Polyamine-Mediated Growth Regulation in Microalgae: Integrating Redox Balance and Amino Acids Pathway into Metabolic Engineering
Abstract
1. Introduction
2. Polyamines’ Biology in Microalgae
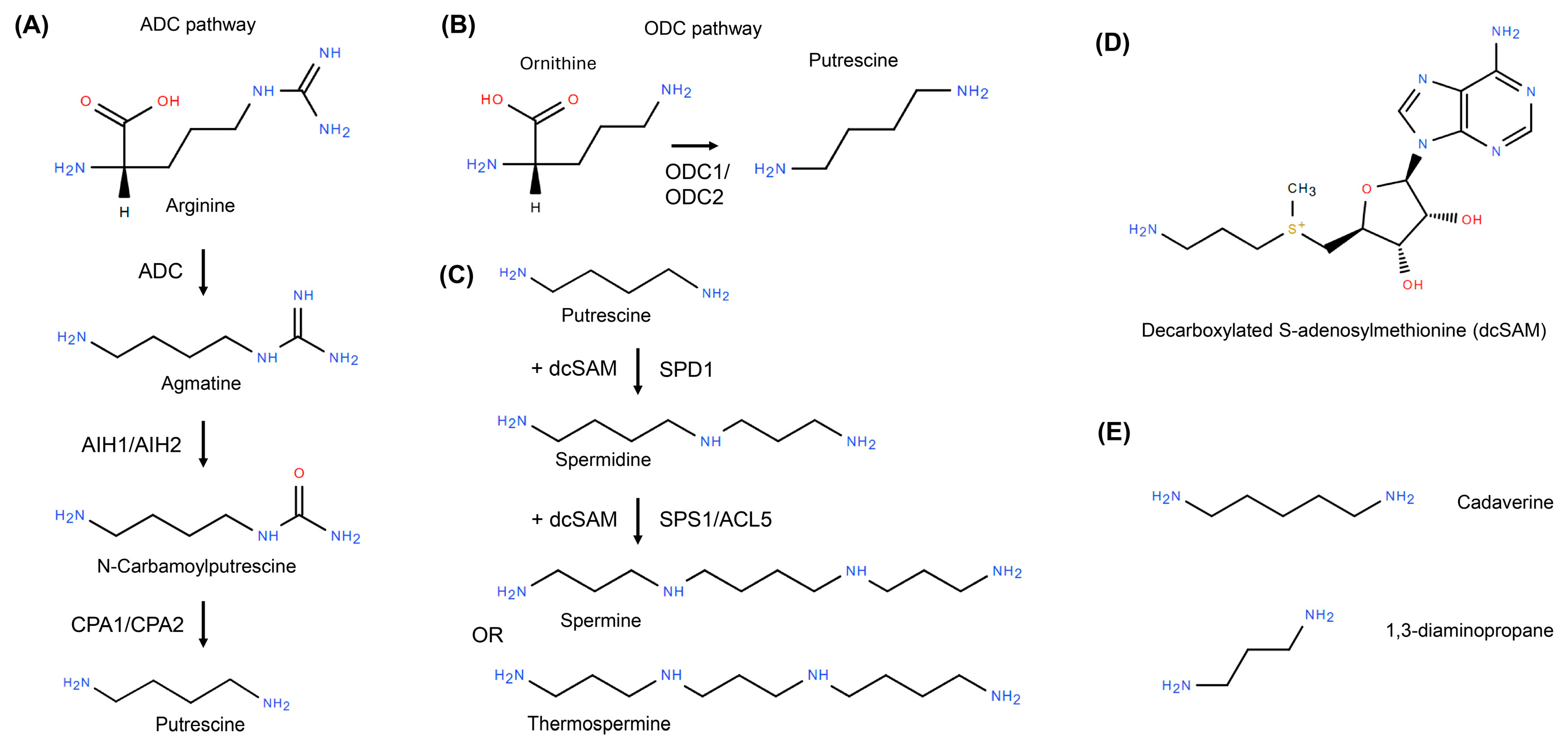
2.1. Biosynthesis Routes
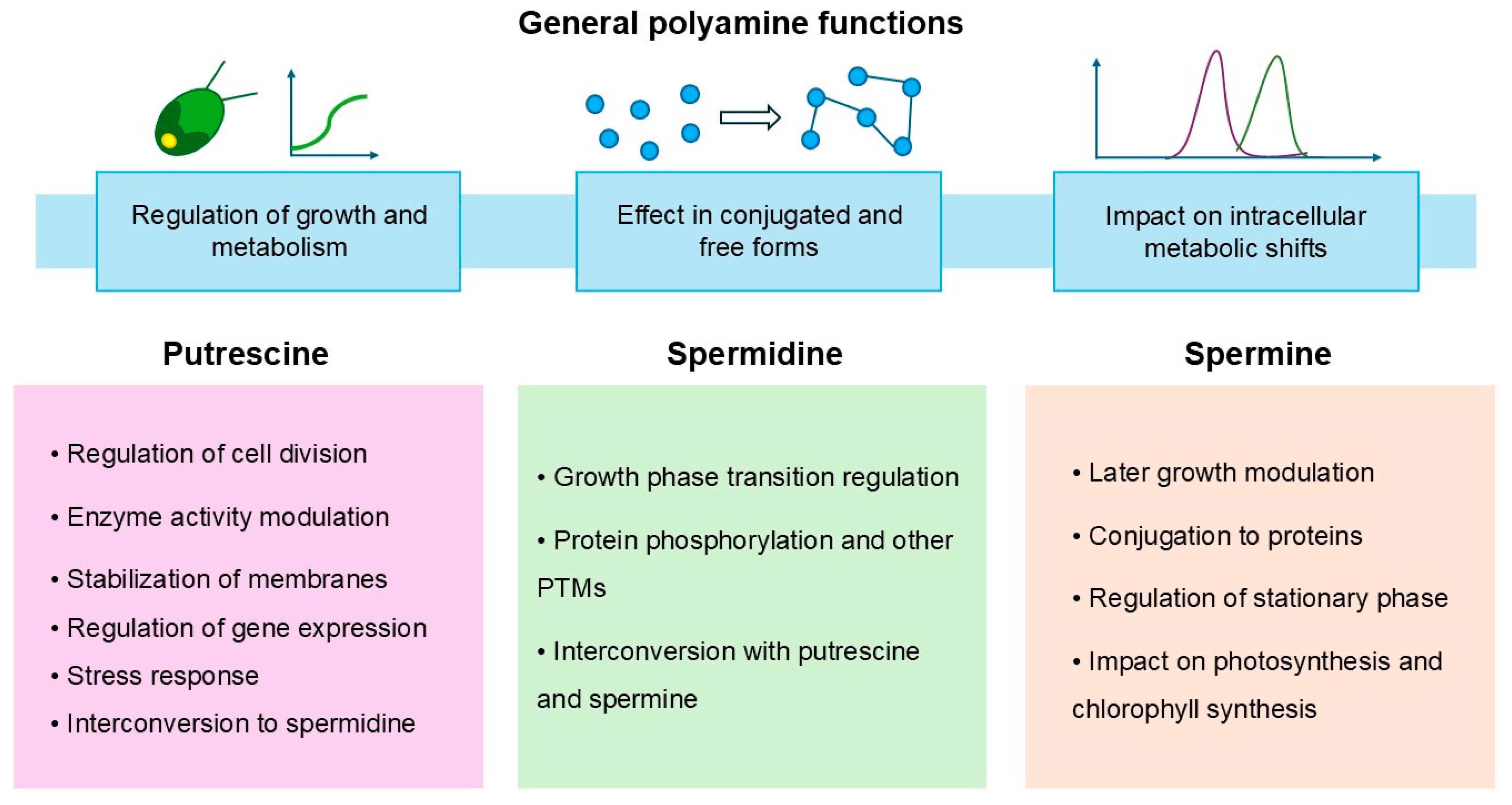
2.2. Functional Roles of Polyamines
2.3. Species-Specific Variation and Structural Roles
3. Polyamine Dynamics and Cell Growth Progression
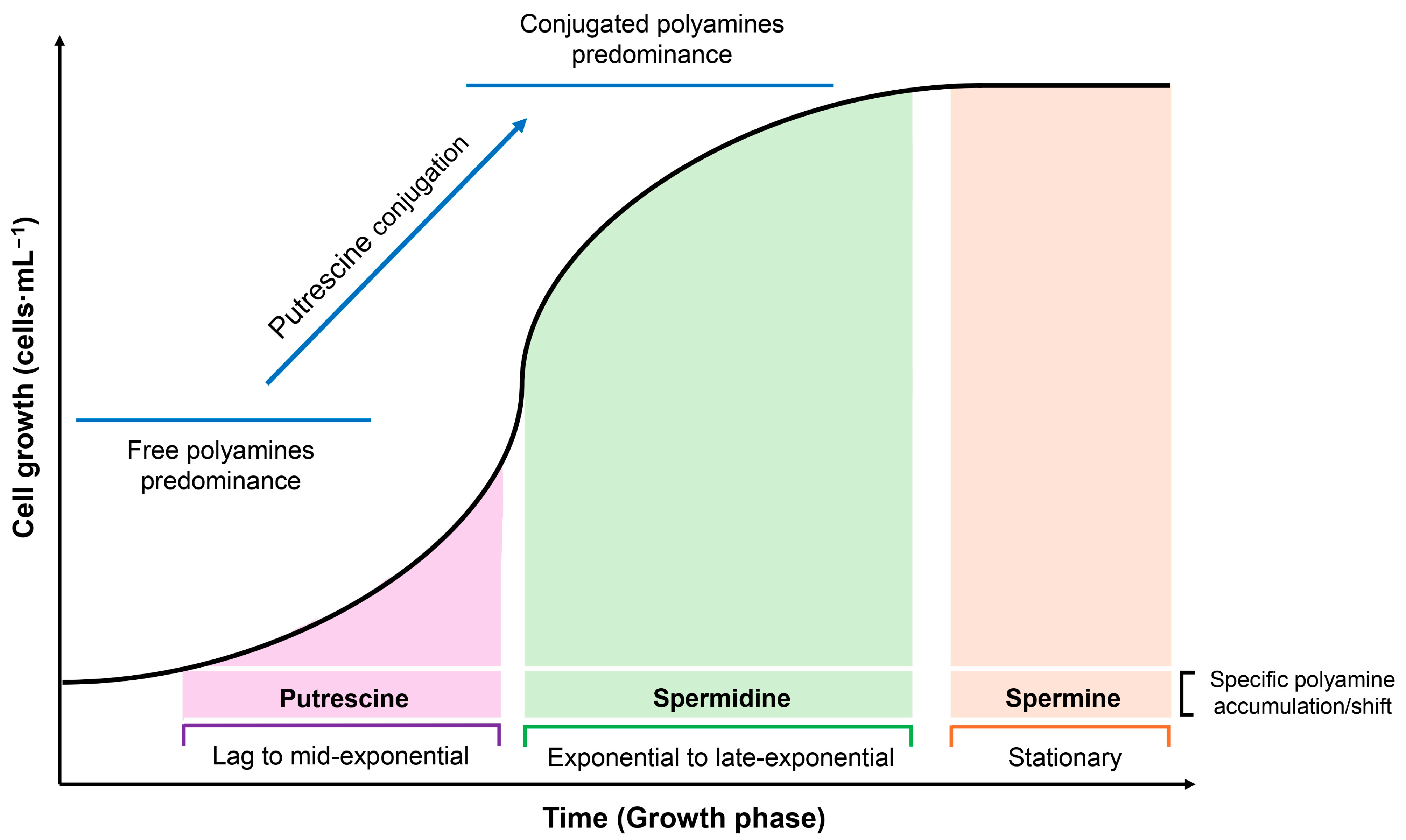
3.1. Intracellular Concentration Shifts and Growth Phase Transitions
3.2. Exogenous Supplementation and Implications in Growth Phenotype
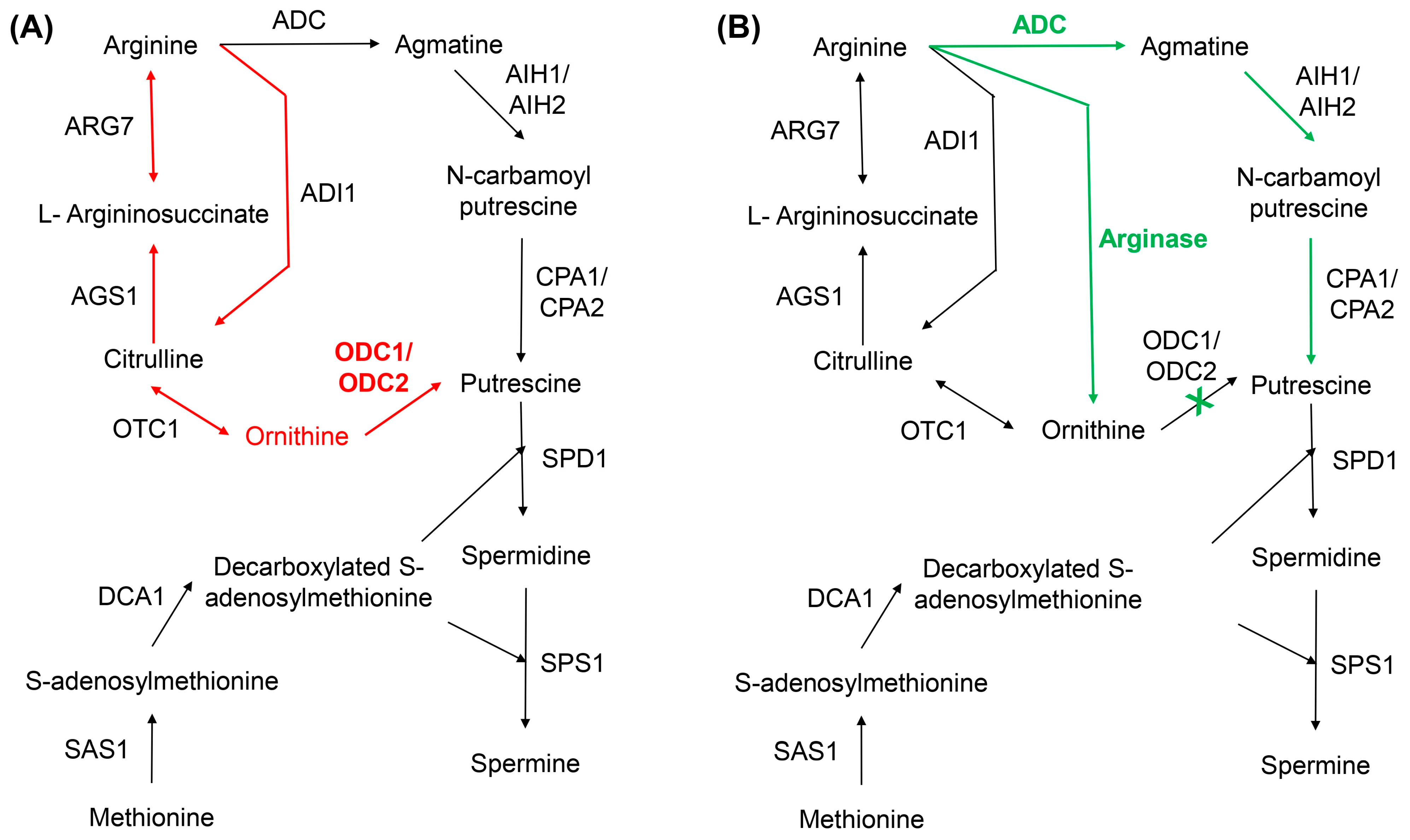
4. Complex Overlap Between Polyamines and Arginine Synthesis Pathways: The Balance of Quiescence and Growth
5. Insights into Growth Regulation from Multi-Omics and Metabolic Modeling
6. Tunable Control by Synthetic Devices
7. Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae Based Production of Single-Cell Protein. Curr. Opin. Biotechnol. 2022, 75, 102705. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as Biostimulants: A New Approach in Agriculture. World J. Microbiol. Biotechnol. 2022, 38, 4. [Google Scholar] [CrossRef] [PubMed]
- Naseema Rasheed, R.; Pourbakhtiar, A.; Mehdizadeh Allaf, M.; Baharlooeian, M.; Rafiei, N.; Alishah Aratboni, H.; Morones-Ramirez, J.R.; Winck, F.V. Microalgal Co-Cultivation—Recent Methods, Trends in Omic-Studies, Applications, and Future Challenges. Front. Bioeng. Biotechnol. 2023, 11, 1193424. [Google Scholar] [CrossRef] [PubMed]
- Winck, F.V.; Páez Melo, D.O.; González Barrios, A.F. Carbon Acquisition and Accumulation in Microalgae Chlamydomonas: Insights from “Omics” Approaches. J. Proteom. 2013, 94, 207–218. [Google Scholar] [CrossRef]
- Venkata Subhash, G.; Rajvanshi, M.; Raja Krishna Kumar, G.; Shankar Sagaram, U.; Prasad, V.; Govindachary, S.; Dasgupta, S. Challenges in Microalgal Biofuel Production: A Perspective on Techno Economic Feasibility under Biorefinery Stratagem. Bioresour. Technol. 2022, 343, 126155. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Preethi; Kavitha, S.; Gunasekaran, M.; Kumar, G. Microalgae Based Biorefinery Promoting Circular Bioeconomy-Techno Economic and Life-Cycle Analysis. Bioresour. Technol. 2020, 302, 122822. [Google Scholar]
- Bachrach, U. The Early History of Polyamine Research. Plant Physiol. Biochem. 2010, 48, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Introduction to the Thematic Minireview Series: Sixty plus Years of Polyamine Research. J. Biol. Chem. 2018, 293, 18681–18692. [Google Scholar] [CrossRef]
- Wu, H.; Min, J.; Ikeguchi, Y.; Zeng, H.; Dong, A.; Loppnau, P.; Pegg, A.E.; Plotnikov, A.N. Structure and Mechanism of Spermidine Synthases. Biochemistry 2007, 46, 8331–8339. [Google Scholar] [CrossRef]
- Fredlund, J.O.; Johansson, M.C.; Dahlberg, E.; Oredsson, S.M. Ornithine Decarboxylase and S-Adenosylmethionine Decarboxylase Expression during the Cell Cycle of Chinese Hamster Ovary Cells. Exp. Cell Res. 1995, 216, 86–92. [Google Scholar] [CrossRef]
- Shene, C.; Asenjo, J.A.; Chisti, Y. Metabolic Modelling and Simulation of the Light and Dark Metabolism of Chlamydomonas reinhardtii. Plant J. 2018, 96, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.L.; Ghamsari, L.; Manichaikul, A.; Hom, E.F.Y.; Balaji, S.; Fu, W.; Shen, Y.; Hao, T.; Palsson, B.; Salehi-Ashtiani, K.; et al. Metabolic Network Reconstruction of Chlamydomonas Offers Insight into Light-Driven Algal Metabolism. Mol. Syst. Biol. 2011, 7, 518. [Google Scholar] [CrossRef]
- Salguero, D.A.M.; Fernández-Niño, M.; Serrano-Bermúdez, L.M.; Melo, D.O.P.; Winck, F.V.; Caldana, C.; Barrios, A.F.G. Development of a Chlamydomonas reinhardtii Metabolic Network Dynamic Model to Describe Distinct Phenotypes Occurring at Different CO2 Levels. PeerJ 2018, 6, e5528. [Google Scholar] [CrossRef]
- Tassoni, A.; Awad, N.; Griffiths, G. Effect of Ornithine Decarboxylase and Norspermidine in Modulating Cell Division in the Green Alga Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2018, 123, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, R.A.; Wittemeier, L.; Einhaus, A.; Baier, T.; Kruse, O. Advanced Pathway Engineering for Phototrophic Putrescine Production. Plant Biotechnol. J. 2022, 20, 1968–1982. [Google Scholar] [CrossRef] [PubMed]
- Inal, M.S.; Unal, D.; Unal, B.T.; Ozturk, M. Effect of Putrescine on Low-Temperature Acclimation in Chlamydomonas reinhardtii. Phyton-Int. J. Exp. Bot. 2022, 91, 583–598. [Google Scholar] [CrossRef]
- Winck, F.V.; Páez Melo, D.O.; Riaño-Pachón, D.M.; Martins, M.C.M.; Caldana, C.; González Barrios, A.F. Analysis of Sensitive CO2 Pathways and Genes Related to Carbon Uptake and Accumulation in Chlamydomonas reinhardtii through Genomic Scale Modeling and Experimental Validation. Front. Plant Sci. 2016, 7, 43. [Google Scholar] [CrossRef]
- Murakami, Y.; Hayashi, S.-I. Role of Antizyme in Degradation of Ornithine Decarboxylase in HTC Cells. Biochem. J. 1985, 226, 893–896. [Google Scholar] [CrossRef]
- Cohen, E.; Arad, S.M.; Heimer, Y.H.; Mizrahi, Y. Polyamine Biosynthetic Enzymes in the Cell Cycle of Chlorella: Correlation between Ornithine Decarboxylase and DNA Synthesis at Different Light Intensities. Plant Physiol. 1984, 74, 385–388. [Google Scholar] [CrossRef]
- Voigt, J.; Bohley, P. Cell-Cycle-Dependent Regulation of Ornithine Decarboxylase Activity in the Unicellular Green Alga Chlamydomonas reinhardtii. Physiol. Plant 2000, 110, 419–425. [Google Scholar] [CrossRef]
- Park, J.J.; Wang, H.; Gargouri, M.; Deshpande, R.R.; Skepper, J.N.; Holguin, F.O.; Juergens, M.T.; Shachar-Hill, Y.; Hicks, L.M.; Gang, D.R. The Response of Chlamydomonas reinhardtii to Nitrogen Deprivation: A Systems Biology Analysis. Plant J. 2015, 81, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.d.F.R.; Giraldi, L.A.; Winck, F.V. From Feasting to Fasting: The Arginine Pathway as a Metabolic Switch in Nitrogen-Deprived Chlamydomonas reinhardtii. Cells 2023, 12, 1379. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K.; Fatima, T.; Handa, A.K.; Mattoo, A.K. Differential Association of Free, Conjugated, and Bound Forms of Polyamines and Transcript Abundance of Their Biosynthetic and Catabolic Genes During Drought/Salinity Stress in Tomato (Solanum lycopersicum L.) Leaves. Front. Plant Sci. 2021, 12, 743568. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Hwang, D.-F. Polyamine Profile in the Paralytic Shellfish Poison-Producing Alga Alexandrium minutum. J. Plankton Res. 2002, 24, 275–279. [Google Scholar] [CrossRef]
- Treves, H.; Murik, O.; Kedem, I.; Eisenstadt, D.; Meir, S.; Rogachev, I.; Szymanski, J.; Keren, N.; Orf, I.; Tiburcio, A.F.; et al. Metabolic Flexibility Underpins Growth Capabilities of the Fastest Growing Alga. Curr. Biol. 2017, 27, 2559–2567.e3. [Google Scholar] [CrossRef]
- Kotzabasis, K.; Senger, H. Free, Conjugated and Bound Polyamines during the Ceil Cycle in Synchronized Cultures of Scenedesmus Obliquus. Z. Naturforsch C J. Biosci. 1994, 49, 181–185. [Google Scholar] [CrossRef]
- Galston, A.W.; Sawhney, R.K. Polyamines in Plant Physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef]
- Tavladoraki, P.; Cona, A.; Federico, R.; Tempera, G.; Viceconte, N.; Saccoccio, S.; Battaglia, V.; Toninello, A.; Agostinelli, E. Polyamine Catabolism: Target for Antiproliferative Therapies in Animals and Stress Tolerance Strategies in Plants. Amino Acids 2012, 42, 411–426. [Google Scholar] [CrossRef]
- Xu, B.; Bo, Y.; Sun, X.; Wang, H.; Guo, H.; Zhou, C.; Ruan, R.; Yan, X.; Cheng, P. Review of the Effect of Polyamines in Microalgae When Ingested by Shellfish. Algal Res. 2021, 58, 102409. [Google Scholar] [CrossRef]
- Kumar, M.; Kuzhiumparambil, U.; Ralph, P.J.; Contreras-Porcia, L. Polyamines: Stress Metabolite in Marine Macrophytes. In Algal Green Chemistry: Recent Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 243–255. ISBN 9780444640413. [Google Scholar]
- Bridoux, M.C.; Ingalls, A.E. Structural Identification of Long-Chain Polyamines Associated with Diatom Biosilica in a Southern Ocean Sediment Core. Geochim. Cosmochim. Acta 2010, 74, 4044–4057. [Google Scholar] [CrossRef]
- Theiss, C.; Bohley, P.; Bisswanger, H.; Voigt, J. Uptake of Polyamines by the Unicellular Green Alga Chlamydomonas reinhardtii and Their Effect on Ornithine Decarboxylase Activity. J. Plant Physiol. 2004, 161, 3–14. [Google Scholar] [CrossRef]
- Hayashi, S.-I.; Murakami, Y. Rapid and Regulated Degradation of Ornithine Decarboxylase. Biochem. J. 1995, 306, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.-I.; Murakami, Y.; Matsufuji, S. Ornithine Decarboxylase Antizyme: A Novel Type of Regulatory Protein. Trends Biochem. Sci. 1996, 21, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Campos, J.L.; Figueras, X.; Besford, R.T. Recent Advances in the Understanding of Polyamine Functions during Plant Development. Plant Growth Regul. 1993, 12, 331–340. [Google Scholar] [CrossRef]
- Yoon, S.; Bay, B.H.; Matsumoto, K. Harnessing Microalgae as Sustainable Cell Factories for Polyamine-Based Nanosilica for Biomedical Applications. Molecules 2025, 30, 1666. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Żyłkiewicz, B. Phytohormones as Regulators of Heavy Metal Biosorption and Toxicity in Green Alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Benkő, P.; Gémes, K.; Fehér, A. Polyamine Oxidase-Generated Reactive Oxygen Species in Plant Development and Adaptation: The Polyamine Oxidase—NADPH Oxidase Nexus. Antioxidants 2022, 11, 2488. [Google Scholar] [CrossRef]
- Fuell, C.; Elliott, K.A.; Hanfrey, C.C.; Franceschetti, M.; Michael, A.J. Polyamine Biosynthetic Diversity in Plants and Algae. Plant Physiol. Biochem. 2010, 48, 513–520. [Google Scholar] [CrossRef]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of Amine Oxidases in Plant Development and Defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef]
- Bridoux, M.C.; Keil, R.G.; Ingalls, A.E. Analysis of Natural Diatom Communities Reveals Novel Insights into the Diversity of Long Chain Polyamine (LCPA) Structures Involved in Silica Precipitation. Org. Geochem. 2012, 47, 9–21. [Google Scholar] [CrossRef]
- Bachrach, U.; Wang, Y.-C.; Tabib, A. Polyamines: New Cues in Cellular Signal Transduction. J. Physiol. Sci. 2001, 16, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Molecular Machines Encoded by Bacterially-Derived Multi-Domain Gene Fusions That Potentially Synthesize, N-Methylate and Transfer Long Chain Polyamines in Diatoms. FEBS Lett. 2011, 585, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Bridoux, M.C.; Annenkov, V.V.; Keil, R.G.; Ingalls, A.E. Widespread Distribution and Molecular Diversity of Diatom Frustule Bound Aliphatic Long Chain Polyamines (LCPAs) in Marine Sediments. Org. Geochem. 2012, 48, 9–20. [Google Scholar] [CrossRef]
- Dornemann, D.; Navakoudis, E.; Kotzabasis, K. Changes in the Polyamine Content of Plastidal Membranes in Light-and Dark-Grown Wildtype and Pigment Mutants of the Unicellular Green Alga Scenedesmus Obliquus and Their Possible Role in Chloroplast Photodevelopment. J. Photochem. Photobiol. B 1996, 36, 293–299. [Google Scholar] [CrossRef]
- Treves, H.; Siemiatkowska, B.; Luzarowska, U.; Murik, O.; Fernandez-Pozo, N.; Moraes, T.A.; Erban, A.; Armbruster, U.; Brotman, Y.; Kopka, J.; et al. Multi-Omics Reveals Mechanisms of Total Resistance to Extreme Illumination of a Desert Alga. Nat. Plants 2020, 6, 1031–1043. [Google Scholar] [CrossRef]
- Treves, H.; Küken, A.; Arrivault, S.; Ishihara, H.; Hoppe, I.; Erban, A.; Höhne, M.; Moraes, T.A.; Kopka, J.; Szymanski, J.; et al. Carbon Flux through Photosynthesis and Central Carbon Metabolism Show Distinct Patterns between Algae, C3 and C4 Plants. Nat. Plants 2022, 8, 78–91. [Google Scholar] [CrossRef]
- Krö, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-Specific Polyamines from Diatoms Control Silica Morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar] [CrossRef]
- Sumper, M.; Lehmann, G. Silica Pattern Formation in Diatoms: Species-Specific Polyamine Biosynthesis. ChemBioChem 2006, 7, 1419–1427. [Google Scholar] [CrossRef]
- Sumper, M.; Brunner, E.; Lehmann, G. Biomineralization in Diatoms: Characterization of Novel Polyamines Associated with Silica. FEBS Lett. 2005, 579, 3765–3769. [Google Scholar] [CrossRef]
- Frigeri, L.G.; Radabaugh, T.R.; Haynes, P.A.; Hildebrand, M. Identification of Proteins from a Cell Wall Fraction of the Diatom Thalassiosira Pseudonana: Insights into Silica Structure Formation. Mol. Cell. Proteom. 2006, 5, 182–193. [Google Scholar] [CrossRef]
- Teng, W.; Shang, X.; Sun, J. The Effect of Silicate on Polyamine Oxidase Genes in Skeletonema Dohrnii. Mar. Environ. Res. 2025, 204, 106860. [Google Scholar] [CrossRef] [PubMed]
- Slocum, R.D.; Galston, A.W. Changes in Polyamine Biosynthesis Associated with Postfertilization Growth and Development in Tobacco Ovary Tissues. Plant Physiol. 1985, 79, 336–343. [Google Scholar] [CrossRef]
- Adlakha, R.C.; Villanueva, V.R.; Calvayrac, R.; Edmunds, L.N. Arrest of Cell Division Blocks the Utilization of Polyamines in Synchronized Cultures of Photoautotrophically Grown Euglena. Arch. Biochem. Biophys. 1980, 201, 660–668. [Google Scholar] [CrossRef]
- Nishibori, N.; Nishijima, T. Changes in Polyamine Levels during Growth of a Red-Tide Causing Phytoplankton Chattonella Antiqua (Raphidophyceae). Eur. J. Phycol. 2004, 39, 51–55. [Google Scholar] [CrossRef]
- Liu, Q.; Nishibori, N.; Imai, I.; Hollibaugh, J.T. Response of Polyamine Pools in Marine Phytoplankton to Nutrient Limitation and Variation in Temperature and Salinity. Mar. Ecol. Prog. Ser. 2016, 544, 93–105. [Google Scholar] [CrossRef]
- Cai, G.; Della Mea, M.; Faleri, C.; Fattorini, L.; Aloisi, I.; Serafini-Fracassini, D.; Del Duca, S. Spermine Either Delays or Promotes Cell Death in Nicotiana tabacum L. Corolla Depending on the Floral Developmental Stage and Affects the Distribution of Transglutaminase. Plant Sci. 2015, 241, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Kaldenhoff, R. Achievements and Challenges of Genetic Engineering of the Model Green Alga Chlamydomonas reinhardtii. Algal Res. 2020, 50, 101986. [Google Scholar] [CrossRef]
- Strenkert, D.; Schmollinger, S.; Gallaher, S.D.; Salomé, P.A.; Purvine, S.O.; Nicora, C.D.; Mettler-Altmann, T.; Soubeyrand, E.; Weber, A.P.M.; Lipton, M.S.; et al. Multiomics Resolution of Molecular Events during a Day in the Life of Chlamydomonas. Proc. Natl. Acad. Sci. USA 2019, 116, 2374–2383. [Google Scholar] [CrossRef]
- Sinzger-D’Angelo, M.; Startceva, S.; Koeppl, H. Bye Bye, Linearity, Bye: Quantification of the Mean for Linear CRNs in a Random Environment. J. Math. Biol. 2023, 87, 43. [Google Scholar] [CrossRef]
- Beigbeder, A.; Vavadakis, M.; Navakoudis, E.; Kotzabasis, K. Influence of Polyamine Inhibitors on Light-Independent and Light-Dependent Chlorophyll Biosynthesis and on the Photosynthetic Rate. J. Photochem. Photobiol. 1995, 28, 235–242. [Google Scholar] [CrossRef]
- Czerpak, R.; Bajguz, A.; Piotrowska, A.; Dobrogowska, R.; Matejczyk, M.; Wiesławski, W. Biochemical Activity of Di- and Polyamines in the Green Alga Chlorella Vulgaris beijerinck (Chlorophyceae). Acta Soc. Bot. Pol. 2003, 72, 19–24. [Google Scholar] [CrossRef]
- Freudenberg, R.A.; Wittemeier, L.; Einhaus, A.; Baier, T.; Kruse, O. The Spermidine Synthase Gene SPD1: A Novel Auxotrophic Marker for Chlamydomonas reinhardtii Designed by Enhanced CRISPR/Cas9 Gene Editing. Cells 2022, 11, 837. [Google Scholar] [CrossRef] [PubMed]
- Theiss, C.; Bohley, P.; Voigt, J. Regulation by Polyamines of Ornithine Decarboxylase Activity and Cell Division in the Unicellular Green Alga Chlamydomonas reinhardtii. Plant Physiol. 2002, 128, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, R.A.; Baier, T.; Einhaus, A.; Wobbe, L.; Kruse, O. High Cell Density Cultivation Enables Efficient and Sustainable Recombinant Polyamine Production in the Microalga Chlamydomonas reinhardtii. Bioresour. Technol. 2021, 323, 124542. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahn, J.W.; Park, E.J.; Choi, J.L. Overexpression of S-Adenosylmethionine Synthetase in Recombinant Chlamydomonas for Enhanced Lipid Production. J. Microbiol. Biotechnol. 2023, 33, 310–318. [Google Scholar] [CrossRef]
- Li, B.; Liang, J.; Phillips, M.A.; Michael, A.J. A Hybrid Biosynthetic-Catabolic Pathway for Norspermidine Production. Biochem. J. 2024, 481, 1241–1253. [Google Scholar] [CrossRef]
- Sumiya, N.; Fujiwara, T.; Era, A.; Miyagishima, S.Y. Chloroplast Division Checkpoint in Eukaryotic Algae. Proc. Natl. Acad. Sci. USA 2016, 113, E7629–E7638. [Google Scholar] [CrossRef]
- Pokora, W.; Aksmann, A.; Baścik-Remisiewicz, A.; Dettlaff-Pokora, A.; Rykaczewski, M.; Gappa, M.; Tukaj, Z. Changes in Nitric Oxide/Hydrogen Peroxide Content and Cell Cycle Progression: Study with Synchronized Cultures of Green Alga Chlamydomonas reinhardtii. J. Plant Physiol. 2017, 208, 84–93. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavandosque, L.L.; Vischi Winck, F. Polyamine-Mediated Growth Regulation in Microalgae: Integrating Redox Balance and Amino Acids Pathway into Metabolic Engineering. SynBio 2025, 3, 8. https://doi.org/10.3390/synbio3020008
Lavandosque LL, Vischi Winck F. Polyamine-Mediated Growth Regulation in Microalgae: Integrating Redox Balance and Amino Acids Pathway into Metabolic Engineering. SynBio. 2025; 3(2):8. https://doi.org/10.3390/synbio3020008
Chicago/Turabian StyleLavandosque, Leandro Luis, and Flavia Vischi Winck. 2025. "Polyamine-Mediated Growth Regulation in Microalgae: Integrating Redox Balance and Amino Acids Pathway into Metabolic Engineering" SynBio 3, no. 2: 8. https://doi.org/10.3390/synbio3020008
APA StyleLavandosque, L. L., & Vischi Winck, F. (2025). Polyamine-Mediated Growth Regulation in Microalgae: Integrating Redox Balance and Amino Acids Pathway into Metabolic Engineering. SynBio, 3(2), 8. https://doi.org/10.3390/synbio3020008






