Thermal Stability of Iron- and Silicon-Substituted Hydroxyapatite Prepared by Mechanochemical Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Mechanochemical Synthesis
2.2. Ex Situ Annealing
2.3. Sample Characterization
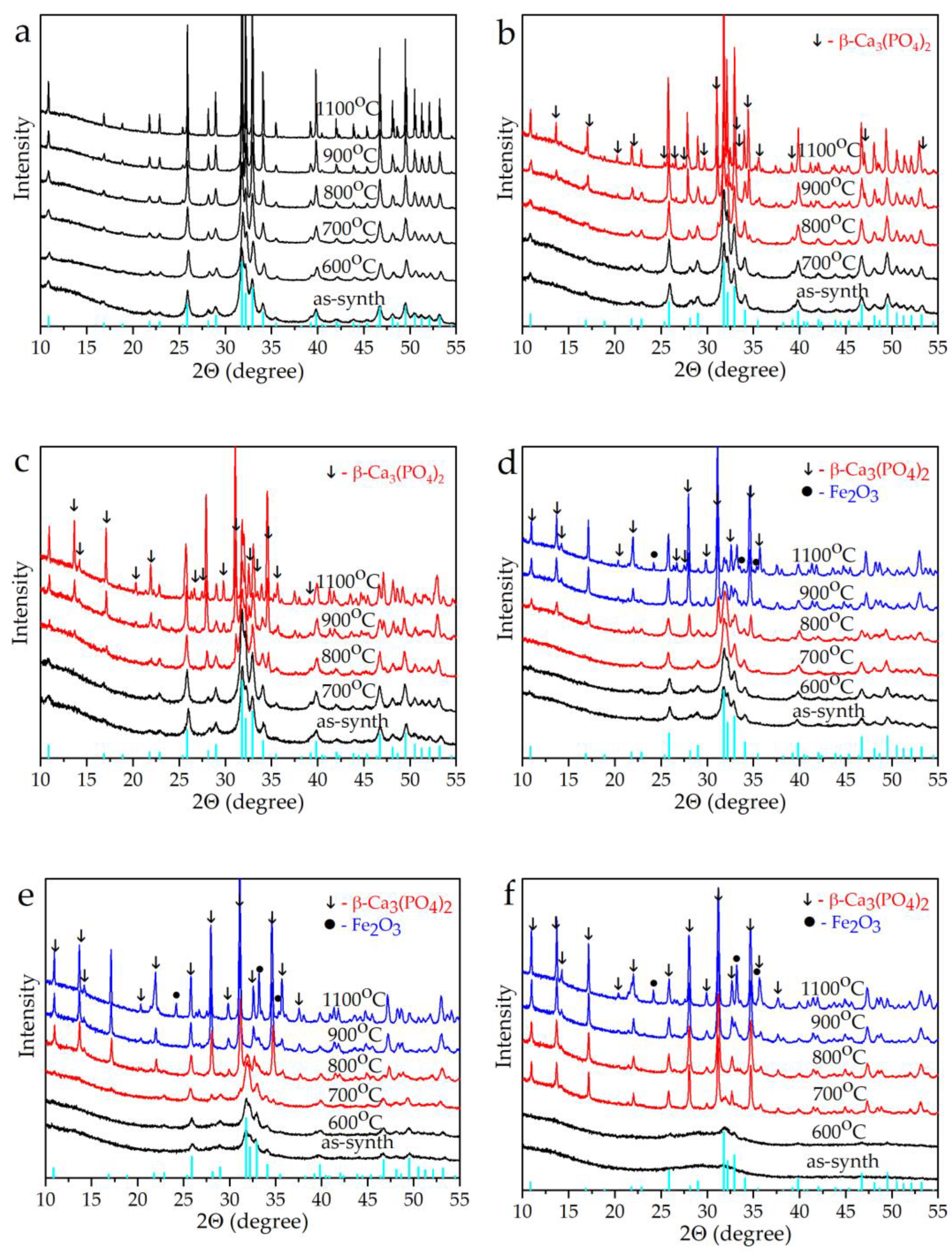
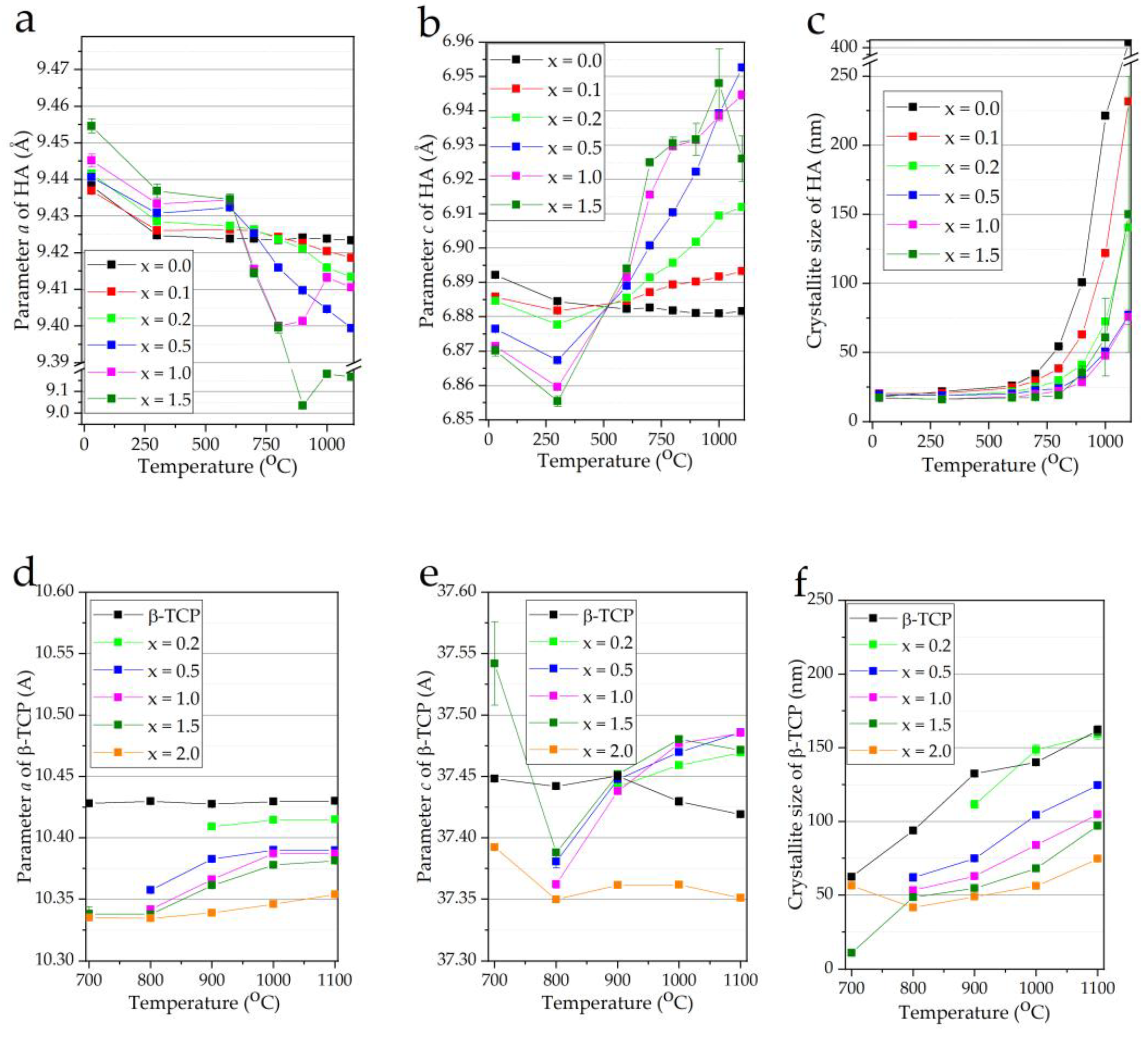
3. Results and Discussion
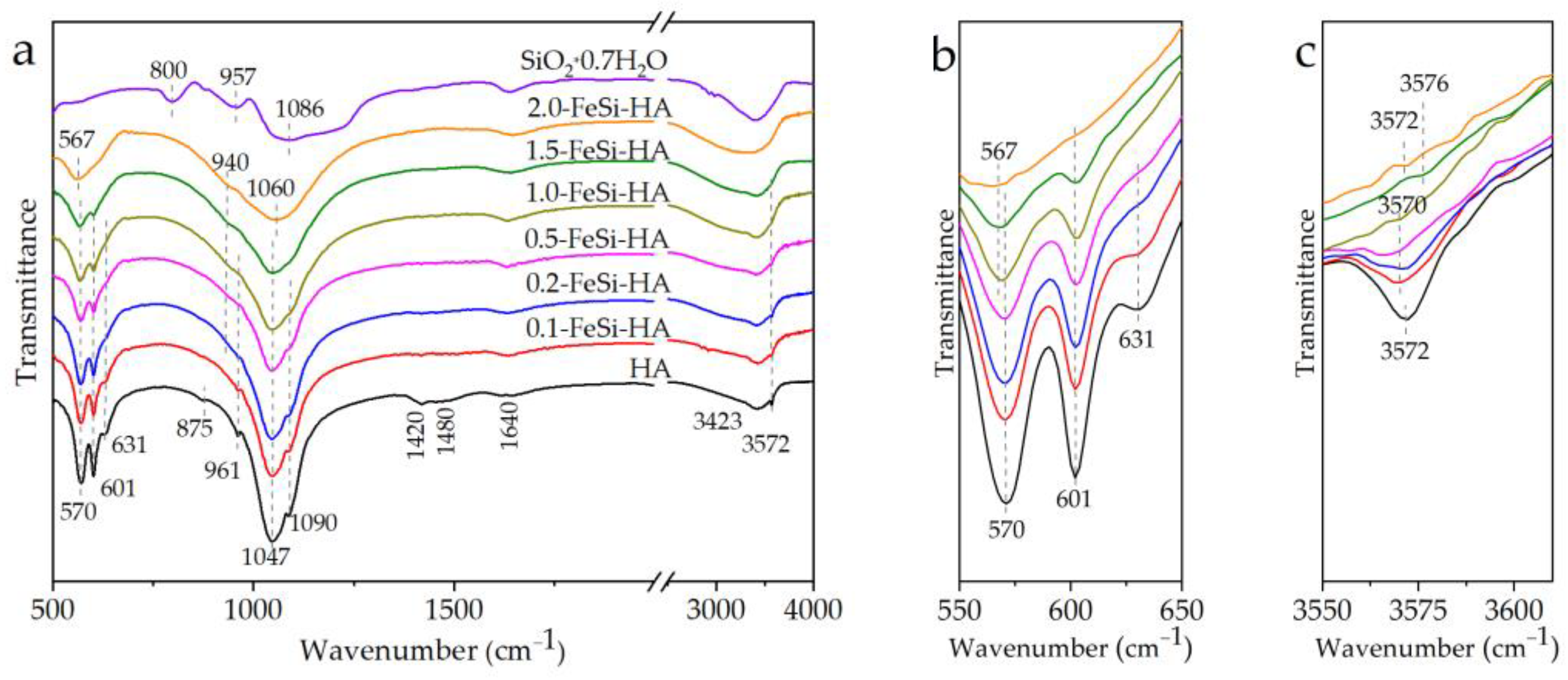
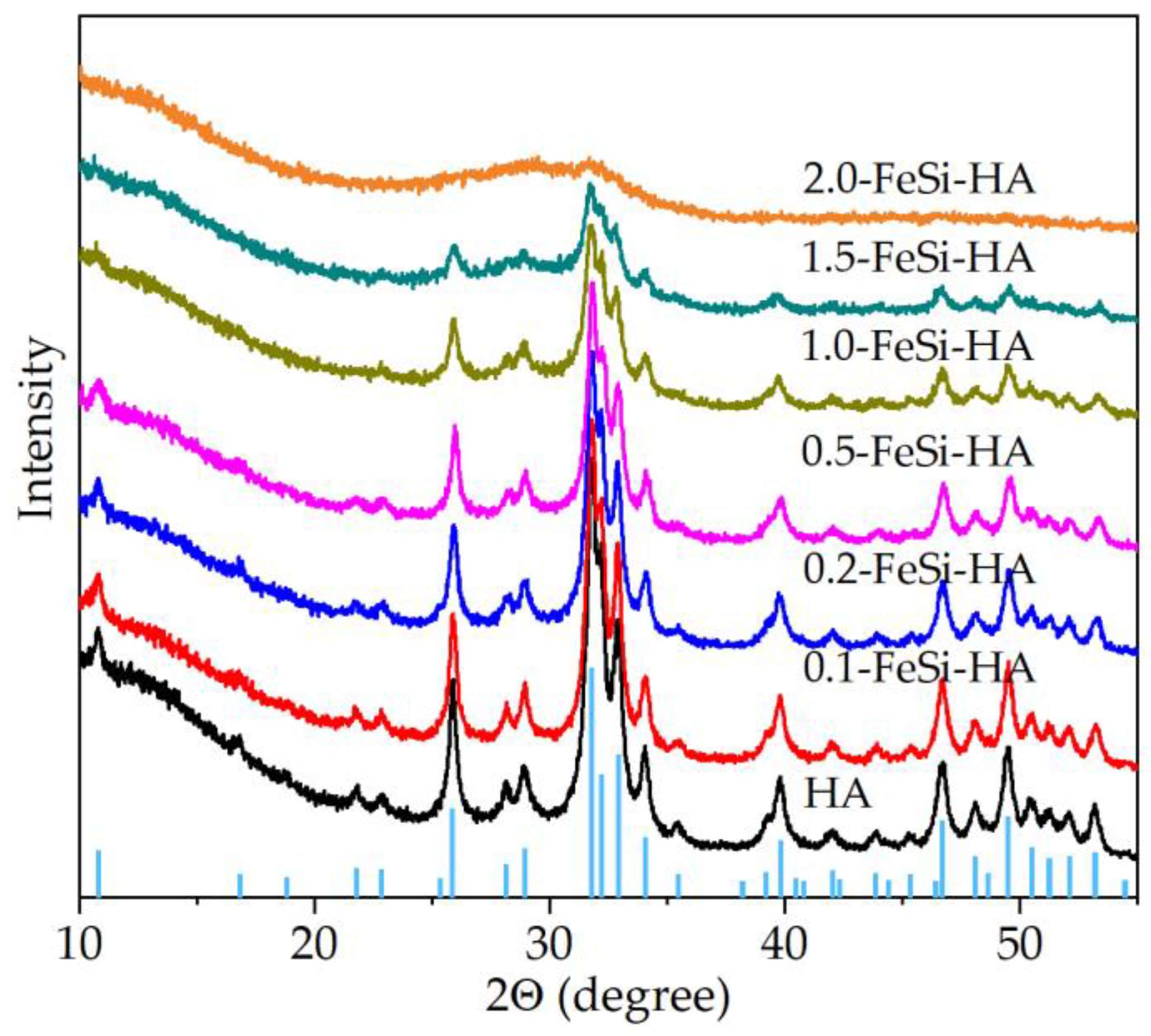
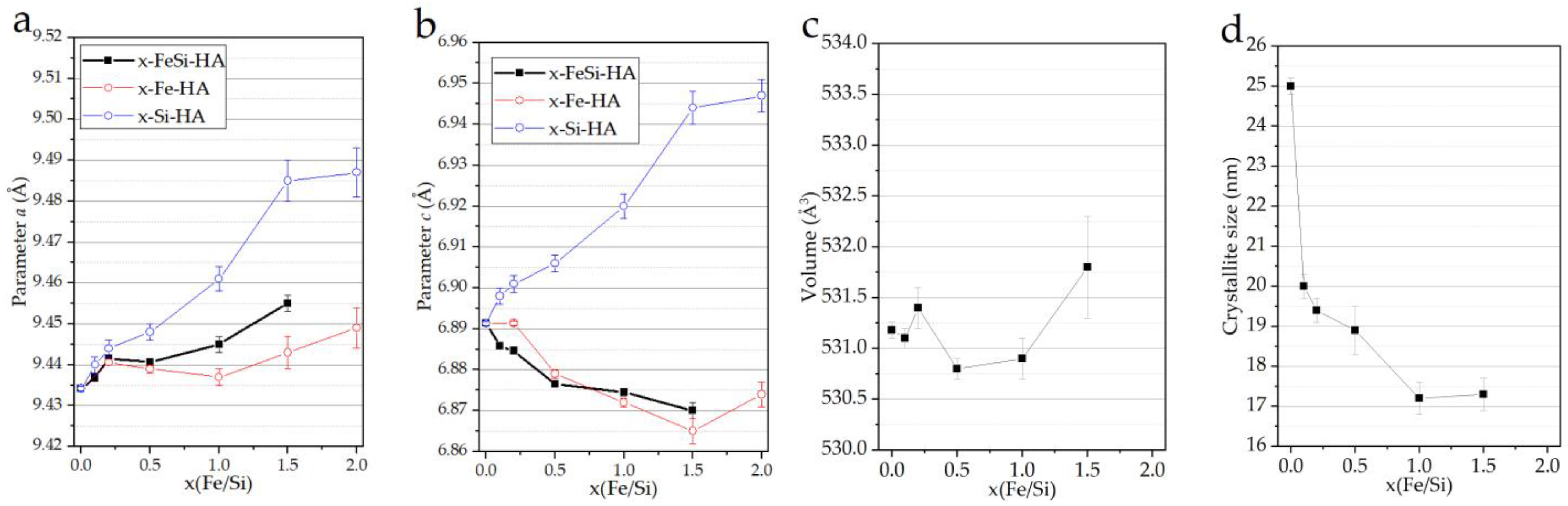
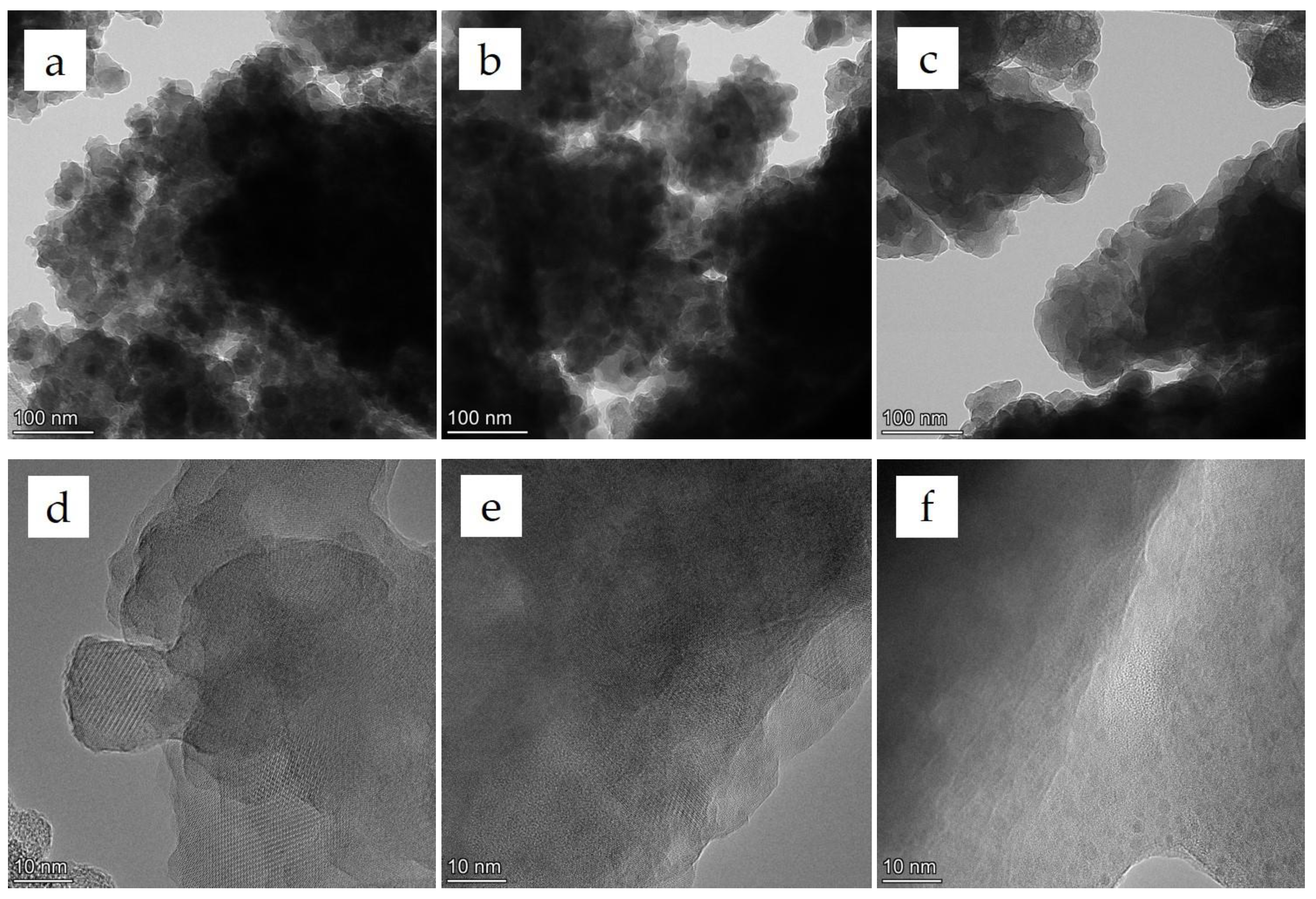
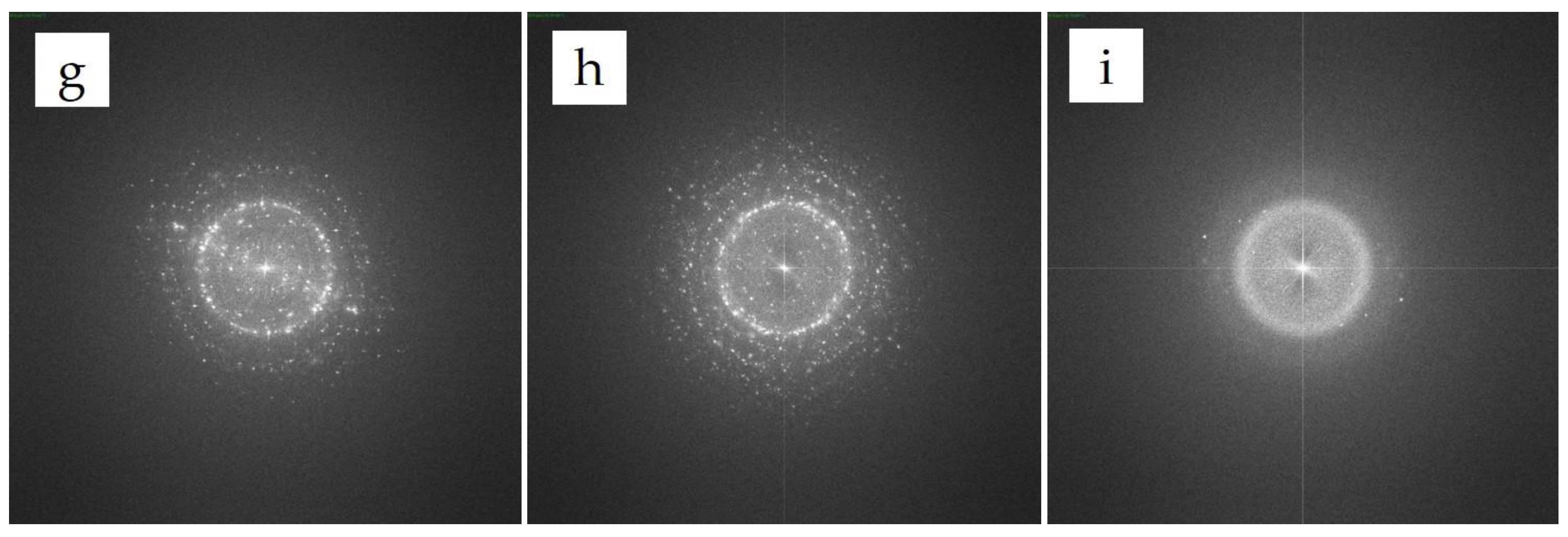
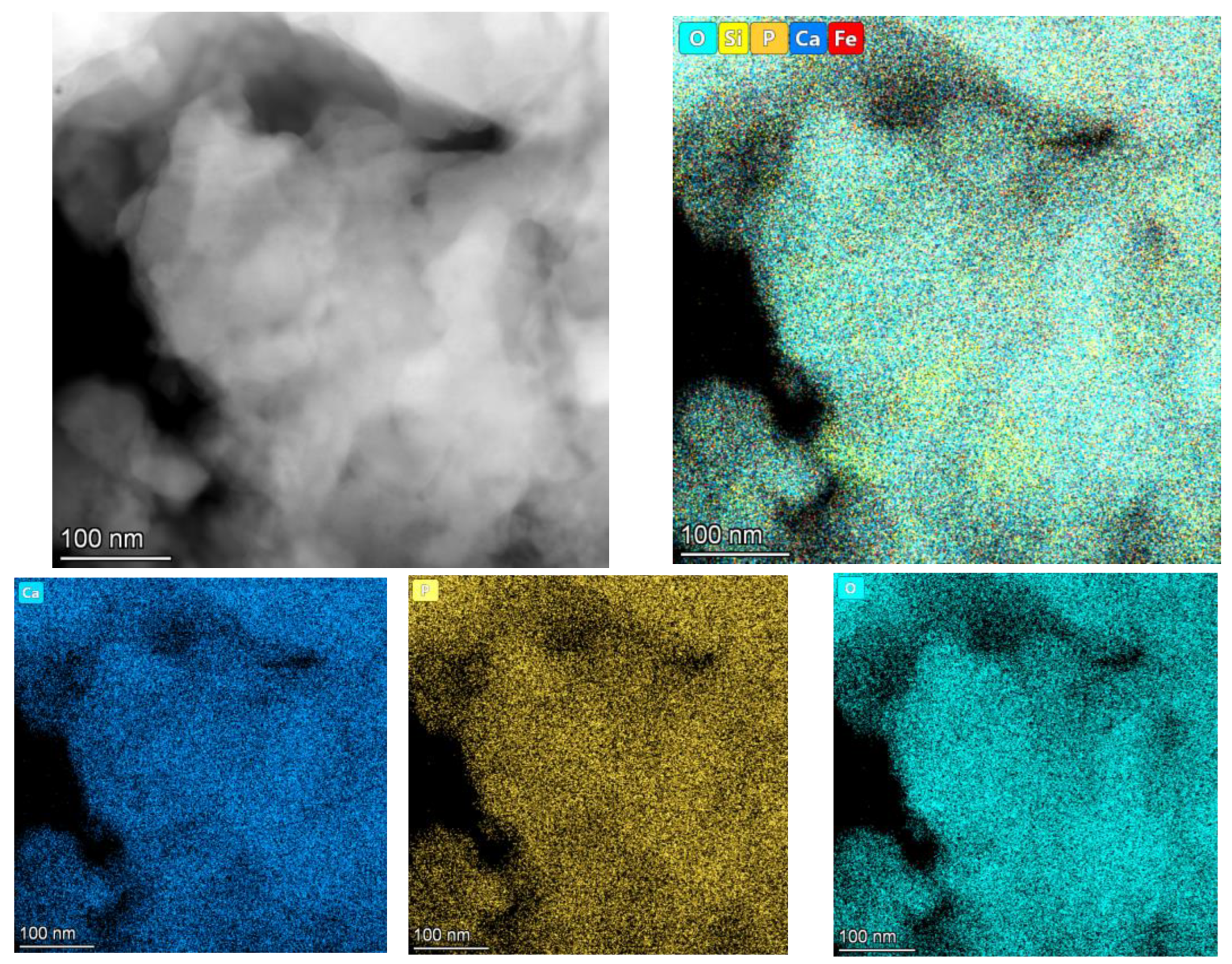
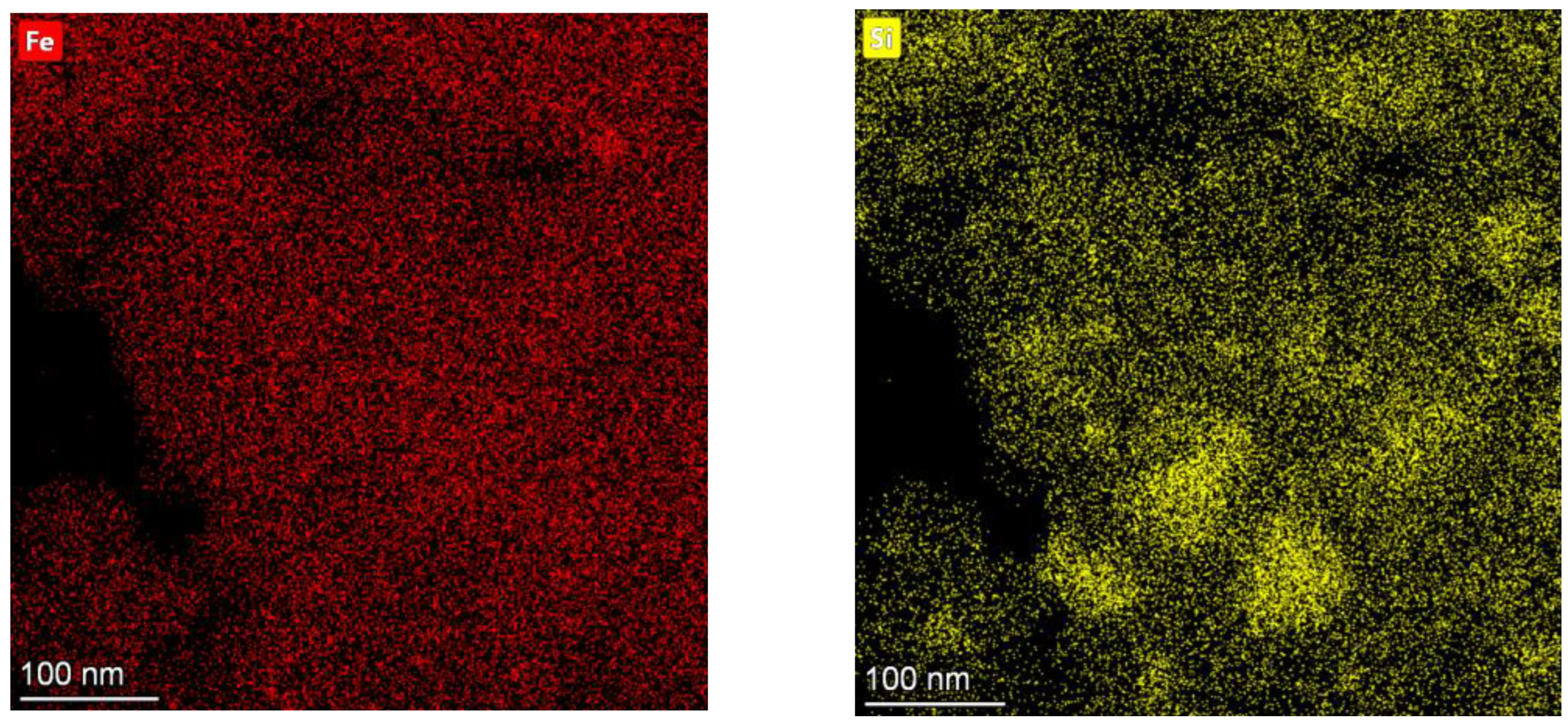
3.1. Analyses of the As-Prepared Samples
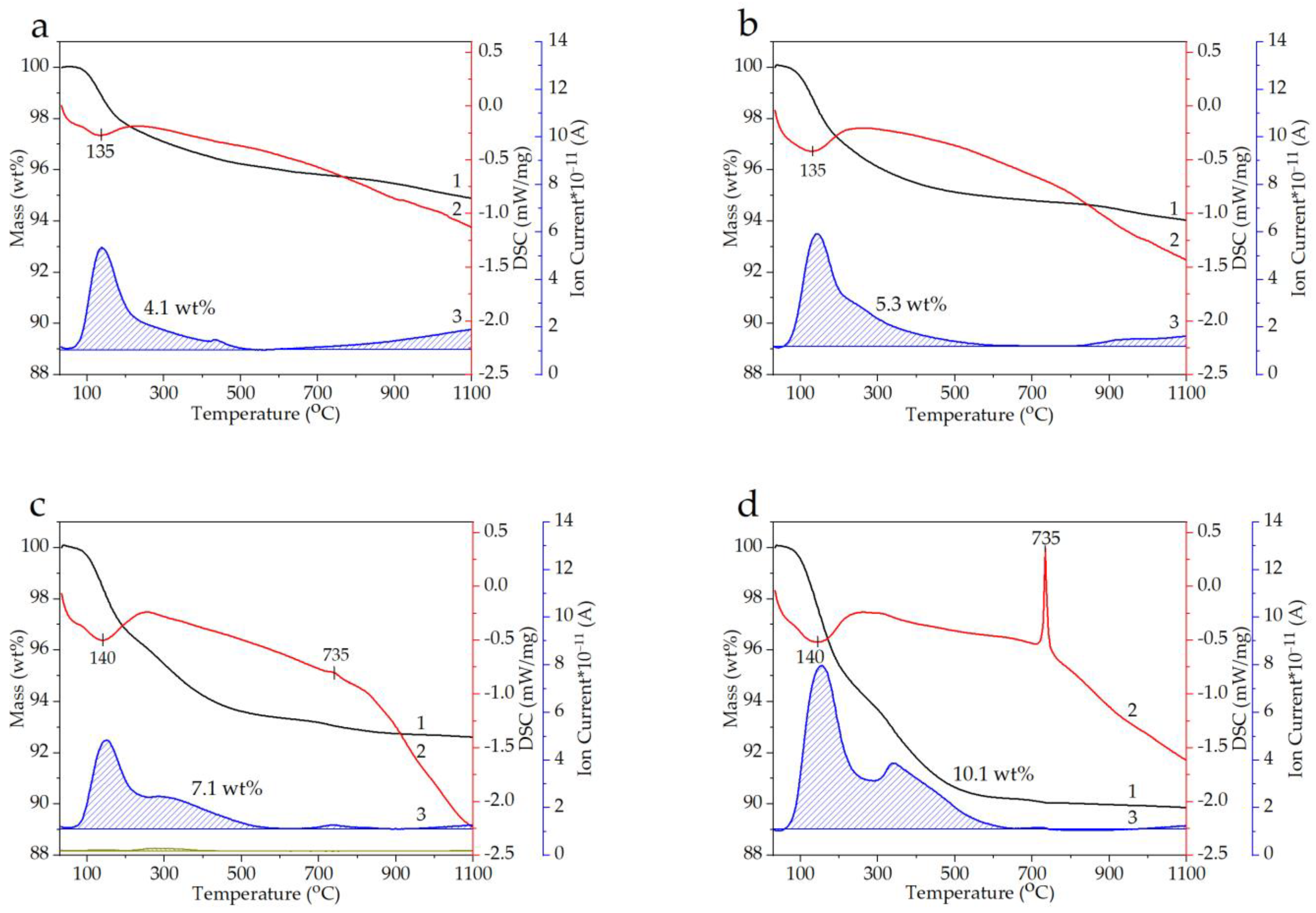
3.2. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, R.S.; Kayser, M.V.; Ali, S.Y. Calcium phosphate microcrystal deposition in the human intervertebral disc. J. Anat. 2006, 208, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Chaikina, M.V.; Bulina, N.V.; Vinokurova, O.B.; Gerasimov, K.B.; Prosanov, I.Y.; Kompankov, N.B.; Lapina, O.B.; Papulovskiy, E.S.; Ishchenko, A.V.; Makarova, S.V. Possibilities of Mechanochemical Synthesis of Apatites with Different Ca/P Ratios. Ceramics 2022, 5, 404–422. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Zein, S.H.S.; Othman, M.R.; Yang, F.; Jansen, J.A. Nanophase hydroxyapatite as a biomaterial in advanced hard tissue engineering: A review. Tissue Eng. B Rev. 2013, 19, 431–441. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Rózycka, D. Substituted hydroxyapatites with antibacterial properties. Biomed Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of Apatite and Other Calcium Orthophosphates; Studies in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 1994; pp. 1–404. ISBN 0-444-81582-1. [Google Scholar]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Ripamonti, A.; Roveri, N.; Romanello, M.; Noris Suarez, K.; Moro, L. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J. Inorg. Biochem. 1997, 68, 45–51. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: A requirement in bone formation independent of vitamin D1. Calcif. Tissue Int. 1981, 33, 27–34. [Google Scholar] [CrossRef]
- Patel, N.; Best, S.M.; Bonfield, W.; Gibson, I.R.; Hing, K.A.; Damien, E.; Revell, P.A. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med. 2002, 13, 1199–1206. [Google Scholar] [CrossRef]
- Hing, K.A.; Revell, P.A.; Smith, N.; Buckland, T. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 2006, 27, 5014–5026. [Google Scholar] [CrossRef]
- Balas, F.; Pérez-Pariente, J.; Vallet-Regí, M. In vitro bioactivity of silicon-substituted hydroxyapatites. J. Biomed. Mater. Res. A 2003, 66, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, A.; Cacciotti, I.; Campagnolo, L.; Bianco, A. Silicon-Substituted Hydroxyapatite for Biomedical Applications. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 95, pp. 343–373. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, J.H.; Kim, Y.T.; Riu, D.H.; Jung, S.J.; Lee, Y.J.; Chung, S.C.; Kim, Y.H. Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors. Biomaterials 2003, 24, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Chen, D.L.; Ru, H.Q.; Yue, X.Y.; Yu, L.; Zhang, C.P. Toughening mechanism in iron-containing hydroxyapatite/titanium composite. Biomaterials 2010, 31, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Pon-On, W.; Charoenphandhu, N.; Teeraporpuntakit, J.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Physicochemical and biochemical properties of ironloaded silicon substituted hydroxyapatite (FeSiHAp). Mater. Chem. Phys. 2013, 141, 850–860. [Google Scholar] [CrossRef]
- Kaygili, O.; Dorozhkin, S.V.; Ates, T.; Al-Ghamdi, A.A.; Yakuphanoglu, F. Dielectric properties of Fe doped hydroxyapatite prepared by sol gel method. Ceram. Int. 2016, 40, 9395–9402. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Intrinsically superparamagnetic Fe-hydroxyapatite nanoparticles positively influence osteoblast-like behavior. J. Nanobiotechnol. 2012, 10, 1–10. [Google Scholar] [CrossRef]
- Iafisco, M.; Sandri, M.; Panseri, S.; Delgado-Lopez, J.M.; Gomez-Morales, J.; Tampieri, A. Magnetic bioactive and biodegradable hollow Fe-doped hydroxyapatite coated poly(L-lactic) acid micro-nanospheres. Chem. Mater. 2013, 25, 2610–2617. [Google Scholar] [CrossRef]
- Sarath Chandra, V.; Baskar, G.; Suganthi, R.V.; Elayaraja, K.; Ahymah Joshy, M.I.; Sofi Beaula, W.; Mythili, R.; Venkatraman, G.; Narayana Kalkura, S. Blood compatibility of iron-doped nanosize hydroxyapatite and its drug release. ACS Appl. Mater. Interfaces 2012, 4, 1200–1210. [Google Scholar] [CrossRef]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Banobre-Lopez, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- Bulina, N.V.; Chaikina, M.V.; Andreev, A.S.; Lapina, O.B.; Ishchenko, A.V.; Prosanov, I.Y.; Gerasimov, K.B.; Solovyov, L.A. Mechanochemical synthesis of SiO44-substituted hydroxyapatite, part II—Reaction mechanism, structure, and substitution limit. Eur. J. Inorg. Chem. 2014, 2014, 4810–4825. [Google Scholar] [CrossRef]
- Marchat, D.; Zymelka, M.; Coelho, C.; Gremillard, L.; Joly-pottuz, L.; Babonneau, F.; Esnouf, C.; Chevalier, J.; Bernache-assollant, D. Accurate characterization of pure silicon-substituted hydroxyapatite powders synthesized by a new precipitation route. Acta. Biomater. 2013, 9, 6992–7004. [Google Scholar] [CrossRef] [PubMed]
- Glimcher, M.J. The Nature of the Mineral Phase in Bone: Biological and Clinical Implications. In Metabolic Bone Disease and Clinically Related Disorders, 3rd ed.; Avioli, L.V., Krane, S.M., Eds.; Academic Press: Camdridge, MA, USA, 1998; pp. 23–50. [Google Scholar] [CrossRef]
- Boskey, A. Bone mineral crystal size. Osteoporos. Int. 2003, 14, 16–21. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Bonel, G.; Legros, R. Types of “H2O” in human enamel and in precipitated apatites. Calcif. Tissue Res. 1978, 26, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Eremina, N.V.; Makarova, S.V.; Isaev, D.D.; Bulina, N.V. Soft mechanochemical synthesis and thermal stability of hydroxyapatites with different types of substitution. Chim. Techno. Acta 2022, 9, 3–9. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Gross, K.A.; Pluduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim. 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Bulina, N.V.; Makarova, S.V.; Baev, S.G.; Matvienko, A.A.; Gerasimov, K.B.; Logutenko, O.A.; Bystrov, V.S. A study of thermal stability of hydroxyapatite. Minerals 2021, 11, 1310. [Google Scholar] [CrossRef]
- Singh, R.K.; Srivastava, M.; Prasad, N.K.; Awasthi, S.; Dhayalan, A.; Kannan, S. Iron doped β -Tricalcium phosphate: Synthesis, characterization, hyperthermia effect, biocompatibility and mechanical evaluation. Mater. Sci. Eng. C 2017, 78, 715–726. [Google Scholar] [CrossRef]

| Degree of Substitution (x) | Sample Designation | Equations for Plausible Chemical Reactions |
|---|---|---|
| 0 | HA | 6.0CaHPO4 + 4.0CaO Ca10(PO4)6(OH)2 + 2H2O |
| 0.1 | 0.1−FeSi−HA | 5.8CaHPO4 + 4.1CaO + 0.1FePO4·2H2O + 0.1SiO2·0.7H2O Ca9.9Fe0.1(PO4)5.9(SiO4)0.1(OH)1.9O0.05 + 2.17H2O |
| 0.2 | 0.2−FeSi−HA | 5.6CaHPO4 + 4.2CaO + 0.2FePO4·2H2O + 0.2SiO2·0.7H2O Ca9.8Fe0.2(PO4)5.8(SiO4)0.2(OH)1.8O0.1 + 2.34H2O |
| 0.5 | 0.5−FeSi−HA | 5.0CaHPO4 + 4.5CaO + 0.5FePO4·2H2O + 0.5SiO2·0.7H2O Ca9.5Fe0.5(PO4)5.5(SiO4)0.5(OH)1.5O0.25 + 2.85H2O |
| 1.0 | 1.0−FeSi−HA | 4.0CaHPO4 + 5.0CaO + 1.0FePO4·2H2O + 1.0SiO2·0.7H2O Ca9.0Fe1.0(PO4)5.0(SiO4)1.0(OH)1.0O0.5 + 3.70H2O |
| 1.5 | 1.5−FeSi−HA | 3.0CaHPO4 + 5.5CaO + 1.5FePO4·2H2O + 1.5SiO2·0.7H2O Ca8.5Fe1.5(PO4)4.5(SiO4)1.5(OH)0.5O0.75 + 4.55H2O |
| 2.0 | 2.0−FeSi−HA | 2.0CaHPO4 + 6.0CaO + 2.0FePO4·2H2O + 2.0SiO2·0.7H2O Ca8.0Fe2.0(PO4)4.0(SiO4)2.0O + 5.40H2O |
| Sample | Concentration (at.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Composition of Synthesized Materials | Expected Composition | |||||||
| Ca | Fe | P | Si | Ca | Fe | P | Si | |
| HA | 66 | - | 34 | - | 63 | - | 37 | - |
| 0.5−FeSi−HA | 59 | 3 | 35 | 3 | 59 | 3 | 35 | 3 |
| 1.0−FeSi−HA | 58 | 8 | 29 | 5 | 57 | 6 | 31 | 6 |
| 2.0−FeSi−HA | 44 | 11 | 33 | 12 | 50 | 12 | 26 | 12 |
| Sample | Phase | 700 °C | 800 °C | 900 °C | 1000 °C | 1100 °C |
|---|---|---|---|---|---|---|
| HA | HA | 100 | 100 | 100 | 100 | 100 |
| 0.1−FeSi−HA | HA | 100 | 100 | 92 | 89 | 87 |
| β−TCP | 0 | 0 | 8 | 11 | 13 | |
| 0.2−FeSi−HA | HA | 100 | 95 | 76 | 70 | 66 |
| β−TCP | 0 | 5 | 24 | 30 | 34 | |
| 0.5−FeSi−HA | HA | 100 | 85 | 57 | 43 | 37 |
| β−TCP | 0 | 15 | 43 | 57 | 63 | |
| 1.0−FeSi−HA | HA | 97 | 67 | 32 | 14 | 12 |
| β−TCP | 3 | 33 | 67 | 81 | 83 | |
| Fe2O3 | 0 | 0 | 1 | 5 | 6 | |
| 1.5−FeSi−HA | HA | 79 | 31 | 7 | 2 | 1 |
| β−TCP | 21 | 69 | 93 | 90 | 90 | |
| Fe2O3 | 0 | 0 | 1 | 8 | 9 | |
| 2.0−FeSi−HA | HA | 15 | 6 | 7 | 3 | 1 |
| β−TCP | 85 | 94 | 91 | 89 | 89 | |
| Fe2O3 | 0 | 0 | 1 | 8 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarova, S.V.; Bulina, N.V.; Vinokurova, O.B.; Ishchenko, A.V. Thermal Stability of Iron- and Silicon-Substituted Hydroxyapatite Prepared by Mechanochemical Method. Powders 2023, 2, 372-386. https://doi.org/10.3390/powders2020022
Makarova SV, Bulina NV, Vinokurova OB, Ishchenko AV. Thermal Stability of Iron- and Silicon-Substituted Hydroxyapatite Prepared by Mechanochemical Method. Powders. 2023; 2(2):372-386. https://doi.org/10.3390/powders2020022
Chicago/Turabian StyleMakarova, Svetlana V., Natalia V. Bulina, Olga B. Vinokurova, and Arcady V. Ishchenko. 2023. "Thermal Stability of Iron- and Silicon-Substituted Hydroxyapatite Prepared by Mechanochemical Method" Powders 2, no. 2: 372-386. https://doi.org/10.3390/powders2020022
APA StyleMakarova, S. V., Bulina, N. V., Vinokurova, O. B., & Ishchenko, A. V. (2023). Thermal Stability of Iron- and Silicon-Substituted Hydroxyapatite Prepared by Mechanochemical Method. Powders, 2(2), 372-386. https://doi.org/10.3390/powders2020022







