3.1. Hot-Pressed Alumina Ceramics
Hot-pressing of high-purity α-Al
2O
3 powder results in dense alumina ceramics (
Table 1). An SEM study of etched surfaces of ceramics demonstrated their features [
19]. The mean grain size of aluminas logically increases with an increase in hot-pressing temperature, but its increase to 6.8 μm when the temperature of hot-pressing changes from 1300 °C to 1600 °C does not significantly affect the properties. However, at a hot-pressing temperature above 1600 °C, the average grain size of alumina ceramics increases to 9.2 μm, and all properties decrease (
Table 1).
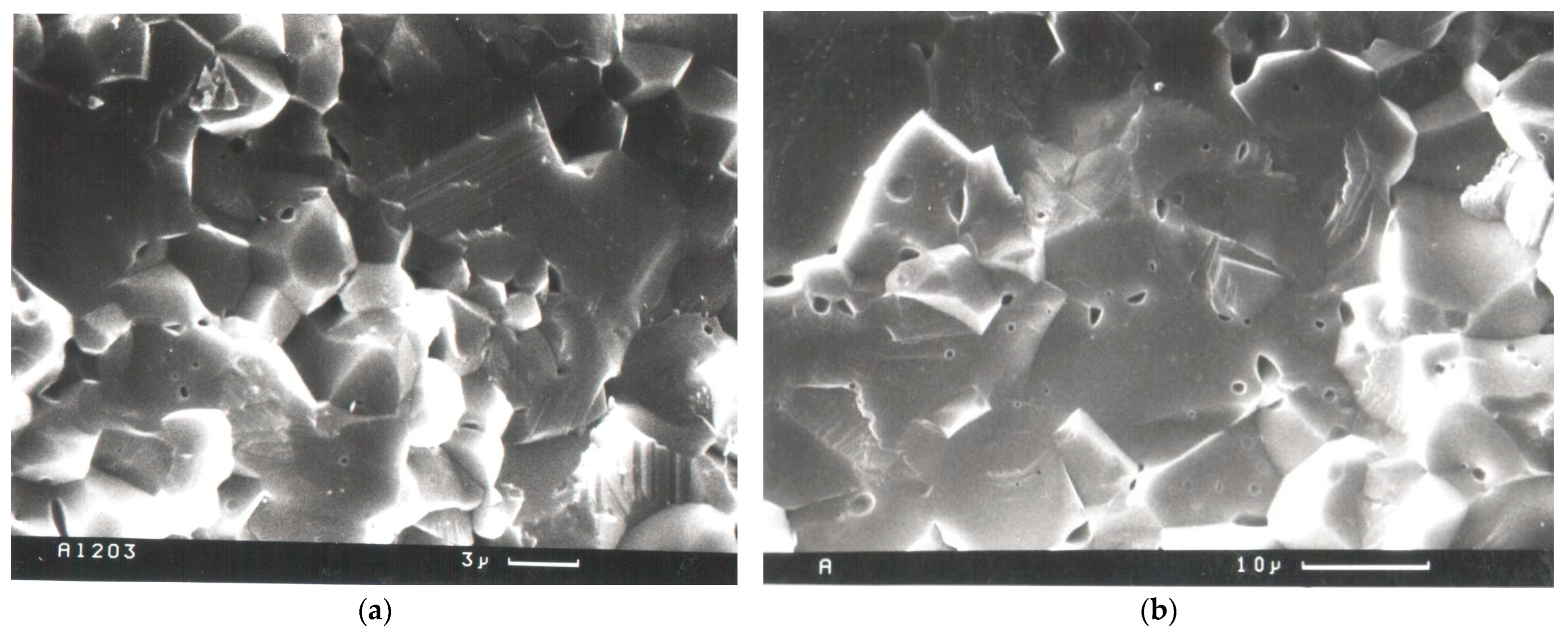
The SEM image of the fracture surface of ceramics produced at a temperature of 1550 °C (
Table 1, sample 4) demonstrated predominantly intergranular fractures, since crystallite facets are clearly visible (
Figure 1a). This material with a grain size of about 6.6 µm (
Figure 1a) has fine intracrystalline closed porosity and coarse interparticle porosity inside. Increasing the hot-pressing temperature up to 1720 °C (
Table 1, sample 8) results in an increase in grain size up to 21.9 µm in full-density aluminas with fine intracrystalline closed porosity inside the samples (
Figure 1b). The presence of chipped areas on the fracture surface of such a material indicates transgranular failure and transgranular crack propagation (
Figure 1b).
There is also a clear trend towards a decrease in the porosity for all hot-pressed samples with an increase in the temperature, despite a significant decrease in the dwell time (
Figure 2a). Only at a low temperature of 1350 °C does doubling the dwell time lead to the lowest porosity of 0.7% and a grain size of 3.5 µm (
Table 1, Sample 2). Despite the better densification of ceramics due to the reduction in porosity, a steady tendency of the deterioration of the hardness and fracture toughness with an increase in the hot-pressing temperature up to 1720 °C was observed (
Figure 2b). In addition, it turned out that hardness is more sensitive to temperature growth than fracture toughness (
Figure 2b). Consequently, it is possible to say that temperature is the most important parameter of hot-pressing. The dwell time and porosity show a negligible effect on hardness and fracture toughness under present conditions.
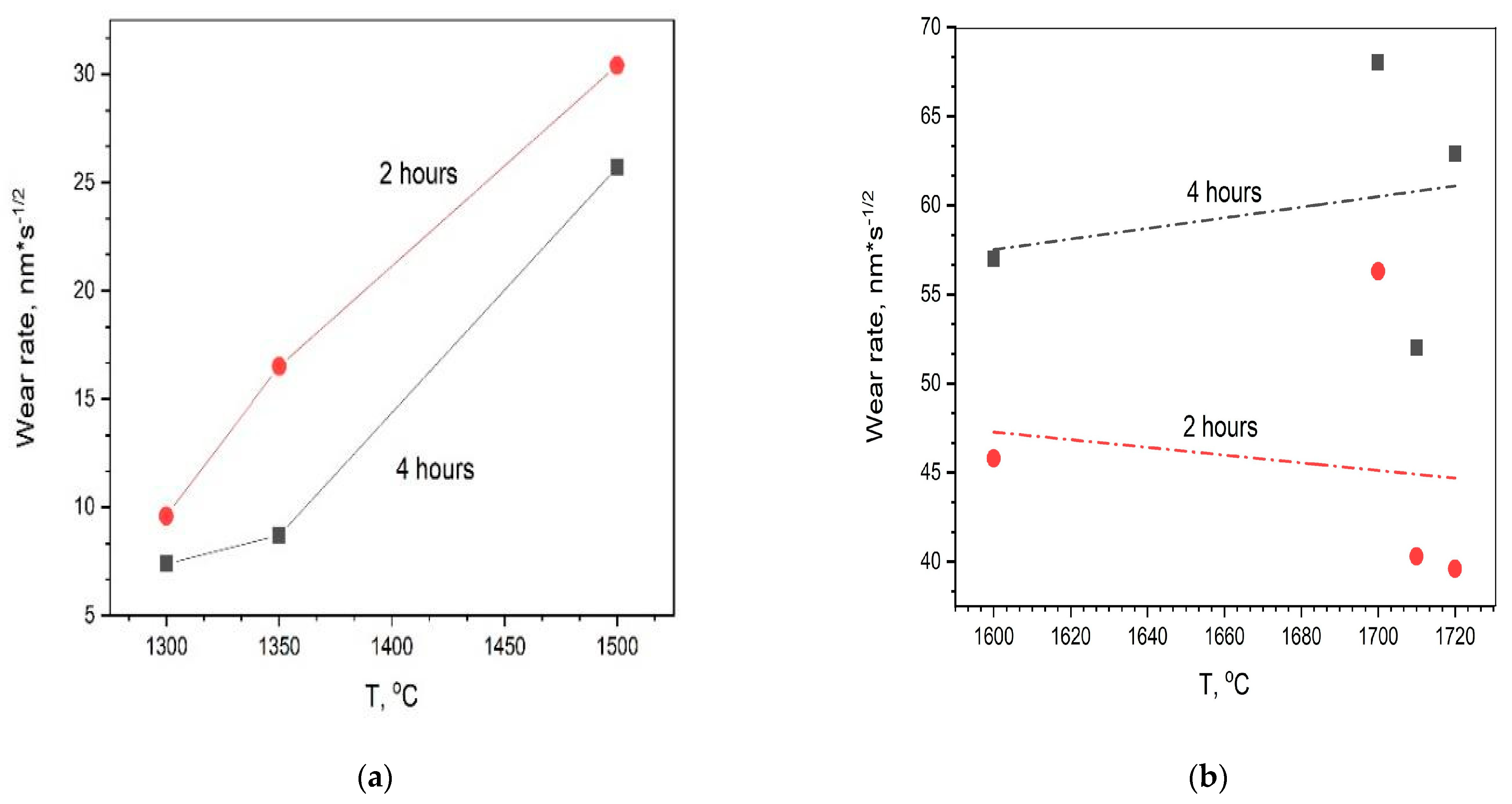
It is interesting to note that the wear rate of the samples obtained at low temperatures of 1300–1500 °C (
Figure 3a) increases after 4 h of testing in contrast to the samples produced at higher temperatures (
Figure 3b). The highest hot-pressing temperature of 1720 °C leads to a drop in the wear rate of the samples by approximately half after 4 h of testing (
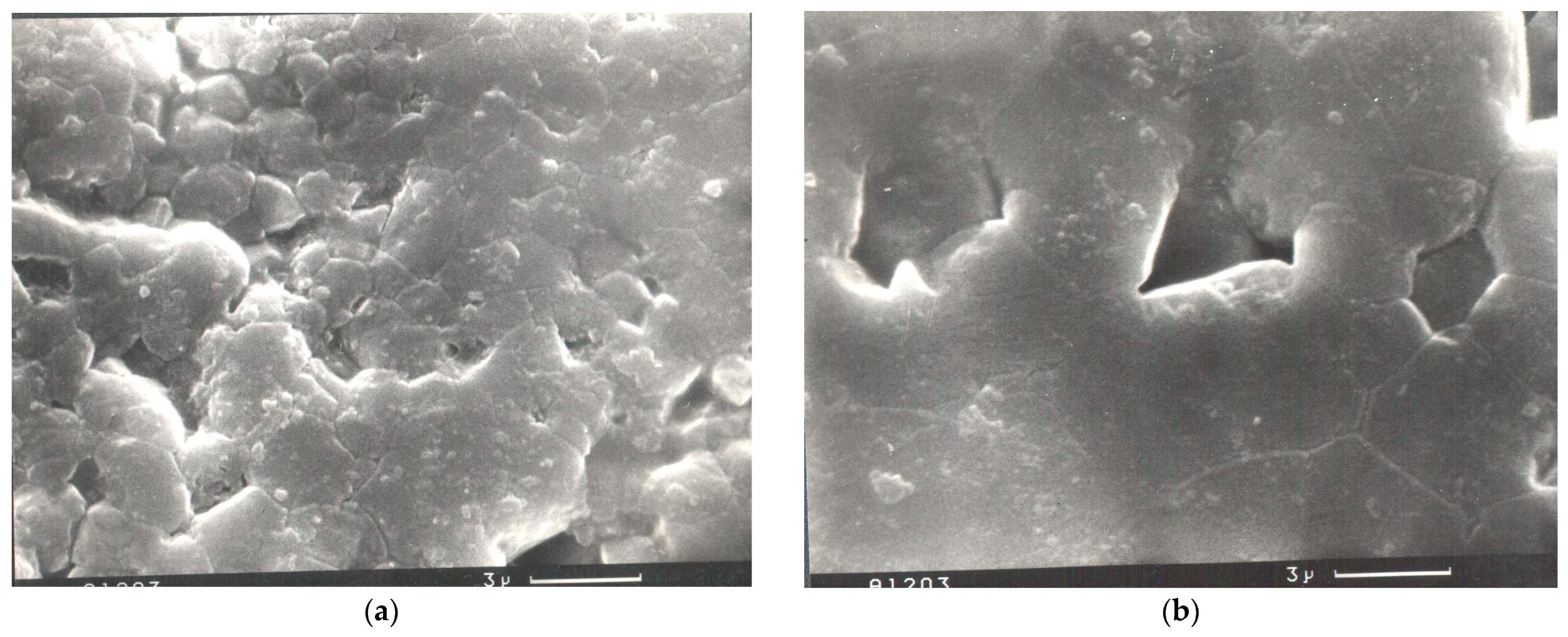
Table 1). Scanning electron microscope (SEM) analysis of the surface structure of the best alumina sample (
Table 1, sample 1) has demonstrated the presence of nanosized grains of 0.5 μm (
Figure 4a), enriched in carbon (
Figure 5a). The grains of the bulk counterpart had a size of about 6.8 µm (
Figure 4b) and stored less carbon (
Figure 5b). This study confirms the positive effect of the fine-grained structure on the strengthening of the surface of alumina ceramics formed at low temperatures.
It is known that alumina is typically considered to be diamagnetic. When aluminum and oxygen bond to form Al2O3, the aluminum atoms lose three electrons to achieve a stable configuration, becoming positively charged ions (Al3+), and the oxygen atoms gain two electrons to form negatively charged ions (O2−). There are no unpaired electrons in this configuration. However, nanostructured powders of aluminum oxide (Al2O3) can exhibit different properties compared to their bulk counterparts due to their unique structural features and increased surface area-to-volume ratio. One of these properties can be the paramagnetic behavior, which arises due to several factors:
Defects and Surface Effects: Nanostructuring introduces a large number of defects, such as vacancies, dislocations, and grain boundaries, into the material. These defects can create localized electronic states within the band structure of Al2O3, which may lead to the presence of unpaired electrons and consequently paramagnetic behavior. Structural heterogeneity and defects in the structure, such as cationic and oxygen vacancies, can also be the cause for their phase transition to the paramagnetic state.
Surface States: The high surface area-to-volume ratio in nanostructured materials results in a larger fraction of atoms located at the surface. Surface atoms can have different electronic configurations compared to those in the bulk, leading to the presence of unpaired electrons and paramagnetic behavior.
Size Effects: At the nanoscale, quantum confinement effects become significant, altering the electronic and magnetic properties of the material. As the size of Al2O3 nanoparticles decreases, the electronic band structure may change, leading to the emergence of paramagnetic behavior.
Overall, the paramagnetic behavior of nanostructured Al
2O
3 is a result of a combination of factors, including defects, size effects, and surface states, which lead to the presence of unpaired electrons and magnetic moments within the material. Therefore, it can be concluded that the strengthened surface layer of alumina samples can be the result of the non-thermal effects of microwaving [
20]. However, exposure at a higher temperature results in several changes: an enlargement of grain size, the calcination of low-melting grain-boundary phases, and the penetration of carbon through the surface. These alterations can disrupt the ordering of dipoles and degrade the surface properties of the alumina samples.
3.2. Alumina Ceramic Composites
Composite ceramics fabricated at a high temperature of 1700 °C with the addition of 5 wt. % SiC, Si
3N
4, SiO
2, and ZrO
2 demonstrated the formation of new structures (
Table 2,
Figure 6). The fracture surface of the ceramic composite produced with the addition of 5 wt. % SiC has an overall smooth appearance and shows a transgranular crack propagation in the structure with a grain size of about 1.3–1.7 μm (
Table 2,
Figure 6a). The incorporation of 5 wt. % Si
3N
4 to the alumina contributes to the formation of a rougher, nonuniform structure on the surface of the fracture. A transgranular crack propagation takes place in a dense structure with a grain size of 1.0–1.2 μm, while some particularly elongated grains of 3 μm were observed due to the intergranular fracture (
Table 2,
Figure 6b). The transgranular fracture also occurs in the fully dense structure of the composite ceramics with grains of 0.9–2.3 μm and the addition of 5 wt. % SiO
2 (
Table 2,
Figure 6c). The propagation of an intergranular crack in the structure with a grain size of about 5.7–6.9 μm was typical only for the fracture surface of a ceramic composite obtained by the addition of 5 wt. % ZrO
2 (
Table 2,
Figure 6d).
An alumina composite with 5 wt. % SiC of the highest hardness was produced at a temperature of 1700 °C and a dwell time of 10 min (
Table 2). The main hardness of alumina composites with the addition of 5 wt. % SiC and Si
3N
4 is slightly higher than for composites with the addition of SiO
2 and ZrO
2. The worst main hardness was displayed by the alumina composite with an addition of 5 wt. % SiO
2 (
Table 2).
The highest and lowest values of fracture toughness of the considered ceramic composites, produced with the addition of 5 wt. % Si
3N
4 and SiO
2, respectively, are explained by the difference in their structures (
Figure 6b,c). For the first structure, there are such hardening mechanisms as crack deflection, its branching, and its arrests due to the presence of elongated Si
3N
4 grains (
Figure 6b), while for the second structure, these mechanisms do not work due to purely transgranular fractures (
Figure 6c). Ceramic composites obtained by the addition of 5 wt. % ZrO
2 and SiC displayed the same fracture toughness, despite the significant difference in fracture surfaces and crack propagation in the structure (
Table 2,
Figure 6a,d).
Based on the data on hardness, fracture toughness, porosity, and mean grain size of the alumina composites, it can be concluded that, in general, the addition of 5 wt. % SiC, Si
3N
4, SiO
2, and ZrO
2 improves the basic properties of alumina ceramics by about 2–6% (
Table 1 and
Table 2). Only the wet erosive wear rate turned out to be the most sensitive to these additives. The formation of grain boundaries in the new compositions and structures in the alumina ceramics by the addition of 5 wt. % SiC, Si
3N
4, SiO
2, and ZrO
2 has sharply affected the wear rate (
Table 1 and
Table 2).
The worn surfaces of ceramic composites made with the addition of various additives vary [
19]. The formation of new structures and, accordingly, their grain boundaries determines the fracture surface and the wear pattern of the ceramic’s surface. The highest value of the wear rate of ceramic composites prepared with the addition of 5 wt. % ZrO
2 is explained by a change in the mechanism of fracture that occurred due to changes in the composition of the grain boundaries (
Table 2,
Figure 6d). Incorporation of ZrO
2 changed the transgranular fracture of pure alumina ceramics produced at high temperatures to intergranular. Since the alumina grain boundaries were weakened by the new composition of Al-O-Zr, grain pullout and chipping became the dominant wear mechanisms. This composite material demonstrates the highest wear rate, close to the wear rate of pure alumina produced at higher temperatures (
Table 1 and
Table 2).
An alumina composite with 5 wt. % SiC, which has the lowest wear rate and an overall smooth appearance of a worn surface, was produced at a temperature of 1700 °C and a dwell time of 10 min (
Table 2) [
19]. Incorporation of SiC inhibited the growth of the alumina grain sizes and preserved the transgranular fracture of pure alumina ceramics prepared at high temperatures. The new Al-O-Si-C composition of the grain boundaries also promoted their strengthening. Therefore, the wear rate of the ceramic composite decreases as a result of the almost simultaneous wear of both the alumina grains and the grain boundary phase (
Table 2).
Incorporation of Si
3N
4 into alumina results in a very rough, worn surface with areas where some separate, elongated grains of Si
3N
4 pulled out from the surface due to an intergranular fracture [
19]. In general, the presence of elongated columnar grains in the matrix should provide additional strengthening of the alumina composite with 5 wt. % Si
3N
4. However, such microstructures did not increase the wear resistance of these nanocomposites compared to the wear resistance of an alumina composite with 5 wt. % SiC (
Table 2). Consequently, the new Al-O-Si-N composition of the grain boundaries is not as strong as Al-O-Si-C.
A very smooth response to wear has been demonstrated by the nanostructured alumina composite with 5 wt% SiO
2 [
19]. Strengthening of the grain boundaries by the Al-O-Si composition results in no fractures having occurred. However, in this case the wear rate was also higher than for the alumina nanocomposites with 5 wt.% SiC (
Table 2). it looks like the main reason lies in the strength of the grain boundaries of the Al-O-Si-C composition.
Although all ceramic composites were obtained at the same temperature (1700 °C), it should be noted that those prepared with a short dwell time (2–10 min) exhibited a lower wear rate after 2 and 4 h of testing (
Table 2). Moreover, hot-pressing at a dwell time of 15–45 min results in a smaller difference in the wear rate (W
4h/W
2h = 0.52–0.73) after 2 and 4 h of testing than at a dwell time of 2–10 min (W
4h/W
2h = 0.47–0.49) for composites made with the addition of 5 wt. % SiC, Si
3N
4 and SiO
2.
The formation of a strengthening surface layer at a short dwell time indirectly points to the nonthermal effect of the microwave electromagnetic field [
20,
21,
22]. It means that materials based on Al
2O
3 nanopowders enriched by Si
3N
4, SiC, and SiO
2 display paramagnetic properties, which can be attributed to several factors, including the electronic structure, the interaction of additives with the host material (Al
2O
3), the formation of new grain boundaries with new properties and defects, and also the specific conditions of the ceramic material synthesis.
Additive Properties: Silicon nitride (Si3N4), silicon carbide (SiC), and silicon dioxide (SiO2) promote a new grain boundary formation with new properties that can introduce defects and result in unpaired electrons within the host Al2O3 lattice and grain boundary phases, which leads to paramagnetic behavior. These additives may also contain their own defects that contribute to paramagnetism, such as crystal lattice defects.
Interaction with the Host Material: The specific interactions between the additives and the host Al2O3 material and the formation of new grain boundaries can influence the manifestation of paramagnetic properties. The additives may facilitate the formation of defect sites on the surface of alumina grains due to new grain boundary formation and within the Al2O3 lattice, thereby promoting paramagnetism. The nature and strength of these interactions can vary depending on the type and concentration of the additives.
Synthesis Conditions: The conditions under which the materials are synthesized can also play a crucial role in determining their magnetic properties. Factors such as temperature, pressure, and the duration of synthesis can affect the formation of defects in grain boundaries within the materials, which in turn can influence their magnetic behavior.
Overall, the presence of specific additives like Si3N4, SiC, and SiO2, along with their interactions with the host Al2O3 material, which contribute to new grain boundary formation, may promote the formation of defect sites and unpaired electrons, leading to paramagnetic properties.
However, for composites made with the addition of 5 wt. % ZrO2, the interaction with the microwave electromagnetic field is of a different nature. The difference in wear rates for a 2 min dwell time after 2 and 4 h of testing was 0.95, whereas it was 0.75 for a 15 min dwell time.
The difference in the nature of interactions between Al2O3 and ZrO2 compared to Al2O3 and Si3N4, SiC, or SiO2 can be attributed to several factors:
Chemical Composition: Zirconium oxide (ZrO2) and silicon nitride (Si3N4), silicon carbide (SiC), or silicon dioxide (SiO2) have different chemical compositions. ZrO2 is an oxide of zirconium, while Si3N4, SiC, and SiO2 are compounds of silicon and nitrogen or carbon. These differences in chemical composition result in different types of chemical bonds, interactions with the Al2O3 lattice, and the formation of grain boundaries of another composition.
Crystal Structure: Zirconium oxide (ZrO2) and aluminum oxide (Al2O3) have different crystal structures. ZrO2 typically adopts a cubic or tetragonal crystal structure at high temperatures, while Al2O3 has a hexagonal close-packed (hcp) crystal structure. Silicon nitride (Si3N4) and silicon carbide (SiC) also have different crystal structures. These differences in crystal structure can affect the types and strengths of interactions between the materials.
Thermal Stability: Zirconium oxide (ZrO2) and aluminum oxide (Al2O3) exhibit different thermal stability characteristics. ZrO2 has a higher melting point and thermal stability compared to Al2O3. These differences in thermal stability can affect the formation and stability of defect sites within the lattice, influencing the nature of interactions with the host material.
Overall, the differences in chemical composition, crystal structure, and thermal stability between ZrO2 and Al2O3 compared to Si3N4, SiC, or SiO2 can result in variations in the nature of interactions with the host Al2O3 lattice, ultimately affecting the manifestation of paramagnetic behavior.
Prolonged dwell time at high temperature, causing an increase in grain size, calcination of low-melting grain-boundary phases, and enrichment with carbon, leads to the ordering of lattice structure, the disappearing of unpaired electrons, and changes in the properties of the surface of the material.