Systematic Review: Antioxidant and Neuroprotective Capacity of Species of the Genus Asplenium (Monilophyta: Aspleniaceae) †
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Živković, S.; Milutinović, M.; Maksimović, V.; Ćirić, A.; Ivanov, M.; Božunović, J.; Banjanac, T.; Mišić, D. Antioxidant and antimicrobial activity of two Asplenium species. S. Afr. J. Bot. 2020, 132, 180–187. [Google Scholar] [CrossRef]
- Jarial, R.; Thakur, S.; Sakinah, M.; Zularisam, A.W.; Sharad, A.; Kanwar, S.S.; Singh, L. Potent anticancer, antioxidant and antibacterial activities of isolated flavonoids from Asplenium nidus. J. King Saud Univ-Sci. 2018, 30, 185–192. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Mishra, S.H. Investigation of mangiferin, as a promising natural polyphenol xanthone on multiple targets of Alzheimer's disease. J. Biol. Act. Prod. Nat. 2014, 4, 111–119. [Google Scholar] [CrossRef]
- Kawpoomhae, K.; Sukma, M.; Ngawhirunpat, T.; Opanasopit, P.; Sripattanaporn, A. Antioxidant and neuroprotective effects of standardized extracts of Mangifera indica leaf. Thai J. Pharm. Sci. 2010, 34, 32–43. [Google Scholar]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chai, T.T.; Wang, X.; Morais-Braga, M.F.; Yang, J.H.; Wong, F.C.; Wang, R.; Yao, H.; Cao, J.; Cornara, L.; et al. Phytochemicals form ferm species: Potential for medicine applications. Phytochem. Rev. 2017, 16, 379–440. [Google Scholar] [CrossRef] [PubMed]
- Umikalsom, Y.; Grayer-Barkmeijer, R.J.; Harborne, J.B. A comparison of the flavonoids in Athyriaceae and Aspleniaceae. Biochem. Syst. Ecol. 1994, 22, 587–594. [Google Scholar] [CrossRef]
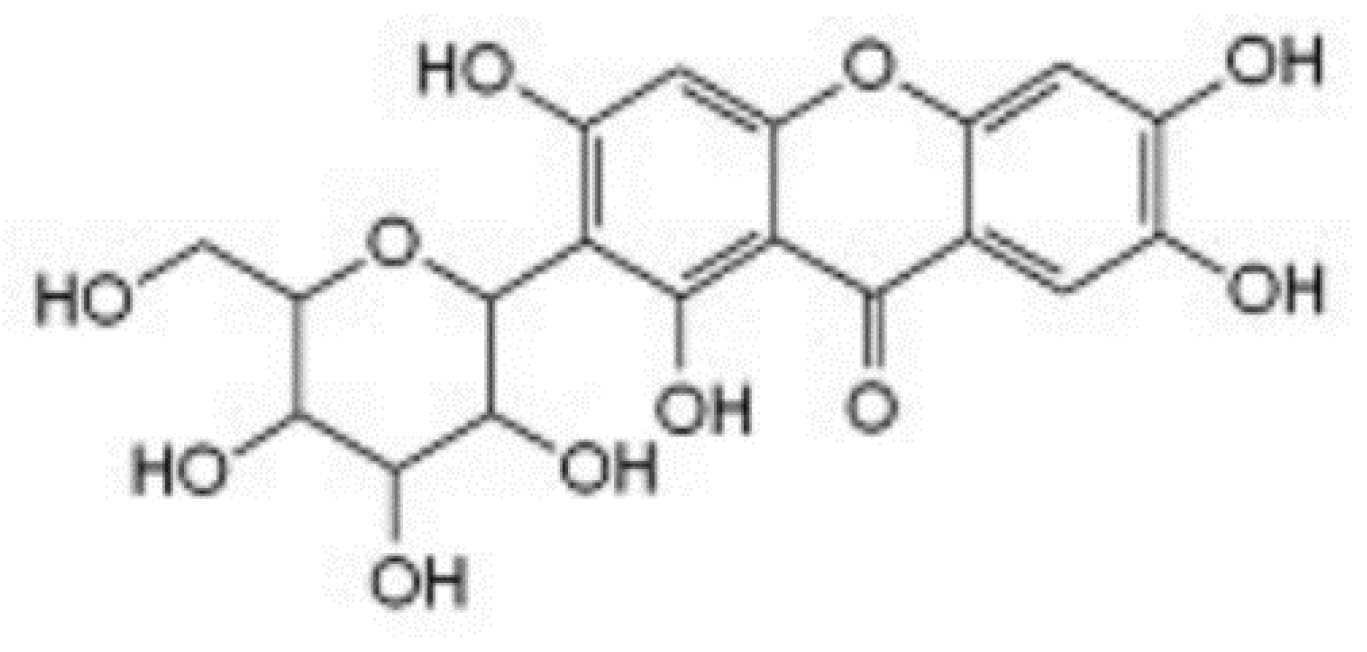
| Compounds | Concentration (mg/g FW) | ||
|---|---|---|---|
| A. adiantum-nigrum | A. ruta-muraria | ||
| Phenolic acids and derivatives | Gallic acid | 0.69 ± 0.01 (Rhizome) | - |
| Protocatechuic acid | 1.61 ± 0.06 (Rhizome) | 1.29 ± 0.01 (Rhizome, Roots), 1.29 ± 0.04 (Fronds) | |
| Gentisic acid | 0.13 ± 0.08 (Fronds) | 0.11 ± 0.01 (Rhizome, Roots), 0.11 ± 0.06 (Fronds) | |
| p-Hydroxybenzoic acid | 0.63 ± 0.05 (Fronds) | 0.44 ± 0.11 (Fronds) | |
| Aesculin | 0.39 ± 0.05 (Fronds) | 0.51 ± 0.03 (Fronds) | |
| Chlorogenic acid | 0.62 ± 0.03 (Rhizome) | 2.05 ± 1.05 (Roots) | |
| Caffeic acid | 0.59 ± 0.06 (Rhizome) | 0.21 ± 0.02 (Fronds) | |
| p-Coumaric acid | 2.82 ± 0.74 (Rhizome) | 1.00 ± 0.08 (Fronds) | |
| Ferulic acid | 0.23 ± 0.01 (Rhizome) | - | |
| Rosmarinic acid | 1.69 ± 0.01 (Roots) | 0.03 ± 0.01 (Fronds) | |
| Flavonoids and derivatives | Epigallocatechin | 3.71 ± 1.33 (Rhizome) | 9.12 ± 1.56 (Rhizome) |
| Epicatechin | - | 1.26 ± 0.01 (Rhizome) | |
| Gallocatechin gallate | - | 10.29 ± 0.67 (Fronds) | |
| Epigallocatechin gallate | 1.15 ± 0.09 (Fronds) | 1.74 ± 0.18 (Fronds) | |
| Rutin | 0.27 ± 0.01 (Roots) | 0.24 ± 0.01 (Roots) | |
| Xanthones | Mangiferin glucoside | 507.69 ± 19.58 (Rhizome) | 0.51 ± 0.01 (Rhizome) |
| Mangiferin | 598.29 ± 20.82 (Rhizome) | 0.70 ± 0.12 (Rhizome) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-González, M.; Simirgiotis, M.; Torres-Benítez, A. Systematic Review: Antioxidant and Neuroprotective Capacity of Species of the Genus Asplenium (Monilophyta: Aspleniaceae). Med. Sci. Forum 2022, 8, 14. https://doi.org/10.3390/IECBS2021-10665
Flores-González M, Simirgiotis M, Torres-Benítez A. Systematic Review: Antioxidant and Neuroprotective Capacity of Species of the Genus Asplenium (Monilophyta: Aspleniaceae). Medical Sciences Forum. 2022; 8(1):14. https://doi.org/10.3390/IECBS2021-10665
Chicago/Turabian StyleFlores-González, Mathias, Mario Simirgiotis, and Alfredo Torres-Benítez. 2022. "Systematic Review: Antioxidant and Neuroprotective Capacity of Species of the Genus Asplenium (Monilophyta: Aspleniaceae)" Medical Sciences Forum 8, no. 1: 14. https://doi.org/10.3390/IECBS2021-10665
APA StyleFlores-González, M., Simirgiotis, M., & Torres-Benítez, A. (2022). Systematic Review: Antioxidant and Neuroprotective Capacity of Species of the Genus Asplenium (Monilophyta: Aspleniaceae). Medical Sciences Forum, 8(1), 14. https://doi.org/10.3390/IECBS2021-10665







