inmet.gov.br/ (accessed on 10 September 2021).
- Kuchenbecker, J.; Fagundes, M. Diversity of insects associated with two common plants in the Brazilian Cerrado: Responses of two guilds of herbivores to bottom-up and top-down forces. Eur. J. Èntomol. 2018, 115, 354–363. [Google Scholar] [CrossRef]
- Warton, D.I.; Hui, F.K.C. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Weibull, W. Wide applicability. J. Appl. Mech. 1951, 103, 293–297. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Zupo, T.; Baeza, M.J.; Fidelis, A. The effect of simulated heat-shock and daily temperature fluctuations on seed germination of four species from fire-prone ecosystems. Acta Bot. Bras. 2016, 30, 514–519. [Google Scholar] [CrossRef]
- Bohn, K.; Pavlick, R.; Reu, B.; Kleidon, A. The Strengths of r- and K-Selection Shape Diversity-Disturbance Relationships. PLoS ONE 2014, 9, e95659. [Google Scholar] [CrossRef]
- Imbert, E. Dispersal by ants in Centaurea corymbosa (Asteraceae): What is the elaiosome for? Plant Species Biol. 2006, 21, 109–117. [Google Scholar] [CrossRef]
- Ohkawara, K.; Akino, T. Seed cleaning behavior by tropical ants and its anti-fungal effect. J. Ethol. 2004, 23, 93–98. [Google Scholar] [CrossRef]
- Kulik, M.M.; Yaklich, R.W. Soybean Seed Coat Structures: Relationship to Weathering Resistance and Infection by the Fungus Phomopsis phaseoli. Crop. Sci. 1991, 31, 108–113. [Google Scholar] [CrossRef]
- Miranda, H.S.; Sato, M.N.; Neto, W.N.; Aires, F.S. Fires in the Cerrado, the Brazilian savanna. In Tropical Fire Ecology: Climate Change, Land Use and Ecosystem Dynamics; Cochrane, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 427–450. [Google Scholar]
- Pivello, V.R.; Oliveras, I.; Miranda, H.S.; Haridasan, M.; Sato, M.N.; Meirelles, S.T. Effect of fires on soil nutrient availability in an open savanna in Central Brazil. Plant Soil 2010, 337, 111–123. [Google Scholar] [CrossRef]
- Corbineau, F.; Xia, Q.; Bailly, C.; El-Maarouf-Bouteau, H. Ethylene, a key factor in the regulation of seed dormancy. Front. Plant Sci. 2014, 5, 539. [Google Scholar] [CrossRef]
- Kępczyński, J. Induction of agricultural weed seed germination by smoke and smoke-derived karrikin (KAR 1), with a particular reference to Avena fatua L. Acta Physiol. Plant. 2018, 40, 87. [Google Scholar] [CrossRef]
- Shayanfar, A.; Ghaderi-Far, F.; Behmaram, R.; Soltani, A.; Sadeghipour, H.R. Impacts of fire cues on germination of Brassica napus L. seeds with high and low secondary dormancy. Plant Biol. 2020, 22, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Okamoto, M.; Tatematsu, K.; Yano, R.; Seo, M.; Kamiya, Y. Abscisic acid and the control of seed dormancy and germination. Seed Sci. Res. 2010, 20, 55–67. [Google Scholar] [CrossRef]
- Kang, J.; Yim, S.; Choi, H.; Kim, A.; Lee, K.P.; Lopez-Molina, L. Abscisic acid transporters cooperate to control seed germination. Nat. Commun. 2015, 6, 8113. [Google Scholar] [CrossRef]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, 874–878. [Google Scholar] [CrossRef]

| Response Variable | Explanatory Variable | Error Distribution | D.f. | Residual Deviance | Residual Df | Dev | F | p |
|---|---|---|---|---|---|---|---|---|
| Time for germination | Predation treatments | Weibull | 2 | --- | 131 | 28.278 | --- | <0.001 |
| Germination percentage | Predation treatments | Quasibinomial | 2 | 249.57 | 207 | 24.169 | 11.912 | <0.001 |
| Response Variable | Explanatory Variable | Error Distribution | D.f. | Residual Deviance | Resid. Df | Dev | F | p |
|---|---|---|---|---|---|---|---|---|
| Time for germination | Elaiosome | Weibull | 2 | --- | 293 | 4.135 | --- | <0.001 |
| Temperature | 1 | --- | 294 | 24.372 | --- | 0.0419 | ||
| Elaiosome * temperature | 2 | --- | 291 | 6.987 | --- | 0.0303 | ||
| Germination percentage | Elaiosome | Quasibinomial | 1 | 506.12 | 418 | 0.04621 | 0.0456 | 0.8311 |
| Temperature | 2 | 503.17 | 416 | 2.95142 | 1.4546 | 0.2347 | ||
| Elaiosome * temperature | 2 | 501.99 | 414 | 1.17516 | 0.5792 | 0.5608 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagundes, M.; dos Santos, H.T.; Cuevas-Reyes, P.; Cornelissen, T. Biotic and Abiotic Interactions Shape Seed Germination of a Fire-Prone Species. Seeds 2022, 1, 16-27. https://doi.org/10.3390/seeds1010003
Fagundes M, dos Santos HT, Cuevas-Reyes P, Cornelissen T. Biotic and Abiotic Interactions Shape Seed Germination of a Fire-Prone Species. Seeds. 2022; 1(1):16-27. https://doi.org/10.3390/seeds1010003
Chicago/Turabian StyleFagundes, Marcilio, Henrique Tadeu dos Santos, Pablo Cuevas-Reyes, and Tatiana Cornelissen. 2022. "Biotic and Abiotic Interactions Shape Seed Germination of a Fire-Prone Species" Seeds 1, no. 1: 16-27. https://doi.org/10.3390/seeds1010003
APA StyleFagundes, M., dos Santos, H. T., Cuevas-Reyes, P., & Cornelissen, T. (2022). Biotic and Abiotic Interactions Shape Seed Germination of a Fire-Prone Species. Seeds, 1(1), 16-27. https://doi.org/10.3390/seeds1010003





