Interaction of Carbon, Titanium, and Boron in Micro-Alloy Steels and Its Effect on Hot Ductility
Abstract
1. Introduction
2. Experimental Procedure
3. Results
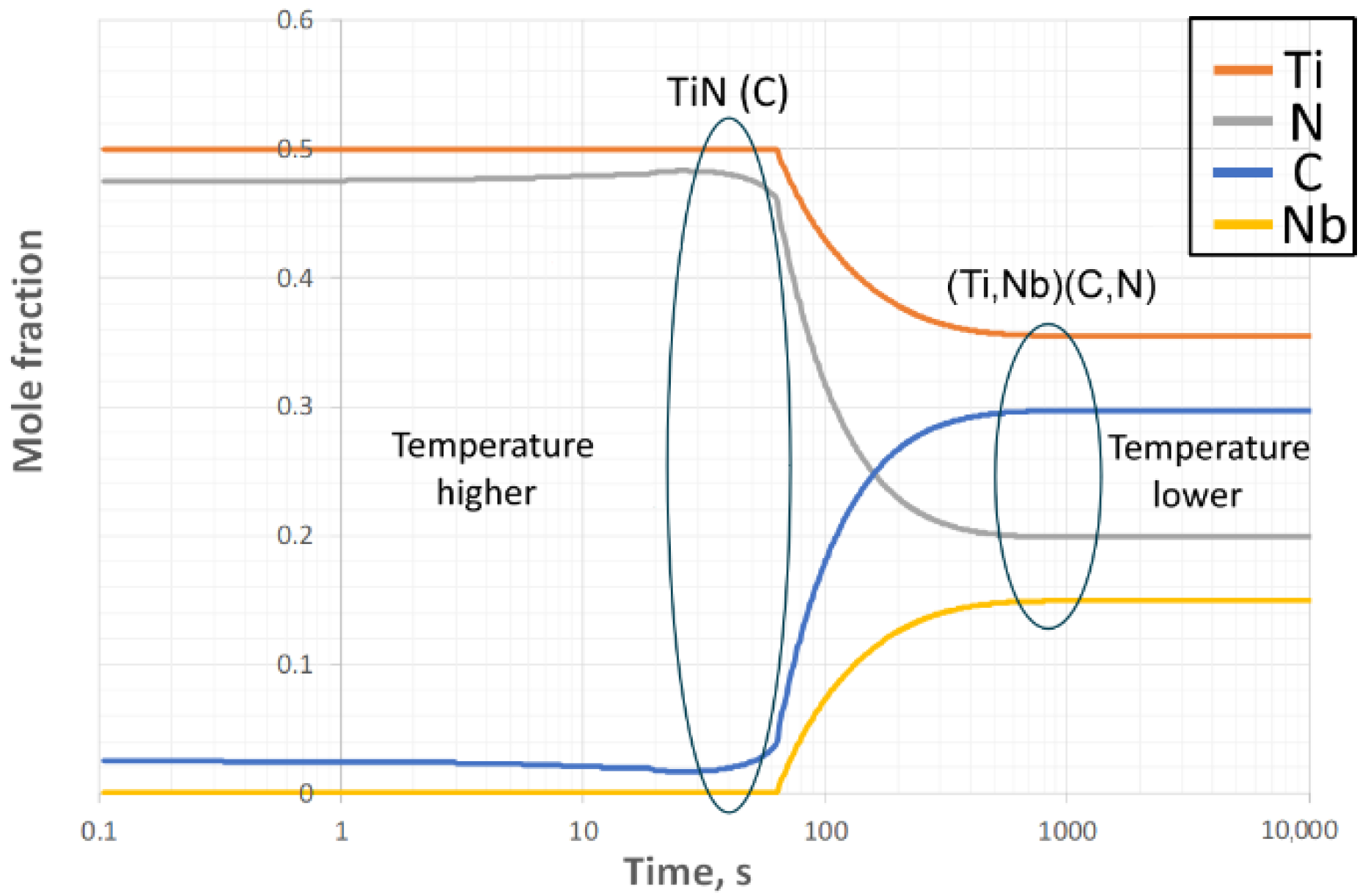
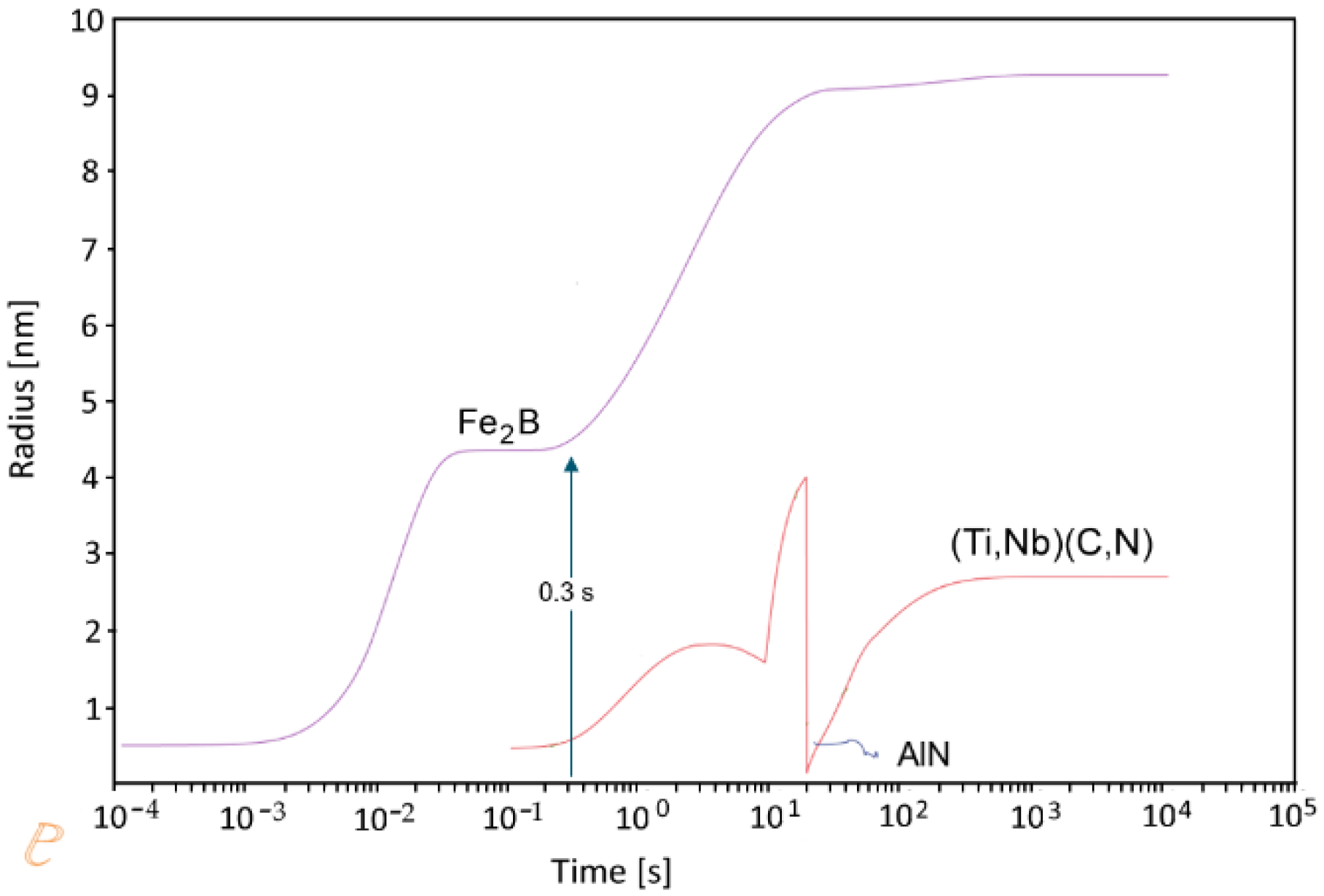
3.1. Thermo-Dynamic and Kinetic Simulations
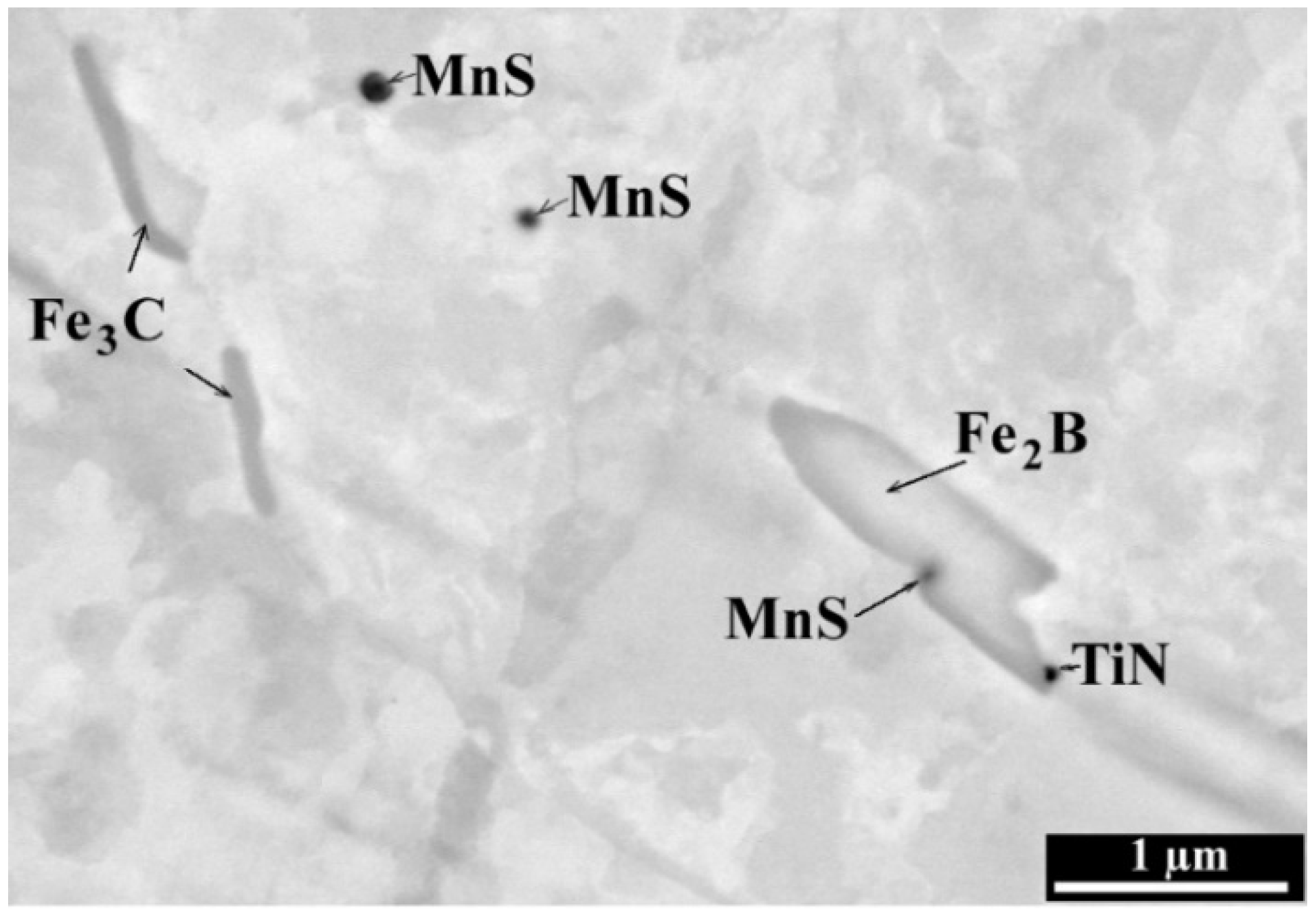
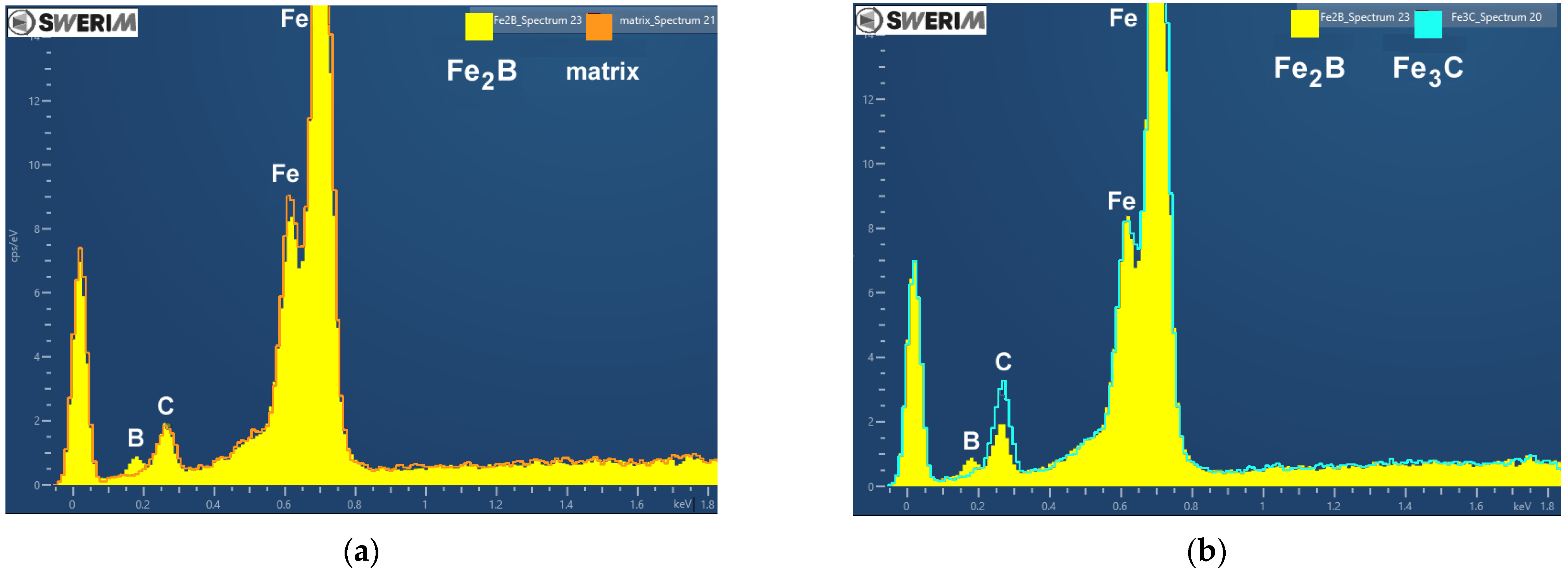
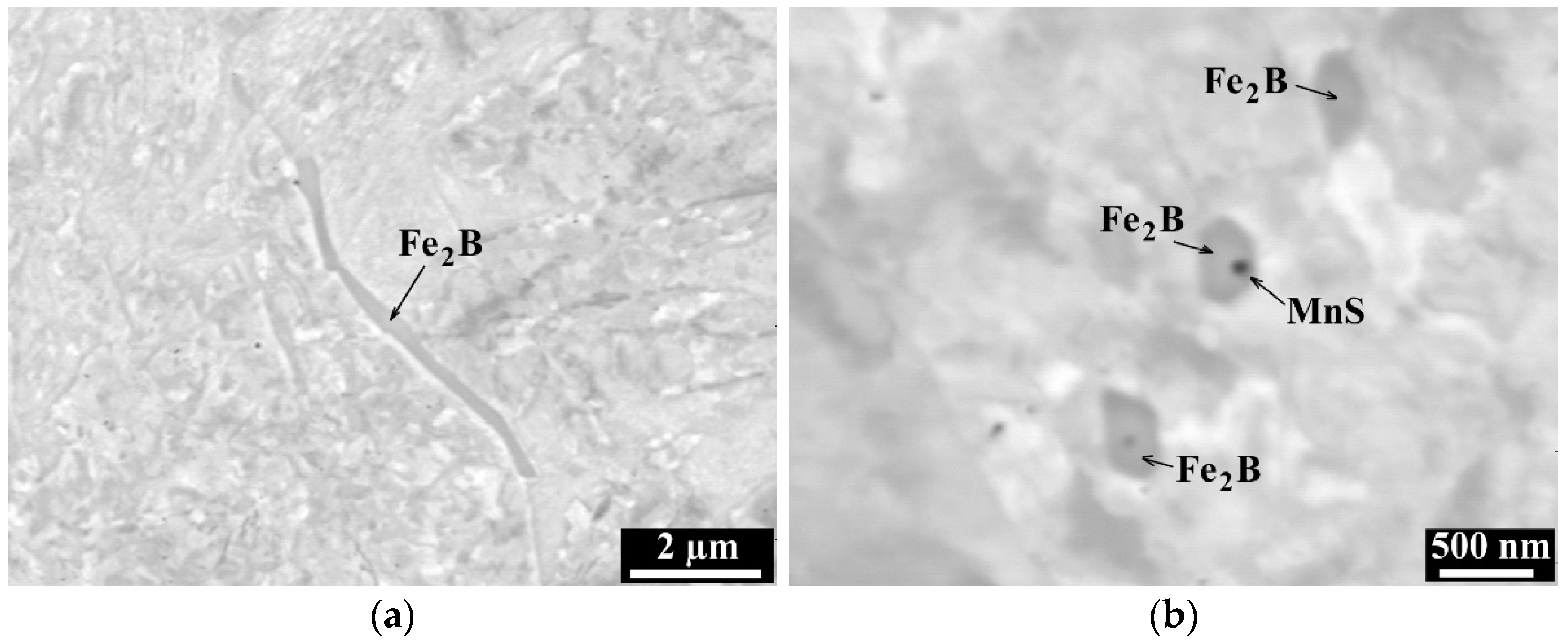
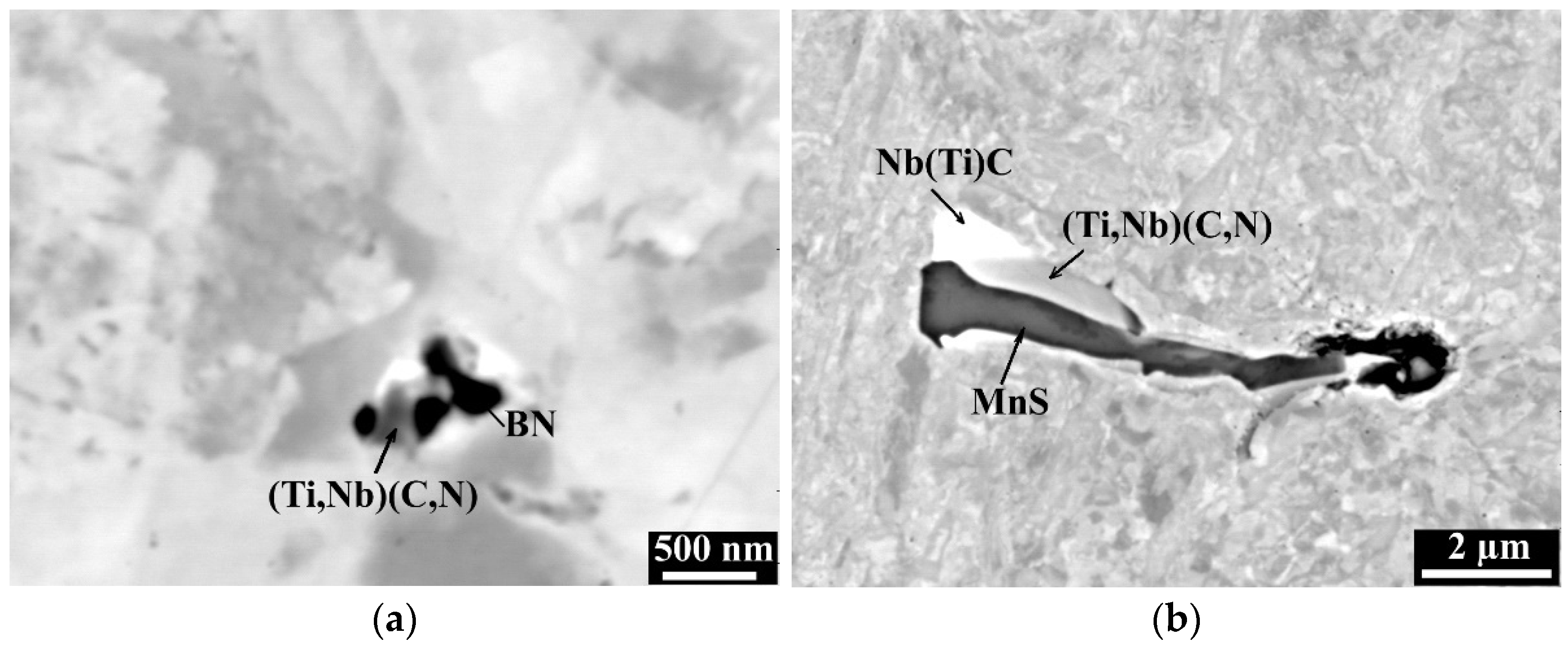
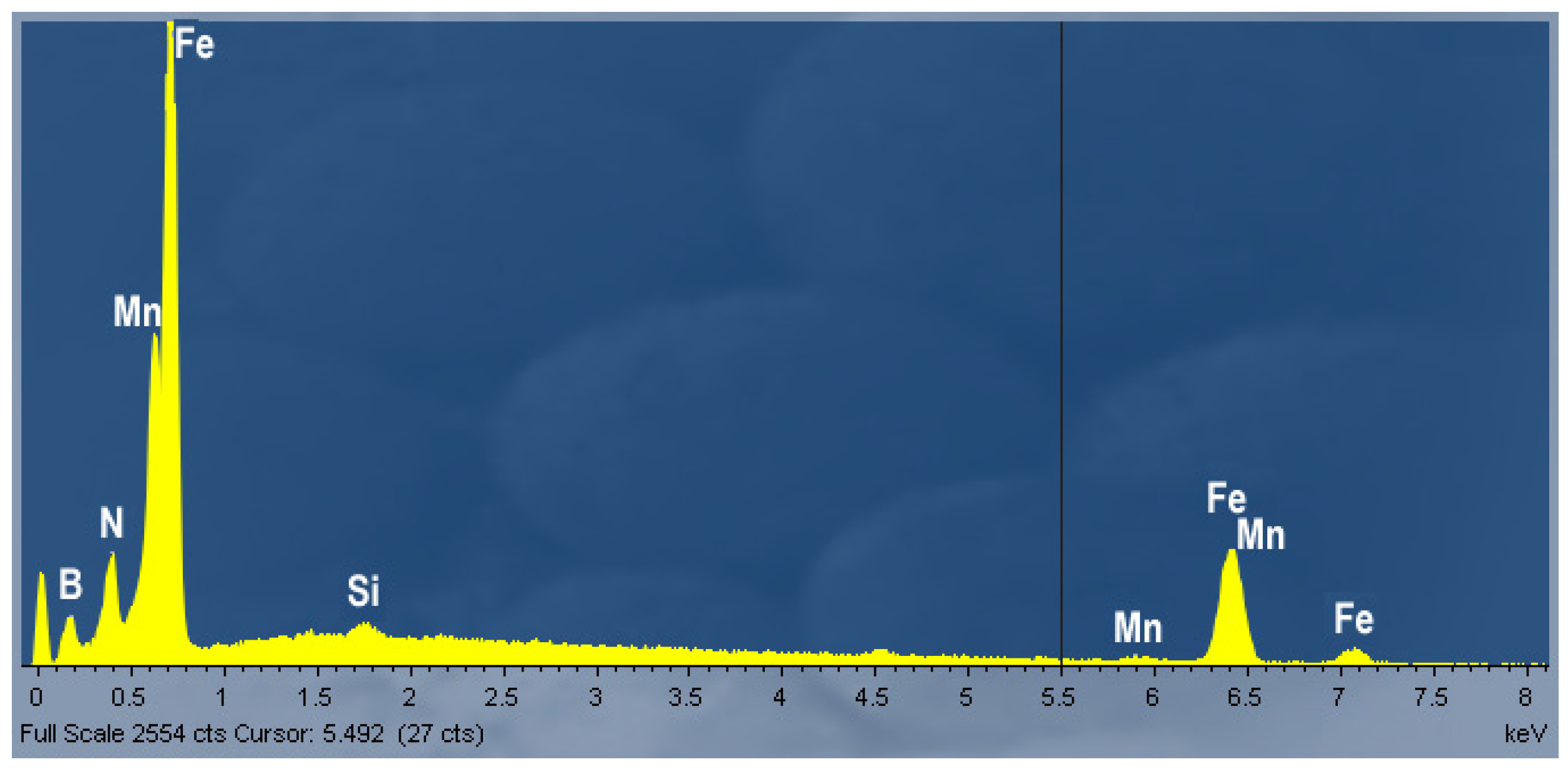
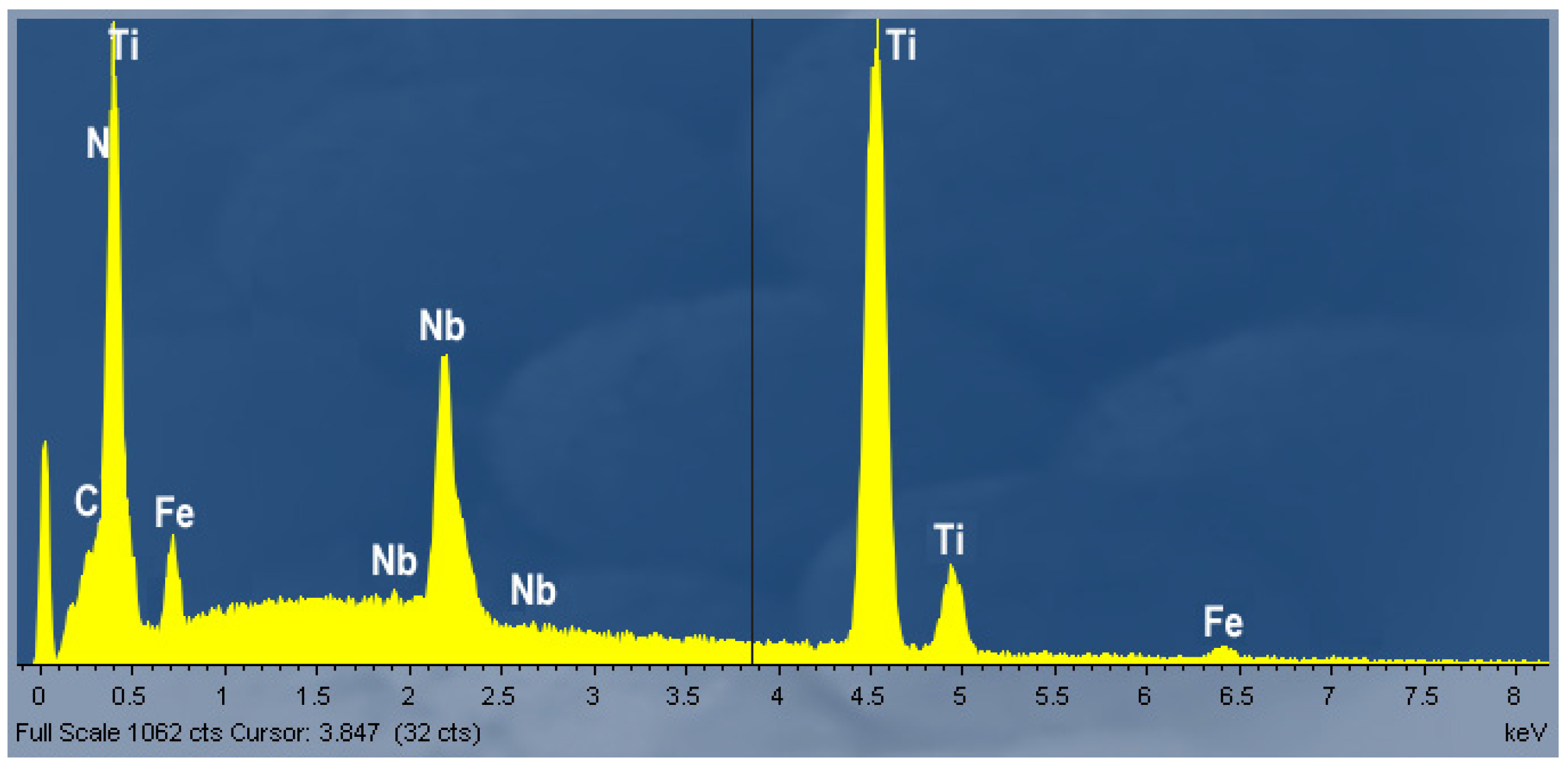
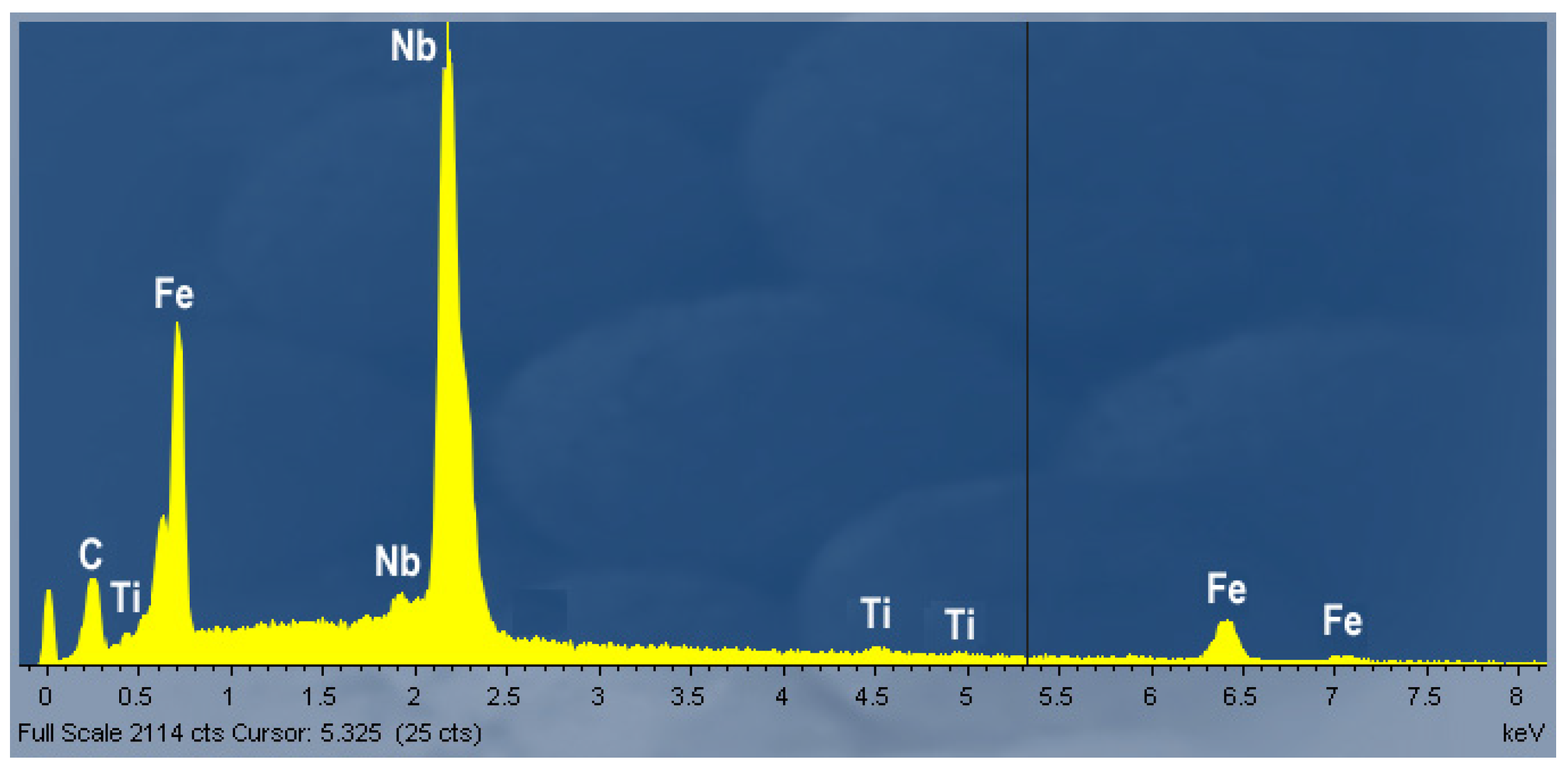
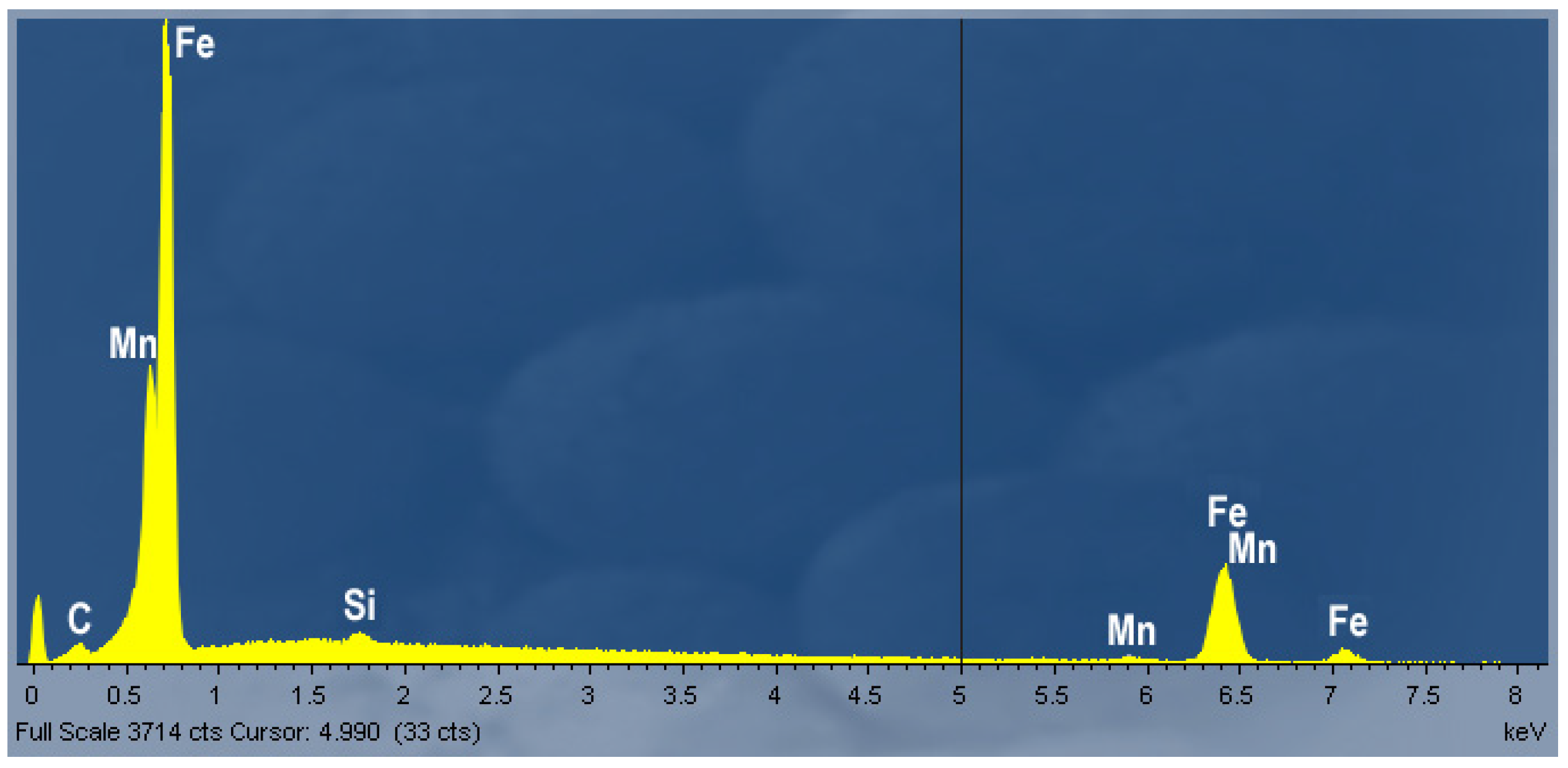
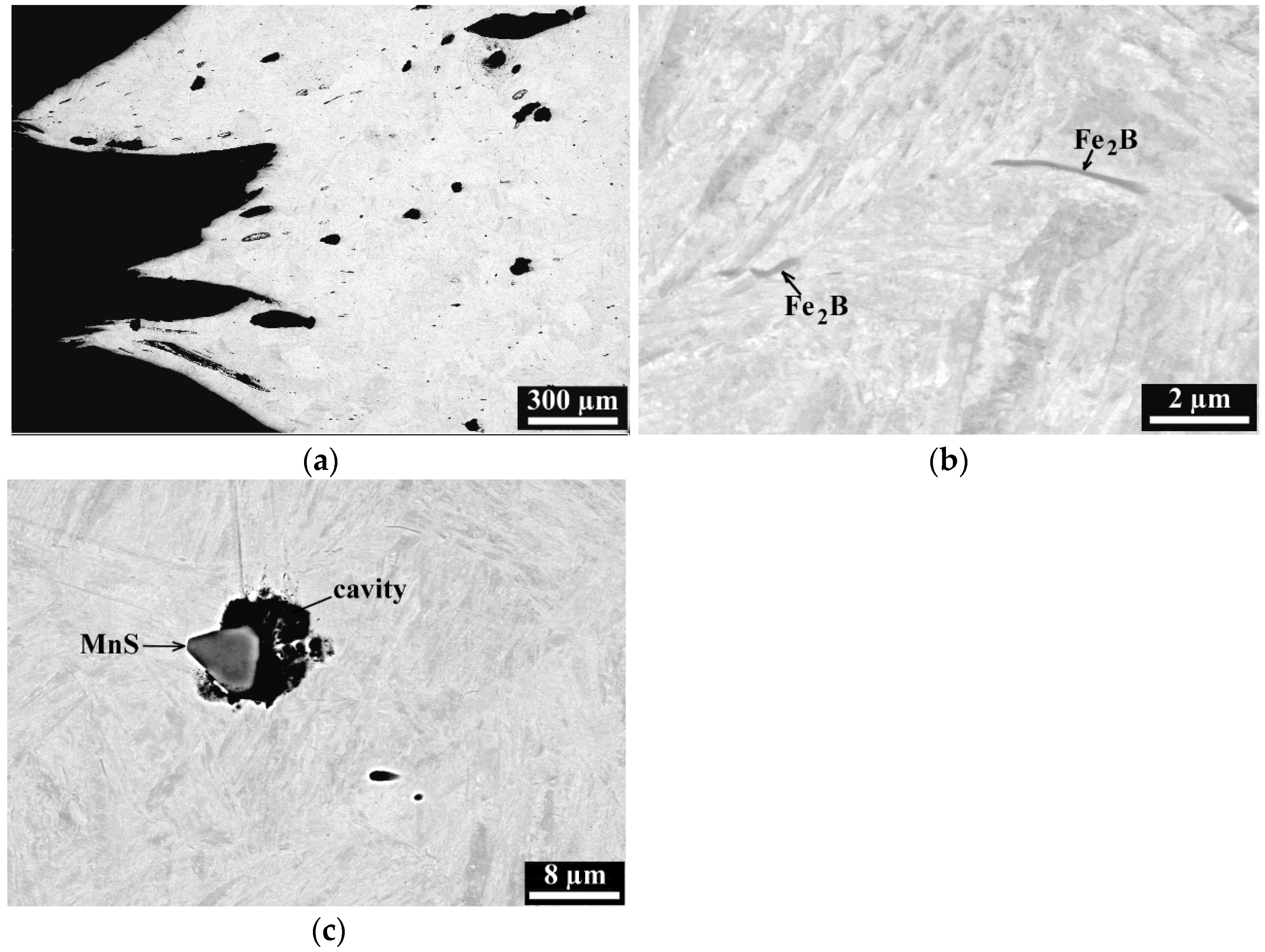
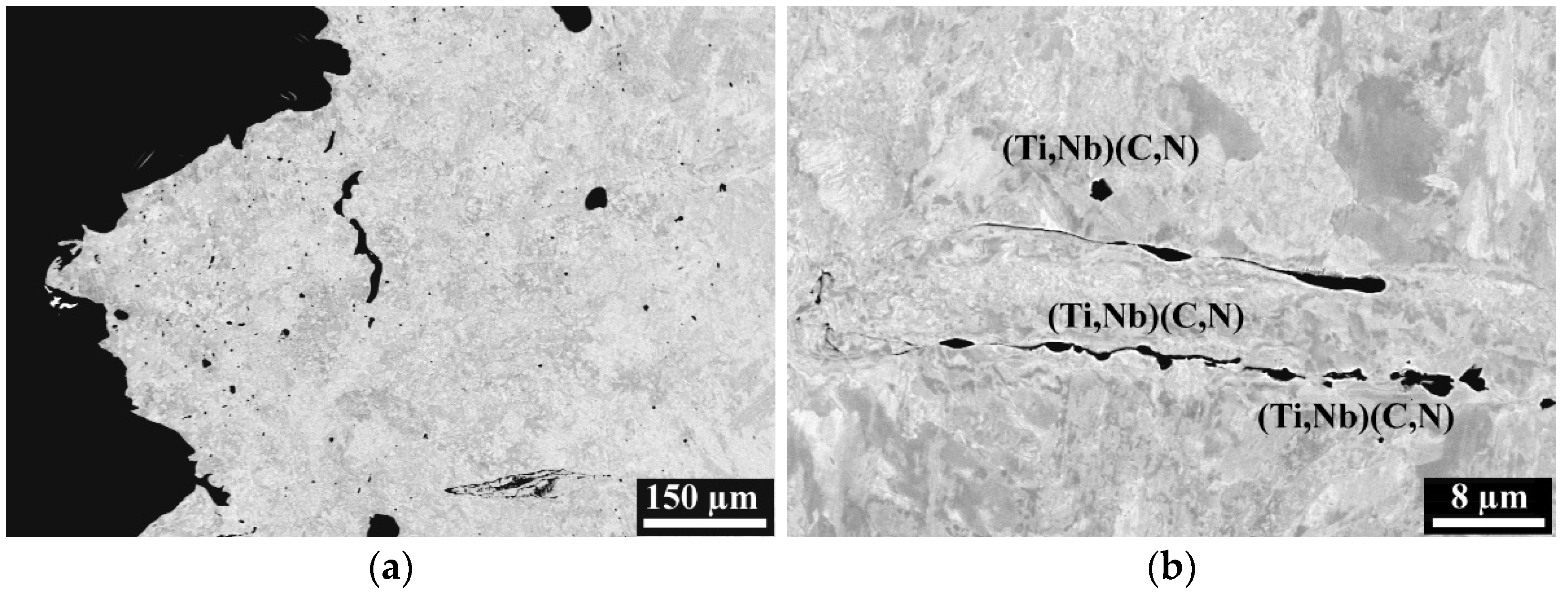
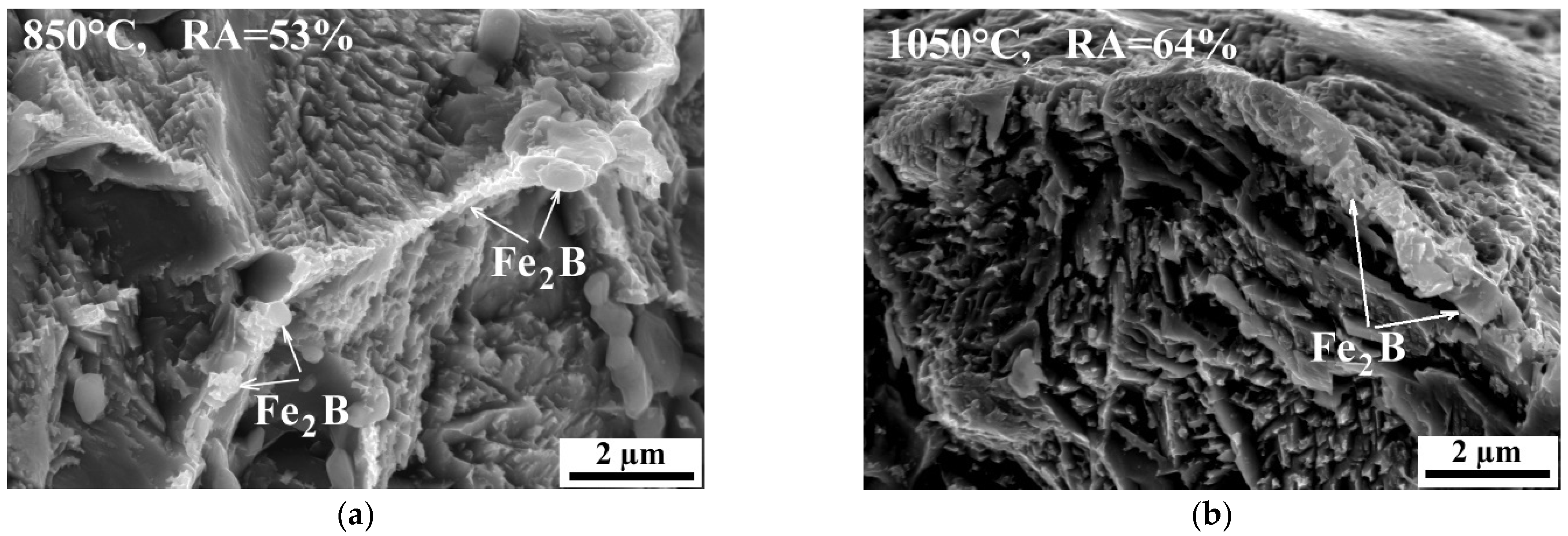
3.2. Metallographic Investigation
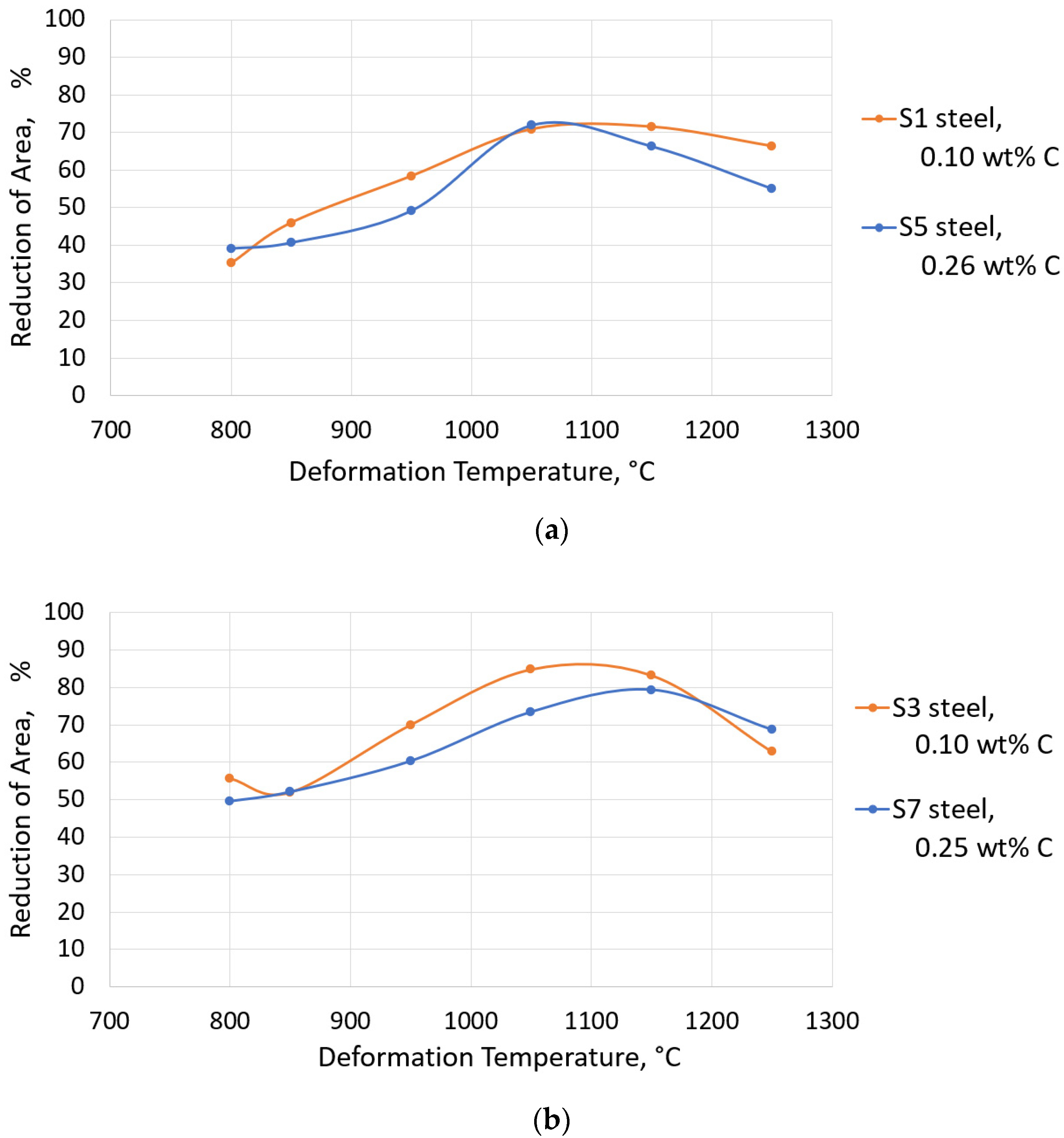
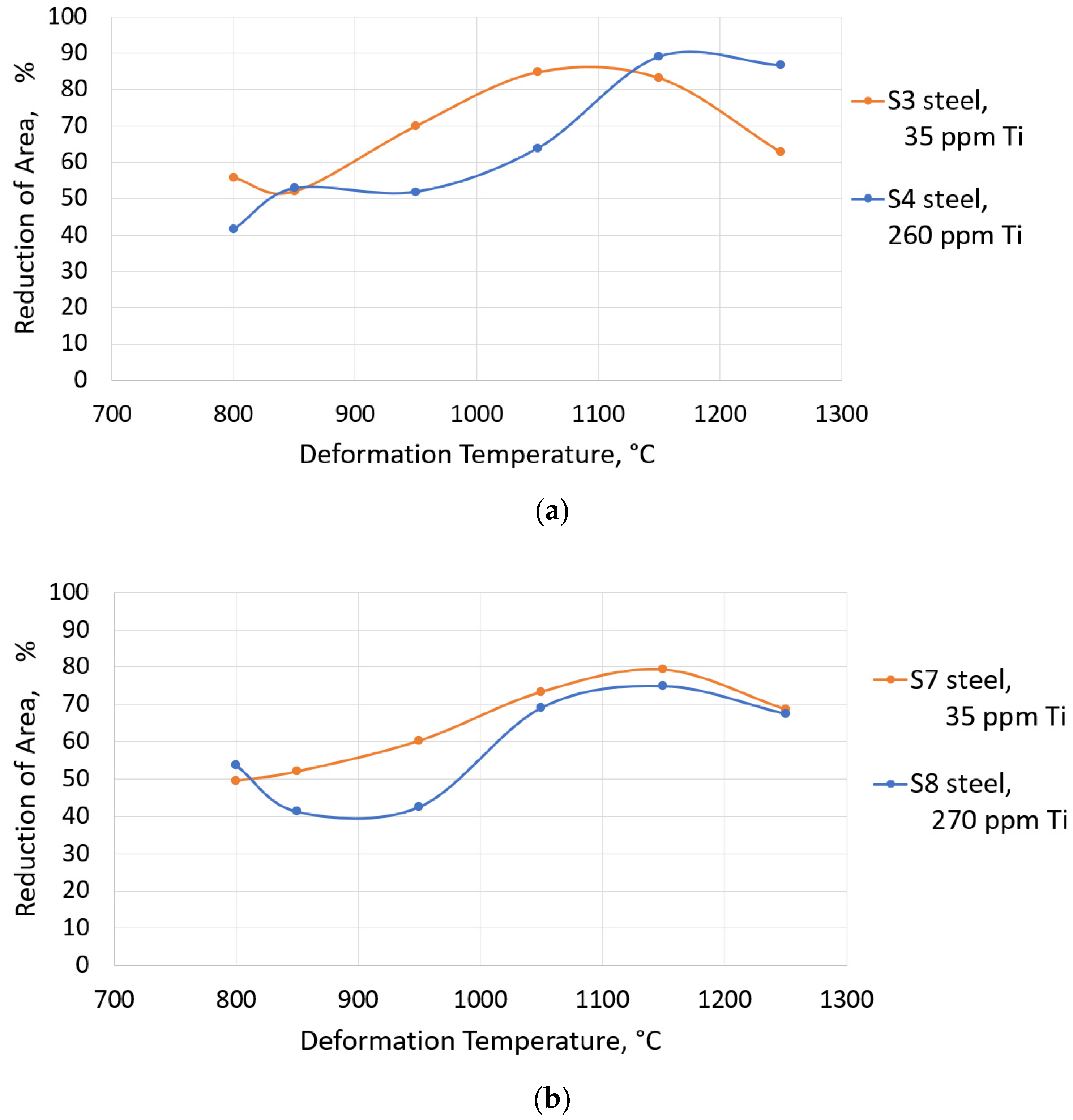
3.3. Hot Ductility Tests
4. Conclusions
- Boron precipitation as iron boride Fe2B, is very beneficial for the hot ductility of steel. This type of precipitate is mostly located along the austenite grain boundaries and as being coherent with the matrix, effectively increases the hot ductility.
- Low nitrogen content (30–35 ppm) in all steels investigated, limited the precipitation of nitrides and carbonitrides.
- Titanium is a very important alloy element protecting boron from binding with nitrogen into BN and the right combination of Ti and B content may give a very good hot ductility.
- Higher Ti contents increased hot ductility in a low ductility zone (1150 °C–1250 °C). However, increasing Ti from 35 ppm to 270 ppm in steel with 0.25 wt% C and 100 ppm B, caused a deterioration in the RA in the entire temperature range. It can be related to the intensified precipitation of (Ti,Nb) carbides/carbonitrides.
- The efficiency of using Ti and B to improve the hot ductility of steel depends on the carbon content. Since the lower carbon content decreases the negative effect of carbides/carbonitrides on the hot ductility, steels with 0.10 wt% C often show better RA than steels with 0.25 wt% C. The addition of Ti to a 0.10 wt% C steel resulted in a higher RA than was possible with an addition of Ti to a 0.25 wt% C steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Chipres, E.; Mejía, I.; Maldonado, C.; Bedolla-Jacuinde, A.; Cabrera, J. Hot ductility behavior of boron microalloyed steels. Mater. Sci. Eng. A 2007, 460–461, 464–470. [Google Scholar] [CrossRef]
- Hannerz, N.E. Critical Hot Plasticity and Transverse Slab Casting with Particular Reference Cracking in Continuous to Composition. Trans. ISIJ 1985, 25, 149–158. [Google Scholar] [CrossRef]
- Song, S.-H.; Guo, A.-M.; Shen, D.-D.; Yuan, Z.-X.; Liu, J.; Xu, T.-D. Effect of boron on the hot ductility of 2.25Cr1Mo steel. Mater. Sci. Eng. A 2003, 360, 96–100. [Google Scholar] [CrossRef]
- Mejía, I.; Salas-Reyes, A.; Calvo, J.; Cabrera, J.M. Effect of Ti and B microadditions on the hot ductility behavior of a High-Mn austenitic Fe–23Mn–1.5Al–1.3Si–0.5C TWIP steel. Mater. Sci. Eng. A 2015, 648, 311–329. [Google Scholar] [CrossRef]
- Komenda, J.; Martin, D.; Lönnqvist, J. The Effect of Boron Addition on Precipitation and Hot Ductility of 1.5Mn-0.1Nb-Ti Carbon Steels in As-Cast Condition. Mater. Sci. Forum 2016, 879, 990–995. [Google Scholar] [CrossRef]
- Cho, K.C.; Mun, D.J.; Kang, M.H.; Lee, J.S.; Kil Park, J.; Koo, Y.M. Effect of Thermal Cycle and Nitrogen Content on the Hot Ductility of Boron-bearing Steel. ISIJ Int. 2010, 50, 839–846. [Google Scholar] [CrossRef]
- Chown, L.; Cornish, L. Investigation of hot ductility in Al-killed boron steels. Mater. Sci. Eng. A 2008, 494, 263–275. [Google Scholar] [CrossRef]
- Hulka, K.; Kern, A.; Schriever, U. Application of Niobium in Quenched and Tempered High-Strength Steels. Mater. Sci. Forum 2005, 500–501, 519–526. [Google Scholar] [CrossRef]
- PMAPIA. Precipitation of Micro Alloy Particles in B and Mn Alloyed Steel Grades and Their InterAction between Elements, Segregation, and Defects during Continuous Casting; RFCS Grant Agreement GA 800644-PMAPIA RFCS 2017; Final Report in Preparation during 2022. Publication Office of the EU. Available online: https://op.europa.eu/en/search-results (accessed on 1 January 2022).
- Zarandi, F.; Yue, S. The Effect of Boron on Hot Ductility of Nb-microalloyed Steels. ISIJ Int. 2006, 46, 591–598. [Google Scholar] [CrossRef][Green Version]
- Mintz, B.; Tuling, A.; Delgado, A. Influence of silicon, aluminium, phosphorus and boron on hot ductility of TRansformation Induced Plasticity assisted steels. Mater. Sci. Technol. 2003, 19, 1721–1726. [Google Scholar] [CrossRef]
- Luitjohan, K.E.; Krane, M.J.M.; Ortalan, V.; Johnson, D.R. Investigation of the metatectic reaction in iron-boron binary alloys. J. Alloy. Compd. 2018, 732, 498–505. [Google Scholar] [CrossRef]
- Abushosha, R.; Vipond, R.; Mintz, B. Influence of titanium on hot ductility of as cast steels. Mater. Sci. Technol. 1991, 7, 613–621. [Google Scholar] [CrossRef]
- Abushosha, R.; Comineli, O.; Mintz, B. Influence of Ti on hot ductility of C–Mn–Al steels. Mater. Sci. Technol. 1999, 15, 278–286. [Google Scholar] [CrossRef]
- Luo, H.; Karjalainen, P.; Porter, D.A.; Liimatainen, H.-M.; Zhang, Y. The Influence of Ti on the Hot Ductility of Nb-bearing Steels in Simulated Continuous Casting Process. ISIJ Int. 2002, 42, 273–282. [Google Scholar] [CrossRef]
- Mintz, B. Influence of nitrogen on hot ductility of steels and its relationship to problem of transverse cracking. Ironmak. Steelmak. 2000, 27, 343–347. [Google Scholar] [CrossRef]
- Jung, J.; Lee, S.-J.; Kim, S.; De Cooman, B.C. Effect of Ti Additions on Micro-Alloyed Nb TRIP Steel. Steel Res. Int. 2011, 82, 857–865. [Google Scholar] [CrossRef]
- Yuan, J.; Xiao, Y.; Min, N.; Li, W.; Zhao, S. The Influence of Precipitate Morphology on the Growth of Austenite Grain in Nb-Ti-Al Microalloyed Steels. Materials 2022, 15, 3176. [Google Scholar] [CrossRef] [PubMed]
- Kvackaj, T.; Bidulská, J.; Bidulský, R. Overview of HSS Steel Grades Development and Study of Reheating Condition Effects on Austenite Grain Size Changes. Materials 2021, 14, 1988. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Han, Y.; Liu, H.-S.; Teng, H.-X.; Qiu, M.-S.; Yang, F. Microstructure evolution and enhanced mechanical properties of a novel Nb-Ti micro-alloyed medium-Mn steel. Mater. Charact. 2022, 187, 111828. [Google Scholar] [CrossRef]

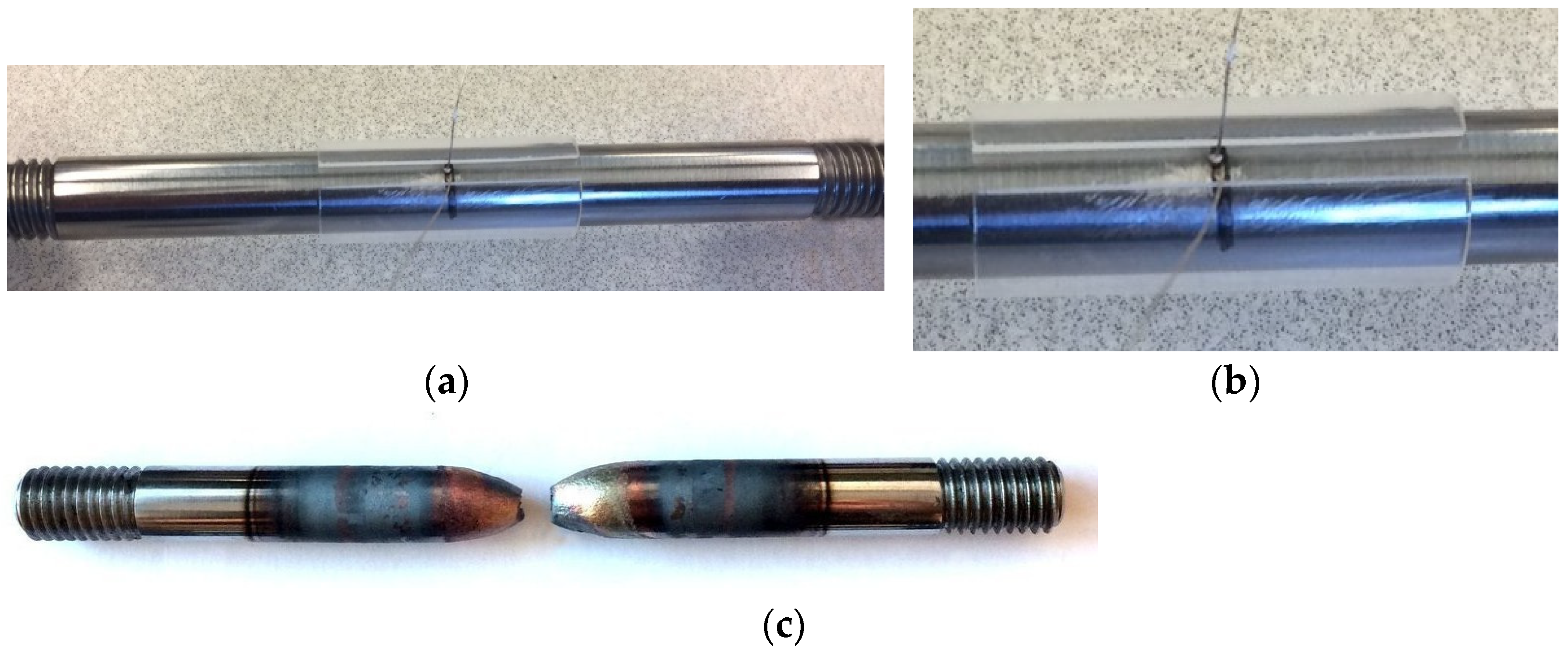
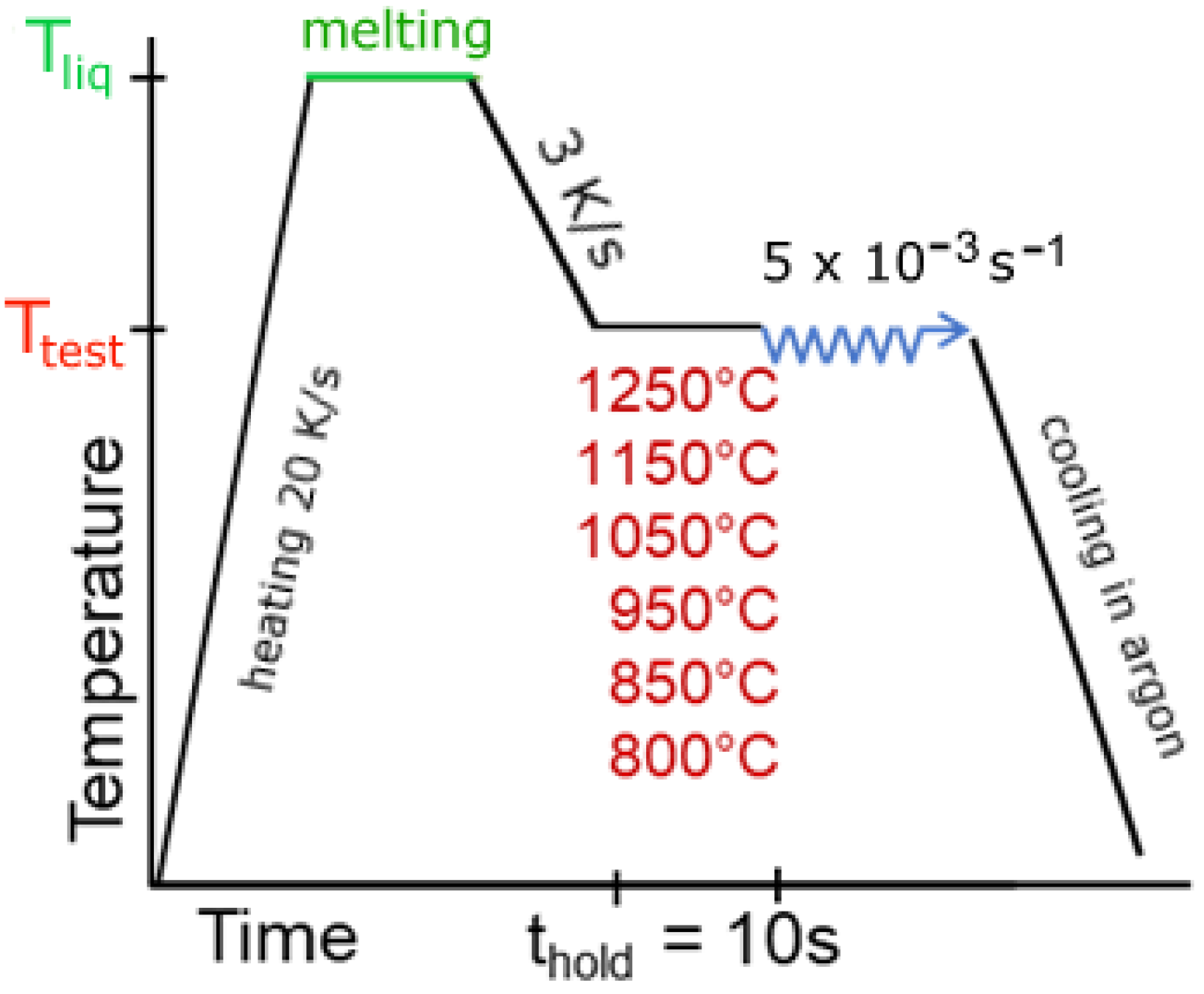
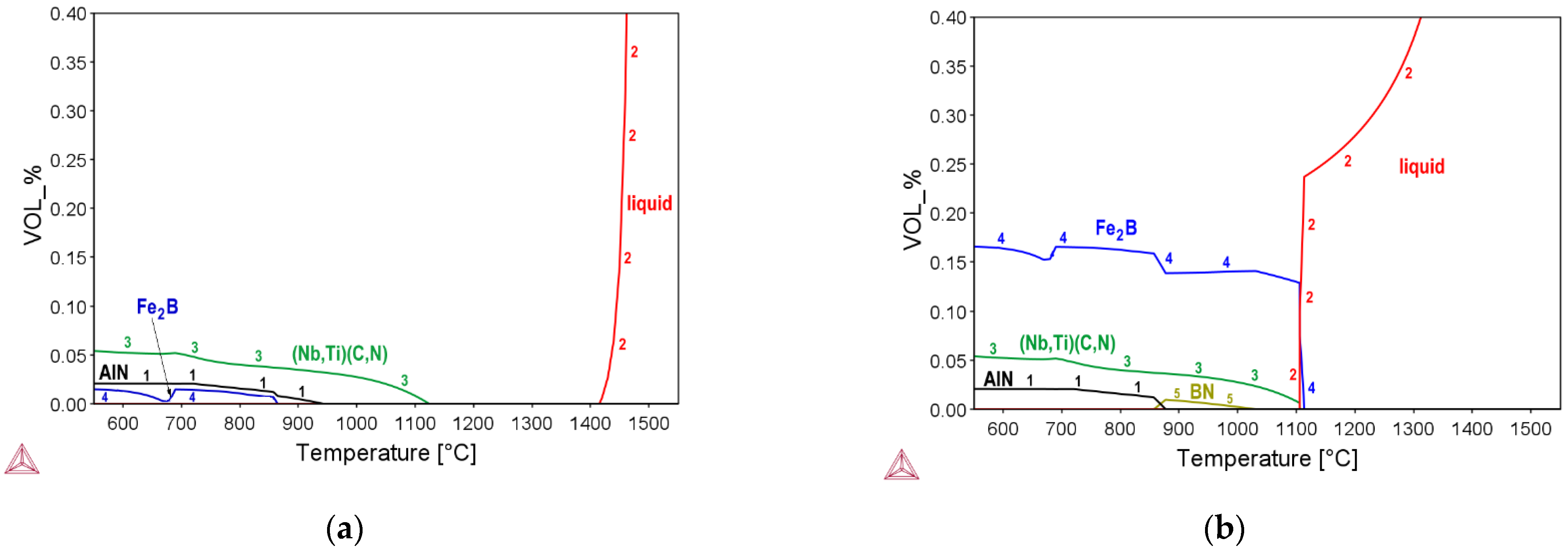

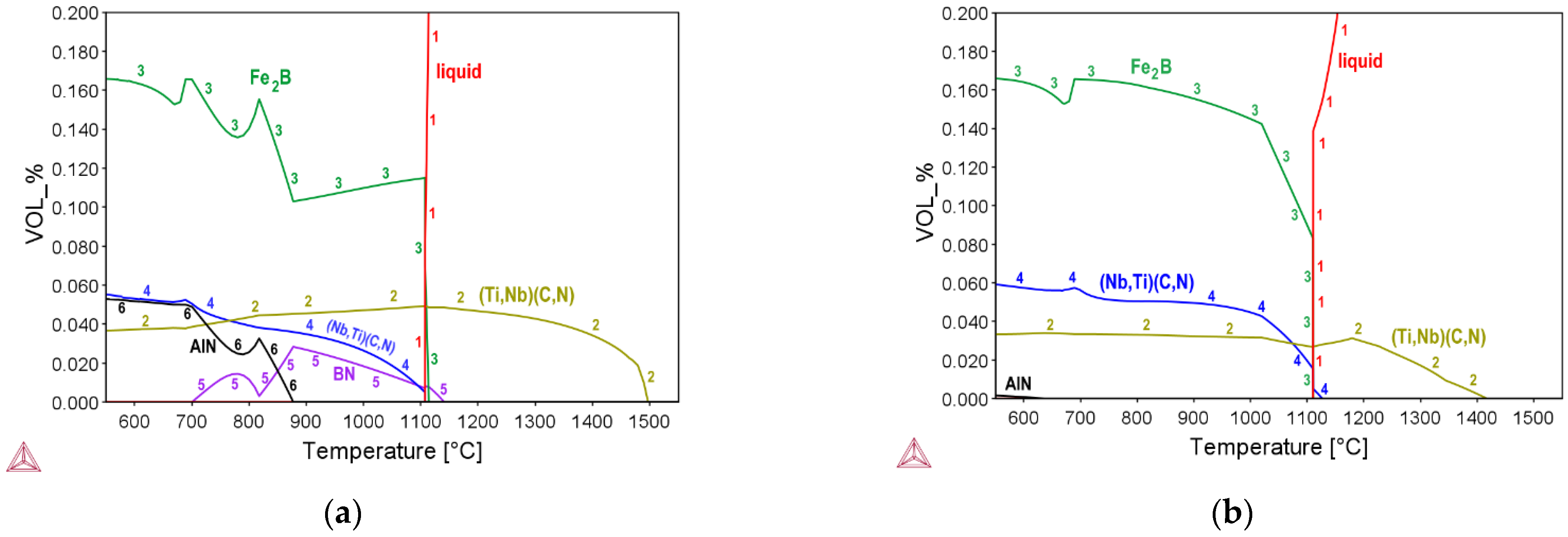


| Steel | C | Si | Mn | P | S | Al | Nb | Ti | B | N | O | Ti:N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | wt% | wt% | wt% | wt% | wt% | wt% | ppm | ppm | ppm | ppm | Ratio | |
| S1 | 0.10 | 0.30 | 1.96 | 0.005 | 0.005 | 0.025 | 0.031 | 35 | - | 35 | 20 | 1.0 |
| S2 | 0.10 | 0.32 | 1.99 | 0.005 | 0.006 | 0.023 | 0.031 | 35 | 58 | 35 | 20 | 1.0 |
| S3 | 0.10 | 0.29 | 1.99 | 0.005 | 0.005 | 0.030 | 0.031 | 35 | 100 | 30 | 20 | 1.16 |
| S4 | 0.10 | 0.30 | 2.01 | 0.005 | 0.006 | 0.030 | 0.032 | 260 | 100 | 30 | 20 | 8.66 |
| S5 | 0.26 | 0.31 | 2.03 | 0.005 | 0.007 | 0.036 | 0.032 | 35 | - | 30 | 20 | 1.16 |
| S6 | 0.26 | 0.31 | 2.01 | 0.005 | 0.008 | 0.037 | 0.033 | 35 | 55 | 30 | 20 | 1.16 |
| S7 | 0.25 | 0.31 | 2.01 | 0.005 | 0.006 | 0.027 | 0.030 | 35 | 100 | 30 | 20 | 1.16 |
| S8 | 0.25 | 0.31 | 2.00 | 0.005 | 0.007 | 0.031 | 0.031 | 270 | 100 | 30 | 20 | 9.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komenda, J.; Luo, C.; Lönnqvist, J. Interaction of Carbon, Titanium, and Boron in Micro-Alloy Steels and Its Effect on Hot Ductility. Alloys 2022, 1, 133-148. https://doi.org/10.3390/alloys1020009
Komenda J, Luo C, Lönnqvist J. Interaction of Carbon, Titanium, and Boron in Micro-Alloy Steels and Its Effect on Hot Ductility. Alloys. 2022; 1(2):133-148. https://doi.org/10.3390/alloys1020009
Chicago/Turabian StyleKomenda, Jacek, Chunhui Luo, and Johan Lönnqvist. 2022. "Interaction of Carbon, Titanium, and Boron in Micro-Alloy Steels and Its Effect on Hot Ductility" Alloys 1, no. 2: 133-148. https://doi.org/10.3390/alloys1020009
APA StyleKomenda, J., Luo, C., & Lönnqvist, J. (2022). Interaction of Carbon, Titanium, and Boron in Micro-Alloy Steels and Its Effect on Hot Ductility. Alloys, 1(2), 133-148. https://doi.org/10.3390/alloys1020009






