Abstract
Background and Objectives: According to guidelines, patent foramen ovale (PFO) closure is recommended for secondary stroke prevention in patients with cryptogenic stroke. Paradoxial embolism from PFO-mediated right to left shunt has been described as the mechanism of stroke in these cases. The aim of the study was to determine whether PFO closure can be associated with improvement of complaints (headaches, fatigue, heart palpitations, dizziness, and visual impairment) and determine its long-term effectiveness on recurrent stroke risk reduction. Materials and Methods: A total of 103 patients were enrolled in a retrospective study and followed-up by phone up to five years after PFO closure. Standardized survey was conducted about their well-being, recurrent cerebrovascular events, and the use of prescribed medication. Patients were also followed up for residual shunts 24 h, 30 days, 1 year, and 2 years after PFO. The pathogenic ischemic stroke subtypes are determined using CCS (Causative Classification System for Ischemic Stroke). Results: Male patients accounted for 43.7% (n = 45). The mean age was—44.4 ± 13 (18–75). The most probable cause for cryptogenic stroke for 53.4% (n = 55) of patients with possible cardio-aortic embolism was PFO. Residual shunts were mostly observed in patients with Amplatzer occluder—87.5% (n = 14). There was correlation between residual shunt and increased risk of transient ischemic attack recurrence (p = 0.067). Five-years after PFO closure recurrent cerebrovascular events were reported in only 5.1% (n = 5) of patients, this difference is statistically relevant (p < 0.001). Out of 51 patients presented with complaints before PFO closure, 25.5% (n = 13) did not present with any complaints after PFO closure. Conclusions: PFO can be considered a possible risk factor for cryptogenic stroke. PFO closure is effective in reducing recurrent cerebrovascular events. Residual shunt after PFO closure increases the risk of transient ischemic attack recurrence. Amplatzer occluder device is associated with a higher risk for residual shunts after PFO closure. PFO closure can be associated with improvement of complaints.
1. Introduction
Patent foramen ovale (PFO) is a normal fetal communication between the right and left atria that closes soon after birth. In 20–34% of the population PFO persists [1]. Usually it is asymptomatic, but is has been associated with decompression illness, systemic arterial embolism, obstructive sleep apnea, and migraine with aura [2]. In some cases, PFO can cause a paradoxical embolism mediated from an interatrial shunt that has been described as the mechanism of cryptogenic stroke, particularly in young adults, therefore, prevention of recurrence is of paramount importance. A cryptogenic stroke is generally defined as a stroke of unknown cause that is now thought to comprise about 25% of all ischemic strokes [3]. The therapeutic possibilities for PFO range from conservative treatment (antithrombotic treatment) to its percutaneous closure using various types of occluders [4]. According to the latest guidelines of American Academy of Neurology (AAN) (2017) in patients younger than 60 years with a PFO and embolic-appearing infarction and no other mechanism of stroke identified, clinicians may recommend closure following a discussion of potential benefits and risks (level C). In patients who opt to receive medical therapy alone without PFO closure, clinicians may recommend an antiplatelet medication such as aspirin or anticoagulation (level C) [5]. Data from 6 major trials (CLOSURE I, PC Trial, RESPECT, CLOSE, REDUCE, and DEFENSE-PFO) demonstrates the superiority of PFO closure over medical management alone in preventing cryptogenic stroke recurrence [6,7].
The aim of the study was to determine whether PFO closure can be associated with improvement of complaints (headaches, fatigue, heart palpitations, dizziness, visual impairment) and determine its long-term effectiveness on recurrent cerebrovascular events (cerebral infarction (CI) or transient ischemic attack (TIA)) risk reduction.
2. Materials and Methods
2.1. Study Design and Patient Population
A single-center (Pauls Stradiņš Clinical University Hospital) prospective–retrospective study was performed on patients who underwent a PFO closure procedure between years 2004 and 2019.
2.2. Evaluations
Transthoracic echocardiogram (TTE) was performed and evaluated by cardiologists.
Ischemic stroke diagnosis was made by neurologists. The pathogenic subtypes of ischemic stroke were determined using CCS (Causative Classification System for Ischemic Stroke) [8] by a radiologist.
2.3. Baseline Study Assessment
At first, a list was obtained from hospitals digital database of patients who underwent PFO closure procedure at Pauls Stradiņš Clinical University Hospital between years 2004 and 2019. Inclusion criteria for study participants:
- -
- Subjects at least 18 years of age.
- -
- Subjects who have been diagnosed with Patent Foramen Ovale (PFO).
- -
- Subjects who have undergone PFO closure procedure at Pauls Stradiņš Clinical University Hospital between years 2004 and 2019.
Afterwards data from hospitals digital database about each patient was collected–personal information (personal code, phone number), what kind of radiological exams were done during hospitalization (CT, CTA, MR, MRA, TTE, Doppler ultrasound) and their findings, type of the atrial septal defect (ostium secundum, ostium primum, sinus venosus, PFO, post-operative iatrogenic defect), date of PFO closure, at what age PFO closure was done, PFO occluder type (amplatzer, cardia, cardia ultra, helex), PFO occluder size, implantation technique (percutaneous, transcatheter), surgeon performing PFO closure procedure, days of hospitalization, intrahospital complications, associated anomalies (abnormal pulmonary vein drainage, mitral valve prolapse, PDA, VSD), was there an atrial septal aneurysm, are there any arrhythmias in patients history (atrial fibrillation, atrial flutter, supraventricular tachycardia, arteriovenous blockage, sinus node dysfunction), atrial fibrillation type (permanent, persistent, paroxysmal), previous cerebrovascular events—what kind (ischemic stroke, transient ischemic attack) and how many, heart failure (class 1–4), previous myocardial infarctions, previously done percutaneous coronary intervention (PCI), does the patient have hypertension and/or diabetes.
When all previously listed information about the patient was collected, pathogenic subtype of ischemic stroke was determined using CCS and patients were followed-up by phone 30 days, 3 months, and 6 months, 1, 2, 3, 4, and 5 years after PFO closure. During the first call (day 30 after PFO closure) standardized survey was conducted—does the patient have any symptoms after stroke (impaired memory, coordination, speech, vision, paresis, headaches), did the patient have any other complaints before PFO closure (headaches, fatigue, dizziness, visual impairment, palpitations), to what floor can they climb to (cardiac stress test), what kind of medication do they use, comorbidities, bad habits (smoking, alcohol), lifestyle (sitting, physical), and height and weight (so that BMI can be calculated). Each time the patient was also called and questioned about recurring cerebrovascular events (ischemic stroke or transient ischemic attack), complaints after PFO closure (headaches, fatigue, dizziness, visual impairment, palpitations), and complications after PFO closure (arrhythmias, shunt).
Patients were also followed up for residual shunts 24 h, 30 days, 1 year, and 2 years after PFO closure by a cardiologist using TT-ECHO-KG and TEE-ECHO-KG bubble study after 6 months
2.4. Statistical Analysis
All previously listed results were summarized using “Microsoft Excel” and analyzed using “IBM SPSS” software. The mean, range, and standard deviation were calculated for quantitative data. Before and after results were compared using the McNemar’s test. Pearson’s Chi-square, Fisher tests, were used to analyze qualitative data. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics of Study Participants
A total of 103 patients were enrolled in the study. Baseline characteristics of study participants are presented in Table 1.

Table 1.
Baseline characteristics of study participants.
Overall, 91 patients were younger than 60 years, 12 patients were 60 years and older—youngest patient being 18 and oldest patient being 75 years of age.
The prevalence of interatrial septal aneurysms was 16.5%.
3.2. Cerebrovascular Event Characteristics before PFO Closure
At least 1 cerebrovascular event before PFO closure was seen in 92.2% (n = 95) of patients. Most common event was CI—84.5% (n = 87), less common was TIA—14.6% (n = 15). Both CI and TIA before PFO closure was seen in 7.4% (n = 7) of patients. One cerebrovascular event before PFO closure was seen in 16.5% of patients, in 14.6%—two events. The biggest number of events was five, which was seen in 1.9% of patients. In 5-year time, only one patient with ASA had recurrent TIA and no patients with ASA had recurrent CI. Cerebrovascular event characteristics before PFO closure are presented in Table 2.

Table 2.
Cerebrovascular event characteristics before PFO closure.
According to CCS for 66.7% (n = 58) of patients the pathogenic subtype of CI was possible cardio-aortic embolism and for 1.1% (n = 1)—evident cardio-aortic embolism. Patients with arterial fibrillation were excluded as that has been identified as the cause of cryptogenic stroke. Therefore, four patients in total were excluded. For 53.4% (n = 55) of patients with possible cardio-aortic embolism the most probable cause for cryptogenic stroke was PFO. Pathogenic subtypes of CI are presented in Table 3.

Table 3.
Pathogenic subtypes of CI.
CT scan was performed in 54.7% (n = 52) of patients who have had a cerebrovascular event. CTA in 47.4% (n = 45) of patients, MR—45.3% (n = 43) and MRA—21.1% (n = 20).
3.3. PFO Closure Procedure Characteristics
The mean age for patients undergoing PFO closure procedure was 44.4 years (SD 13). For 3.9% (n = 4) of patients the PFO closure was unsuccessful—these patients were excluded from the statistics moving forward. Therefore, the procedures success rate is 96.1%. All patients underwent percutaneous device implantation. The most common type of implantation device (occluder) used was Amplatzer—58.6% (n = 58), less frequently used were Cardia Ultrasept device—39.4% (n = 39). Mean septal occluder size was 24.8 (SD 4.2) mm, of which the smallest was 10 mm and the largest 35 mm. Most used occluder size was 25 mm–78.8% (n = 78). The average hospital stay was 2.2 (SD 0.5) days, minimum being 2 days and maximum being 4 days. Residual shunt 24 h post-op was observed in 9.1% of patients, and no patients had intrahospital complications. Residual shunts were mostly observed in patients with Amplatzer occluder—87.5% (n = 14), for patients with Cardia Ultrasept occluder residual shunts were observed in only 1% of patients. PFO closure procedure characteristics are presented in Table 4.

Table 4.
PFO closure procedure characteristics.
3.4. Cerebrovascular Event Characteristics after PFO Closure
All patients used antithrombotic therapy with Acidum Acetylsalicylicum 100 mg once a day for 6 months after PFO closure procedure. Secondary stroke prevention with antiplatelet agents (aspirin) after six months is still being used in 55.6% (n = 55) of patients, oral anticoagulants (dabigatran, rivaroxaban)—6.1% (n = 6). Antithrombotic therapy after the first 6 months was stopped for 59.6% (n = 59) of patients.
After PFO closure patients were followed for up to 5 years. Residual shunts after 30 days were observed in 2% (n = 2) of patients (from 99 patients included), after 1 year in 7.1% (n = 7) (from 99 patients included), and after 2 years in 2.3% (n = 2) (from 86 patients included). In total 16.2% (n = 16) had residual shunt after PFO closure. Frequency of residual shunt after PFO closure are presented in Figure 1.
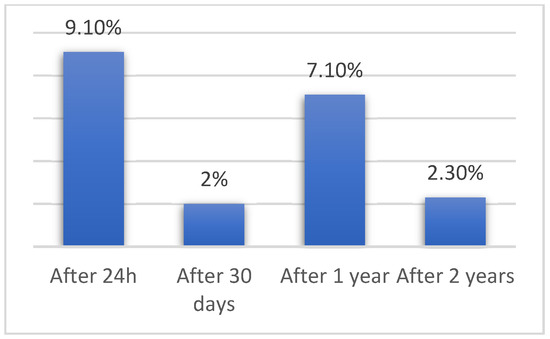
Figure 1.
Frequency of residual shunt after PFO closure.
TIA in 5-year time after PFO closure was observed in only 3% (n = 3) of patients. One patient (1%) had TIA 1 year after PFO closure (from 99 patients included), 1.2% (n = 1) 2 years after (from 86 patients included) and 1.8% (n = 1) 4 years after PFO closure. One patient had a recurrent TIA after PFO closure and 2 patients had TIA after PFO closure, without having had any before (p = 0.393). Number of patients having TIA before and after PFO closure are presented in Figure 2.
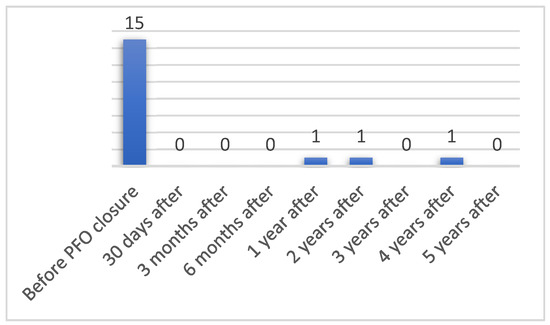
Figure 2.
Number of patients having TIA before and after PFO closure.
CI in 5-year time after PFO closure was observed in only 3% (n = 3) of patients (Figure 3). Two patients (2%) had CS 6 months after PFO closure (from 99 patients included) and 1.4% (n = 1) 3 year after (from 69 patients included). No patients who did not have CI before PFO closure had CI after; however, 3 patients had recurrent CI after PFO closure (p = 0.586). Before PFO closure 92.2% (n = 95) of patients had at least one cerebrovascular event and in five-year time after PFO closure recurrent cerebrovascular events were reported in only 5.1% (n = 5) of patients, this difference is statistically relevant (p < 0.001).
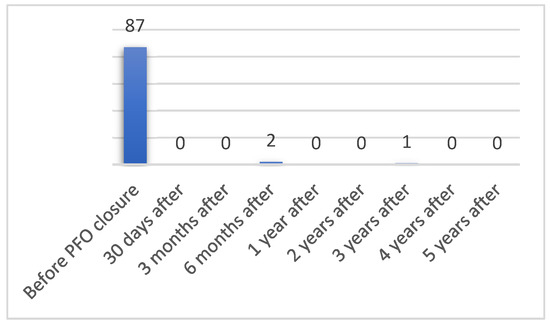
Figure 3.
Number of patients having CI before and after PFO closure.
There was no correlation between residual shunt after PFO closure and increased risk of stroke recurrence in 2 years’ time. None of 16 patients who had a residual shunt after PFO closure experienced recurrent stroke (p = 0.596). However, there was correlation between residual shunt after PFO closure and increased risk of TIA recurrence in 2 years’ time: of 16 patients who had residual shunt after PFO closure, 2 had recurrent TIA (p = 0.067).
3.5. Patient Complaints
Fifty-one patients (51.5%) had complaints before PFO closure (headaches, fatigue, dizziness, visual impairment, and heart palpitations)—some of these complaints were associated with previous CI, but majority of them were independent. Thirty-eight patients (38.4%) complained of headaches (including migraines and chronic headaches), 6.1% (n = 6) complained of fatigue, 18.2% (n = 18) complained of dizziness, 7.1% (n = 7) of visual impairment and 7.1% (n = 7) of heart palpitations.
Four patients (4%) after 30 days experienced heart palpitations (from 99 patients included), after 1 year—6.1% (n = 6) (from 99 patients included), and after 2 years—2.3% (n = 2) (from 86 patients included). Seven patients complained of heart palpitations after PFO closure although they did not have these complaints before, and only for one of seven patient’s complaints of heart palpitation persisted after PFO closure, this difference is not statistically relevant (p = 0.390)
One patient (1%) after 30 days experienced fatigue (from 99 patients included), after 3 months–1% (n = 1) (from 99 patients included), and after 3 years—1% (n = 1) (from 69 patients included). There were no new complaints of heart palpitations observed after PFO closure, and only for two of seven patients’ complaints persisted after PFO closure, this difference is statistically relevant (p = 0.003).
Six patients (6.1%) after 30 days experienced dizziness (from 99 patients included), after 3 months—7.1% (n = 7) (from 99 patients included), after 6 months–8.1% (n = 8) (from 99 patients included), after 1 year–8.1% (n = 8) (from 99 patients included), after 2 years—8.1% (n = 7) (from 86 patients included), after 3 years—4.3% (n = 3) (from 69 patients included), after 4 years—5.4% (n = 3) (from 56 patients included) and after 5 years—4.7% (n = 2) (from 43 patients included). For half of the patients (n = 9) complaints of dizziness persisted after PFO closure, and for five people dizziness was a new complaint after PFO closure, this difference is statistically relevant (p < 0.001).
Two patients (2%) after 30 days experienced visual impairment (from 99 patients included), after 3 months—3% (n = 3) (from 99 patients included), after 6 months—3% (n = 3) (from 99 patients included), after 1 year—3% (n = 3) (from 99 patients included), after 2 years–2.3% (n = 2) (from 86 patients included), after 3 years—1.4% (n = 1) (from 69 patients included) and after 4 years—1.8% (n = 1) (from 56 patients included). There were no new complaints of visual impairment after PFO closure; however, for four patients’ complaints persisted after PFO closure, this difference is statistically relevant (p < 0.001).
Thirteen patients (13.1%) after 30 days experienced headaches (from 99 patients included), after 3 months—15.2% (n = 15) (from 99 patients included), after 6 months—17.2% (n = 17) (from 99 patients included), after 1 year—17.2% (n = 17) (from 99 patients included), after 2 years—18.6 % (n = 16) (from 86 patients included), after 3 years—13% (n = 9) (from 69 patients included), after 4 years—17.9% (n = 10) (from 56 patients included) and after 5 years–18.6% (n = 8) (from 43 patients included). There were two patients with new complaints of headaches after PFO closure, and for only half of the patients (n = 19) complaints of headaches persisted after PFO closure procedure, this difference is statistically relevant (p < 0.001).
Out of 51 patients presented with complaints before PFO closure (headaches, fatigue, heart palpitations, dizziness, visual impairment), 25.5% (n = 13) did not present with any complaints after PFO closure, this difference is statistically relevant (p = 0.017). All patients with headaches and migraines noted that after PFO closure the symptoms were much less frequent and painful.
4. Discussion
Despite this study being with a small number of patients, it demonstrates that PFO percutaneous closure with occluders is safe and effective, with the success rate of 96.1%. From 99 patients included in this study, there were no deaths and no intrahospital complications.
The most used implantation device was Amplatzer occluder (58.6%), the Amplatzer occlude and Gore Cardioform Septal Occluder (not used in our research) are the only devices that has FDA approval for PFO closure in the context of cryptogenic stroke [9]. Residual shunt after PFO closure was observed in 16.2% (n = 16) of patients. In comparison with literature, residual shunts may be observed in up to 25%, with unclear clinical significance [10]. With the Amplatzer occluder 87.5% of residual shunts were observed directly; however, for patients with Cardia Ultrasept occluder residual shunts were observed in 1% of patients. Therefore, Cardia Ultrasept occluder could be considered more efficient than Amplatzer occluder. Some research does suggest that the presence of residual shunt is associated with an increased risk for stroke or TIA recurrence [11]. In our research there was no correlation between residual shunt and increased risk of stroke recurrence; however, there was a correlation between residual shunt and increased risk of TIA recurrence (p = 0.067).
All patients used antithrombotic therapy for 6 months after PFO closure procedure, which is the recommended duration according to local guidelines in Pauls Stradiņš Clinical University Hospital. According to the latest European Society of Cardiology guidelines it is still up for debate whether to use single antiplatelet therapy for at least 5 years after PFO closure or even indefinitely. It is even reasonable to purpose dual antiplatelet therapy for 1 to 6 months after PFO closure [12]. Forty patients (40.4%) continued using antithrombotic therapy after the first 6 months from PFO closure, because they had additional risk factors for recurrent cerebrovascular events.
The prevalence of interatrial septal aneurysms (ASA) was 16.5%. For patients with PFO-associated stroke, ASA is an important predictor of recurrent stroke [13]. In 5 years’ time, only 1 patient with ASA had recurrent TIA.
According to CCS for 66.7% (n = 58) of patients the pathogenic subtype of CI was possible cardio-aortic embolism and for 1.1% (n = 1)—evident cardio-aortic embolism. Patients with arterial fibrillation should be excluded as that has been an identified as the cause of cryptogenic stroke. Therefore, the one patient with evident cardio-aortic embolism, and three patients with possible cardio-aortic embolism were excluded. For 53.4% (n = 55) of patients with possible cardio-aortic embolism the most possible cause for cryptogenic stroke is PFO.
Before PFO closure 92.2% (n = 95) of patients had at least one cerebrovascular event and in five-year time after PFO closure recurrent cerebrovascular events were reported in only 5.1% (n = 5) of patients (p < 0.001). We analyzed each of recurrent cerebrovascular event patients individually—one patient with recurrent TIA had been diagnosed with thrombophilia, three patients with recurrent TIA had a residual shunt, one patient with CI 4 years after PFO closure had other etiological factors for recurrent cerebrovascular event. This clearly shows that PFO closure procedure is effective in reducing recurrent cerebrovascular events. After PFO closure any patients is still at risk of developing recurrent cerebrovascular events, because there are many etiological factors that may contribute to this. As stated previously there is a correlation between residual shunt and increased risk of TIA recurrence, which can be seen in three patients having TIA after PFO closure due to residual shunts.
Out of 51 patients presented with complaints before PFO closure (headaches, fatigue, heart palpitations, dizziness, and visual impairment), 25.5% (n = 13) did not present with any complaints after PFO closure, this difference is statistically relevant (p = 0.017). There are many contributing factors that could play a role in patient’s complaints; therefore, it is hard to say whether these changes are only because of PFO closure, however it can be assumed that PFO closure can be associated with improvement of complaints. A lot of patients with headaches and migraines noted that after PFO closure the symptoms were much less frequent and less painful.
5. Conclusions
PFO can be considered a possible risk factor for cryptogenic stroke. PFO closure is effective in reducing recurrent cerebrovascular events. Residual shunt after PFO closure increases the risk of transient ischemic attack recurrence. Amplatzer occluder device is associated with a higher risk for residual shunts after PFO closure. PFO closure can be associated with improvement of complaints (headaches, fatigue, heart palpitations, dizziness, visual impairment).
Author Contributions
Conceptualization, K.J. and K.L.; methodology, K.J., K.L., and A.R.; formal analysis, K.J., K.L., A.T., and A.B.; investigation, K.L., A.T., and A.B.; resources, K.J., K.L., and A.R..; data curation, K.L. and A.T.; writing—original draft preparation, A.T.; writing—review and editing, K.J.; visualization, K.J., K.L., and A.T.; supervision, K.J. and E.M.; project administration, K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Riga Stradins University (RSU) Research Ethics Committee (protocol code 22-2/310/2021 and approval on 23 April 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We are grateful to Pauls Stradins Clinical University Hospital departments of Neurology and Cardiology staff for stroke and PFO patient care.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calvert, P.A.; Rana, B.S.; Kydd, A.C.; Shapiro, L.M. Patent foramen ovale: Anatomy, outcomes, and closure. Nat. Rev. Cardiol. 2011, 8, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Giblett, J.P.; Williams, L.K.; Kyranis, S.; Shapiro, L.M.; Calvert, P.A. Patent Foramen Ovale Closure: State of the Art. Interv. Cardiol. 2020, 15, e15. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Diener, H.C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J.; Group, C.S.E.I.W. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol 2014, 13, 429–438. [Google Scholar] [CrossRef]
- Martín Mañero, C.; Medina Durán, P.; Morales Delgado, N.; Martín Rioboó, E. Patent foramen ovale. An update for primary care. Semergen 2021, 47, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Messé, S.R.; Gronseth, G.S.; Kent, D.M.; Kizer, J.R.; Homma, S.; Rosterman, L.; Carroll, J.D.; Ishida, K.; Sangha, N.; Kasner, S.E. Practice advisory update summary: Patent foramen ovale and secondary stroke prevention: Report of the Guideline Subcommittee of the American Academy of Neurology. Neurology 2020, 94, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Parra, O.; AliÓ, J. Patent foramen ovale closure in non-lacunar cryptogenic ischemic stroke: Where are we now? J. Geriatr. Cardiol. 2021, 18, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Elzanaty, A.M.; Patel, N.; Sabbagh, E.; Eltahawy, E.A. Patent foramen ovale closure in the management of cryptogenic stroke: A review of current literature and guideline statements. Curr. Med. Res. Opin. 2021, 37, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Arsava, E.M.; Ballabio, E.; Benner, T.; Cole, J.W.; Delgado-Martinez, M.P.; Dichgans, M.; Fazekas, F.; Furie, K.L.; Illoh, K.; Jood, K.; et al. The Causative Classification of Stroke system: An international reliability and optimization study. Neurology 2010, 75, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Collado, F.M.S.; Poulin, M.F.; Murphy, J.J.; Jneid, H.; Kavinsky, C.J. Patent Foramen Ovale Closure for Stroke Prevention and Other Disorders. J. Am. Heart Assoc. 2018, 7, e007146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas, J.L.; Derumeaux, G.; Guillon, B.; Massardier, E.; Hosseini, H.; Mechtouff, L.; Arquizan, C.; Béjot, Y.; Vuillier, F.; Detante, O.; et al. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N. Engl. J. Med. 2017, 377, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; Aggarwal, S.; Cohen, M. Residual Shunt After Patent Foramen Ovale Closure and Long-Term Stroke Recurrence. Ann. Intern Med. 2020, 173, 945. [Google Scholar] [CrossRef] [PubMed]
- Pristipino, C.; Sievert, H.; D’Ascenzo, F.; Louis Mas, J.; Meier, B.; Scacciatella, P.; Hildick-Smith, D.; Gaita, F.; Toni, D.; Kyrle, P.; et al. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur. Heart J. 2019, 40, 3182–3195. [Google Scholar] [CrossRef] [PubMed]
- Turc, G.; Lee, J.Y.; Brochet, E.; Kim, J.S.; Song, J.K.; Mas, J.L.; Investigators, C.a.D.-P.T. Atrial Septal Aneurysm, Shunt Size, and Recurrent Stroke Risk in Patients With Patent Foramen Ovale. J. Am. Coll. Cardiol. 2020, 75, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).