Abstract
Nesting behavior in rodents, used to assess animal welfare/illness and instrumental tasks, is also proposed as valuable for disease monitoring, evaluating potential risk factors and interventions. The reliability of Deacon’s 5-point ordinal scale to score nests at 24 h is well-recognized. However, previous work with the 3xTg-AD mice model of Alzheimer’s disease proposed a 3-day protocol to discard false negatives, thus unveiling genotype-, sex- and age-dependent differences. Here, we propose the size of nesting as a numeric variable, complementary to the ordinal scale, to allow parametric repeated measures analysis for identifying and evaluating temporal patterns in the nest-building process. Thus, nests of male and female mice with normal and AD-pathological aging ‘measured’ during 3-days showed that the nest-building process responded to a linear equation in wild-type animals or when female sex was considered but disrupted in males or the AD-genotype. Genotype per sex interaction indicated the optimal nest-building process in wild-type females, as they build the best nests at 72 h and the worst nests in 3xTg-AD mice at 48 h. On each day, data were consistent with the ordinal scale, but the identification of temporal patterns with the numeric variable confirmed nest-building as a complex process, which is sensitive to sex and genotype.
Keywords:
environment; social; nesting; daily life activities; animal welfare; disease monitoring; aging 1. Introduction
Nesting behavior in rodents is a species-typical ethological behavior used as a naturalistic instrument for measuring animal welfare/illness and behavioral aspects related to instrumental tasks [1,2,3]. It is also proposed as valuable for disease monitoring, evaluating potential risk factors and preventive/therapeutical interventions [4,5,6]. The reliability of Deacon’s scale to score nests at 24 h is well-recognized, and it is based on a 5-point ordinal scale ranging from ‘not noticeably touched nesting material’ to ‘perfect nest’ [7]. In previous work using an animal model of Alzheimer’s disease and wild-type counterparts, we proposed a 3-day protocol to discard false negatives, thus unveiling genotype-, sex- and age-dependent differences [4]. Here, we propose the size of nesting as a numeric variable complementary to the ordinal scale. The present work aims to confirm that measuring the nest size allows the required parametric repeated-measure analysis to identify and evaluate temporal patterns in the nest-building process.
2. Materials and Methods
2.1. Animal Model of Alzheimer’s Disease
Homozygous triple-transgenic 3xTg-AD mice harboring human PS1/M146V, APPSwe and tauP301L transgenes were genetically engineered at the University of California Irvine, as previously described [8]. Briefly, two independent transgenes (encoding human APPSwe and human tauP301L, both under control of the mouse Thy1.2 regulatory element) were co-injected into single-cell embryos harvested from homozygous mutant PS1M146V knock-in (PS1KI) mice. The PS1 knock-in mice were originally generated after embryonic transfer into pure C57BL/6.
2.2. Experimental Subjects and Design
A cohort of twenty-three 16-month-old male and female mice from the Spanish colonies of 3xTg-AD and C57BL/6 wild-type mice (from now, referred to as non-transgenic mice, NTg) from litters of a breeding program established after embryonic transfer to C57BL/6 strain background was used in this study. The sample size was n = 7–8 per genotype and sex group. All animals were housed and maintained (Makrolon, 35 × 35 × 25 cm3) under standard laboratory conditions (12 h light/dark, cycle starting at 8:00 a.m., food and water ad libitum, 22 ± 2 °C and 50–60% humidity) at Universitat Autònoma de Barcelona. Behavioral tests were performed from 9:00 h to 13:00 h. Behavioral assessments were performed blind to the experiment in a counterbalanced manner.
All procedures followed Spanish legislation on ‘Protection of Animals Used for Experimental and Other Scientific Purposes’ and the EU Council directive (2010/63/EU) on this subject. Protocol CEEAH 3588/DMAH 9452 was approved the 8th of March 2019 by Departament de Medi Ambient i Habitatge, Generalitat de Catalunya. The study complies with the ARRIVE guidelines developed by the NC3Rs and aims to reduce the number of animals used [9].
2.3. Behavioral Assessment
Nests of male and female mice with normal and AD-pathological aging were measured using paper nesting material and our 3-days protocol [4]. Animals were individually located in home-cages with clean sawdust bedding supplied with one soft tissue folded in four parts (50 cm × 50 cm × 3 mm). On the next day, 48 and 72 h later, the nests were assessed according to Deacon 5-point ordinal scale from 1 to 5 as follows: 1 = not noticeably touched; 2 = partially torn up; 3 = mostly shredded but often no identifiable site; 4 = identifiable but flat; 5 = perfect or nearby [7]. Pictures of the nests were taken for posterior analysis with KINOVEA 0.8.15 free software and determination of N1 (size of the nest at 24 h), N2 (size of the nest at 48 h), and N3 (size of the nest at 72 h). This new method, presented here, allows obtaining quantitative measures of the nest in a continuous scale (see Figure 1).
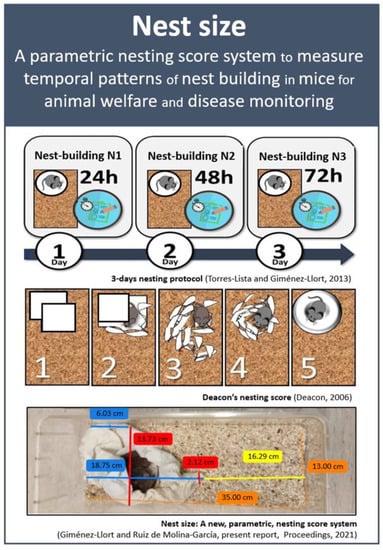
Figure 1.
Nest size: A new, parametric, nesting score system. The 3-days nesting protocol [4] was used and the new score system presented here is complementary to classical Deacon’s nesting score [7]. Pictures of the nests were taken for posterior analysis with KINOVEA 0.8.15 free software and determination of quantitative measures of N1 (size of the nest at 24 h), N2 (size of the nest at 48 h), and N3 (size of the nest at 72 h) in a continuous scale (cm) to allow statistical parametric analysis of the nest building patterns.
2.4. Statistics
Results are expressed as mean ± SEM. SPSS 15.0 (SPSS for Windows, Version 15.0. Chicago, SPSS Inc., Armonk, NY, USA) and GraphPad Prism version 5.0 for Windows (version 5.0, GraphPad Software, La Jolla, CA, USA) were used. Since Deacon’s score data consist of discrete values, non-parametric statistical tests used included the Mann–Whitney U test to compare two groups for each parameter and the Kruskal–Wallis test for global comparison of groups for all the parameters. Fisher’s exact test was used to analyze the incidence. The size of nests was analyzed with RMA, repeated-measures ANOVA with genotype and sex as between factors and day as within factor. One-way analysis of variance (ANOVA) and Bonferroni’s post hoc test and Paired t-test were also used. In all the tests, statistical significance was considered at p < 0.05.
3. Results
Deacon’s nesting scale was used to verify the nesting impairment in the 3xTg-AD mice as previously described. For simplification, Deacon’s nesting score data are not presented. The measurement results showed that the nest-building process responded to a linear equation in the wildtype animals (Only NTg—RM ANOVA N1N2N3—Day factor; lineal equation, F(1,14) = 9.941, *** p = 0.007; quadratic equation, F(1,14) = 0.529, p = 0.476, n.s.) or when female sex was considered (Only Females—RM ANOVA N1N2N3—Day factor; lineal equation F(1,13) = 7.341, p = 0.018; ** quadratic equation, F(1,13) = 0.025, p = 0.877, n.s.).
In contrast, lineal progression was found to be disrupted in males (Only Males—RM ANOVA N1N2N3—Day factor; lineal equation, F(1,16) = 0.593, p = 0.453, n.s.; quadratic equation, F(1,16) = 0.356, p = 0.467, n.s.) or the AD-genotype (Only 3xTg-AD—RM ANOVA N1N2N3—Day factor; lineal equation, F(1,15) = 0.117, p = 0.737, n.s.; quadratic equation, F(1,15) = 0.354, p = 0.478, n.s.).
Genotype per sex interaction indicated that the nest-building process was optimal in wild-type females, as they build the best nests at 72 h, while 3xTg-AD females build the worst nests at 48h (N2, F(1,29) = 5.311, p = 0.029, **).
On each day, data were consistent with the ordinal scale, but the identification of temporal patterns with the numeric variable confirmed nest-building as a complex process, which is sensitive to sex and genotype.
4. Discussion
The present brief report proposes the measurement of nest size as a helpful numerical and parametric variable for identifying and establishing temporal patterns in the nest-building process that is sensitive to genotype and sex and useful for animal welfare and disease monitoring. This measure is presented as complementary to Deacon’s score such that the gold-standard 5-items scale with well-known reliability can allow comparison with other works. Here, the ordinal scale was also used to verify the impairment of nesting, as we previously reported using the five scores in our protocol involving three days [4]. In that work, the possibility of identifying and evaluating the temporal patterns in the time progression of the ordinal score was restricted by the limitations of non-parametric analysis, which can be solved with this complementary measurement. Furthermore, a picture for posterior analysis with free software also provides a work capacity and reliability in the measurement.
In the present work, paper material was used since it is the one that better allows showing the impairments in 3xTg-AD mice [4] as compared to more naturalistic nesting materials that are known to help the animals build better nests [10]. For further validation of the temporal patterns, we are currently performing these measurements using cotton [4]. Similarly, the current data show genotype-, sex- and genotype × sex-dependent patterns in nest building, which would also agree with our previous results using Deacon’s scale [4] and strain and sex differences in the expression of behaviors in non-induced compulsive-like mice [11]. Furthermore, this would also agree with the response to divergent selection for nesting behavior in mus musculus described in the 1980s [12] or the identification of house mice bidirectionally selected for thermoregulatory nest-building behavior [13].
We have previously described nesting as an ethological behavior that could be used as the behavioral indicator of functional derangements shown in the 3xTg-AD mice since the early stages of the disease, which worsens through its advancement [4]. Furthermore, other authors have also reported this to happen in other AD models [14,15,16]. Finally, nesting has been helpful to confirm that beneficial pharmacological [6] and non-pharmacological [17] in cognition and anxiety-like behaviors can also be reflected in specie-typical behaviors of their daily life activity ethogram.
5. Conclusions
In conclusion, the present report confirms that measuring the nest size allows the required parametric repeated measures analysis to identify and evaluate temporal patterns in the nest-building process in both male and female mice modeling AD, with distinctive patterns compared to their age sex-matched wild-type C57BL/6 mice with normal aging.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/IECBS2021-10685/s1.
Author Contributions
Conceptualization, L.G.-L.; methodology, L.G.-L.; resources, L.G.-L.; data curation, A.M.R.d.M.-G.; statistical analysis: A.M.R.d.M.-G.; writing, L.G.-L.; funding acquisition, L.G.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by UAB-GE-260408 to L.G.-L. The colony of 3xTg-AD mice is sustained by ArrestAD H2020 Fet-OPEN-1-2016-2017-737390, European Union’s Horizon 2020 research, and innovation program under grant agreement No. 737390 to L.G.-L.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Departament de Medi Ambient i Habitatge, Generalitat de Catalunya (CEEAH 3588/DMAH 9452) on the 8 March 2019.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We thank Frank M. LaFerla, Institute for Memory Impairments and Neurological Disorders, University of California Irvine, CA, USA, for kindly providing the progenitors of the Spanish colonies of 3xTg-AD and NTg mice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods 2014, 234, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, I.A.; Dahlborn, K. Improving housing conditions for laboratory mice: A review of “environmental enrichment”. Lab. Anim. 2002, 36, 243–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaskill, B.; Pritchett-Corning, K.R. Nest building as an indicator of illness in laboratory mice. J. Appl. Anim. Behav. Sci. 2016, 180, 140–146. [Google Scholar] [CrossRef]
- Torres-Lista, V.; Giménez-Llort, L. Impairment of nesting behaviour in 3xTg-AD mice. Behav. Brain Res. 2013, 247, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Muntsant, A.; Giménez-Llort, L. Impact of social isolation on the behavioral and functional profiles and hippocampal atrophy asymmetry in dementia in times of coronavirus pandemic (COVID-19): A translational neuroscience approach. Front. Psychiatry 2020, 11, 572583. [Google Scholar] [CrossRef] [PubMed]
- Van der Jeugd, A.; Parra-Damas, A.; Baeta-Corral, R.; Soto-Faguás, C.M.; Ahmed, T.; LaFerla, F.M.; Giménez-Llort, L.; D’Hooge, R.; Saura, C. Reversal of memory and neuropsychiatric symptoms and reduced tau pathology by selenium in 3xTg-AD mice. Sci. Rep. 2018, 8, 6431. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.J. Assessing nest building in mice. Nat. Protoc. 2006, 1, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular A and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.E.; Rohr, S.; Dufour, B.D.; Gaskill, B.N.; Pajor, E.A.; Garner, J.P. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 25–31. [Google Scholar] [PubMed]
- Mitra, S.; Bastos, C.P.; Chesworth, S.; Frye, C.; Bult-Ito, A. Strain and sex based characterization of behavioral expressions in non-induced compulsive-like mice. Physiol. Behav. 2017, 168, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, C.B. Response to divergent selection for nesting behavior in mus musculus. Genetics 1980, 96, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Bult, A.; Lynch, C.B. Multiple selection responses in house mice bidirectionally selected for thermoregulatory nest-building behavior: Crosses of replicate lines. Behav. Genet. 1996, 26, 439–446. [Google Scholar] [CrossRef]
- Deacon, R.M.; Koros, E.; Bornemann, K.D.; Rawlins, J.N. Aged Tg2576 mice are impaired on social memory and open field habituation tests. Behav. Brain Res. 2009, 197, 466–468. [Google Scholar] [CrossRef]
- Wesson, D.W.; Wilson, D.A. Age and gene overexpression interact to abolish nesting behaviour in Tg2576 amyloid precursor protein (APP) mice. Behav. Brain Res. 2011, 216, 408–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filali, M.; Lalonde, R.; Rivest, S. Anomalies in social behaviors and exploratory activities in an APPswe/PS1 mouse model of Alzheimer’s disease. Physiol. Behav. 2011, 104, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Torres-Lista, V. Social Nesting, Animal Welfare, and Disease Monitoring. Animals 2021, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).