1. Introduction
Fentanyl, a synthetic opioid approximately 50–100 times more potent than morphine, has become a cornerstone in the management of severe pain, particularly in oncological settings and perioperative care [
1]. Despite its therapeutic utility, fentanyl’s pharmacokinetic profile—characterized by high lipid solubility, rapid central nervous system penetration, and potent μ-opioid receptor agonism—renders it especially susceptible to misuse, dependence, and fatal overdose [
2].
Globally, the surge in fentanyl-related morbidity and mortality has prompted urgent policy and surveillance responses, particularly in North America, where the non-medical use of illicitly manufactured fentanyl has driven a public health crisis [
3,
4]. In contrast, until recently, in countries like Portugal, fentanyl was predominantly accessed through legal prescriptions for legitimate medical purposes. Nonetheless, signs of rising consumption and concern over abuse potential have emerged [
5]. Illicit production and use, the diversion and misuse of medicines, and the online sale of non-controlled new psychoactive substances is a reality in Europe [
6].
The pharmacological and physicochemical properties of fentanyl dosage forms strongly influence their potential for misuse. Immediate-release products offer rapid onset and are often associated with higher abuse liability, while extended-release systems, although intended for steady delivery, may still be tampered with in order to extract the drug [
7]. To address these concerns, the development of abuse-deterrent formulations (ADFs) has gained traction—particularly in the United States—as a harm-reduction strategy. Despite progress in this area, ADFs remain underutilized or unavailable in many European markets [
8]. These technologies incorporate various deterrent mechanisms, including prodrugs, aversive agents, opioid antagonists, or physical and chemical barriers that inhibit common manipulation methods such as crushing, dissolving, or snorting [
7].
This study aims to characterize the pharmaceutical landscape of fentanyl in Portugal, with particular attention being paid to dosage form distribution, consumption trends, and formulation-related abuse potential. By identifying current gaps in abuse deterrence, this research seeks to inform future strategies for safer opioid prescribing, surveillance, and pharmaceutical innovation in the Portuguese context.
3. Results and Discussion
This retrospective observational study aimed to characterize fentanyl consumption in Portugal over an eight-year period (2015–2022). The analysis included all legally dispensed analgesic opioid medicines used in ambulatory settings (
Figure 1).
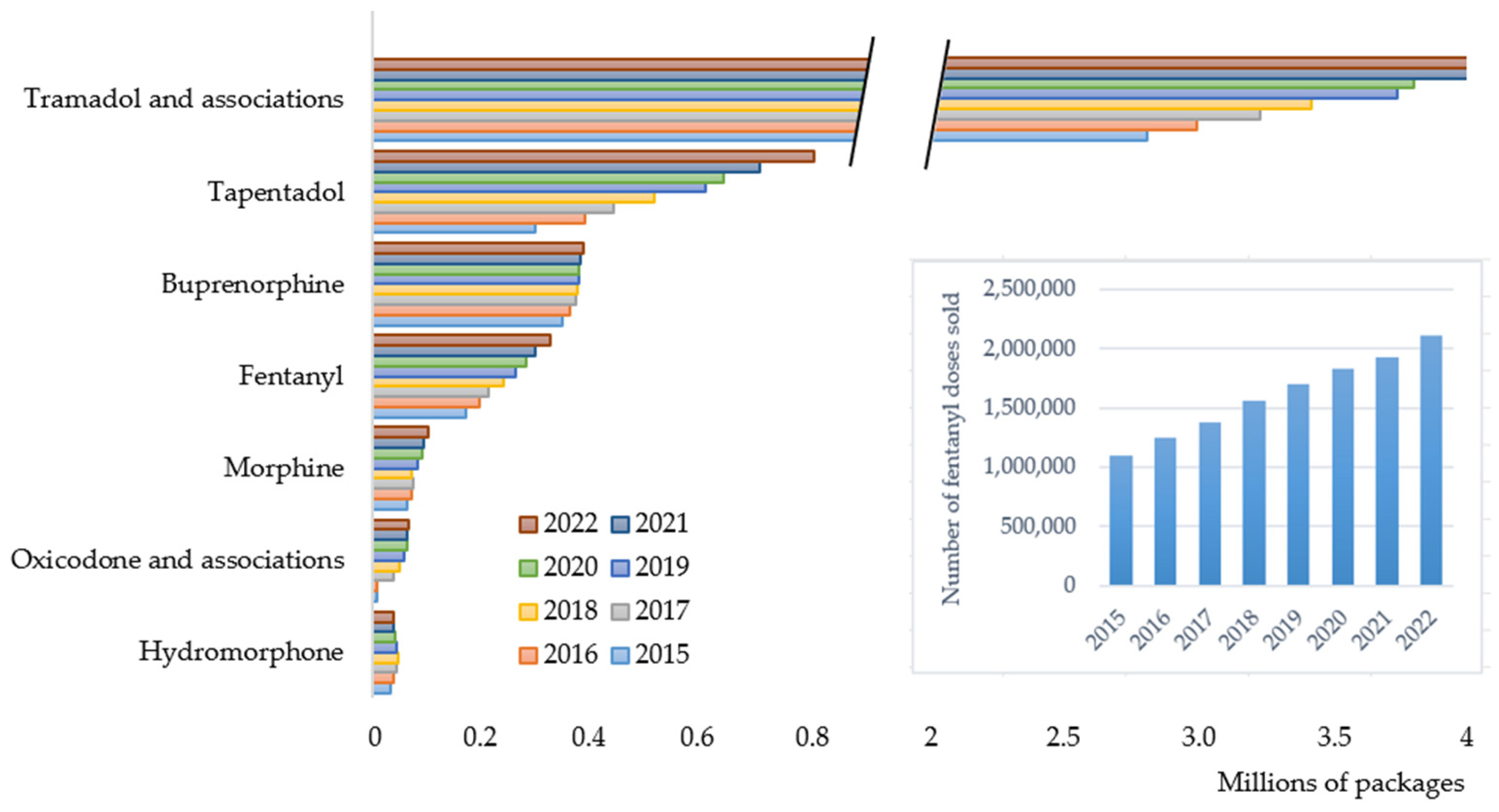
Fentanyl ranked as the fourth most consumed opioid in Portugal, following tramadol (and its fixed-dose combinations), tapentadol, and buprenorphine. Over the study period, fentanyl consumption nearly doubled, increasing by approximately 90% both in terms of packages and doses sold (
Figure 1 and insert). A linear upward trend was observed, with an average annual increase of approximately 22,468 packages/year. The consistent annual growth in fentanyl use emphasizes its increasing role in pain management, despite the availability of alternative analgesics. Remarkably, the rate of increase in fentanyl use outpaced that of traditional strong opioids such as morphine and hydromorphone, suggesting a preferential shift toward fentanyl formulations, possibly due to its high potency, availability of various non-invasive administration routes, and extended-release dosage forms.
A gradual increase in the generic-to-brand ratio was observed both in terms of the number of packages sold and total doses consumed, indicating a growing penetration of generic fentanyl products in the Portuguese ambulatory market. This trend aligns with national and European policies promoting generic use as a cost-containment measure.
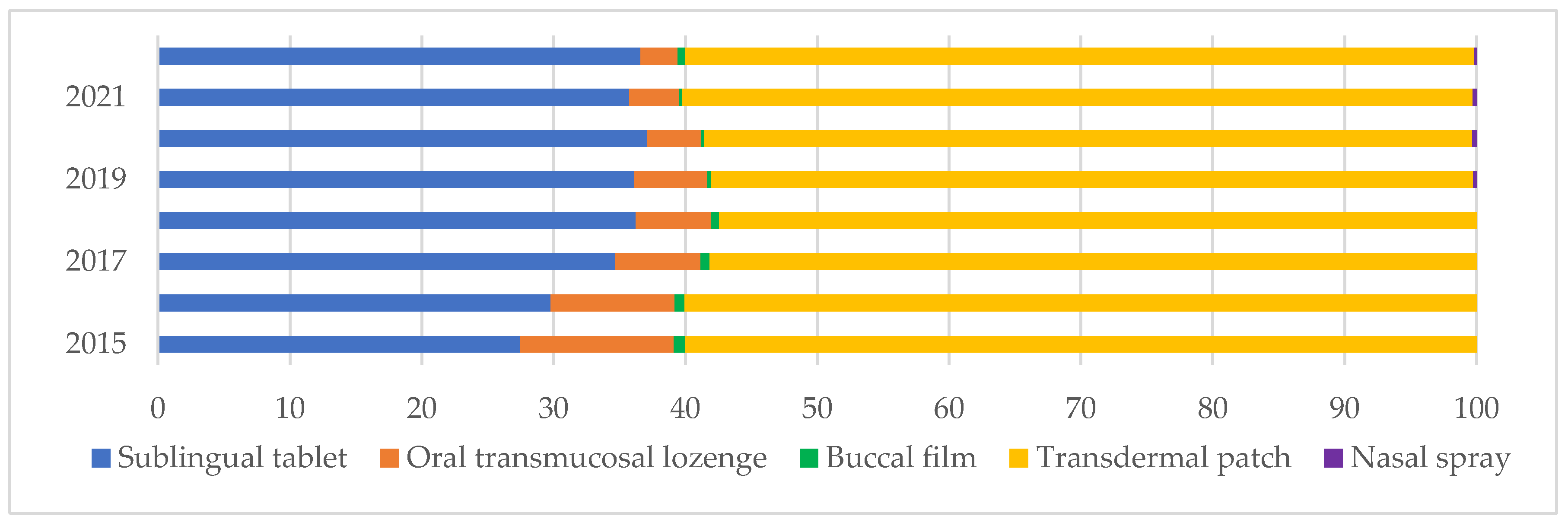
Figure 2 illustrates the share of fentanyl dosage forms sold (in number of doses), emphasizing the predominance of transdermal patches and sublingual tablets, which together consistently account for most of the total fentanyl use throughout the study period. Constant transdermal consumption (58–60%) reflects its established role in chronic pain management, particularly in oncology and palliative care. Sublingual tablets show a notable upward trend, increasing from 27.45% in 2015 to 36.58% in 2022. On the other hand, oral transmucosal lozenges showed a significant decrease from 11.66% in 2015 to 2.81% in 2022. This downward trend might reflect reduced prescribing due to concerns over misuse or risk to children, as they look like lollipops. Buccal films and nasal sprays (introduced in 2019) represent marginal shares of overall consumption, respectively, 0.56 and 0.20% in 2022. This is attributable to factors such as limited prescriber familiarity, higher costs, recent market entry, specialized therapeutic indications, and restricted access. Notably, the three least consumed are exclusively available as brand-name medicines.
Dosage forms that are rapid-acting, highly potent, and approved for outpatient use present an increased risk of non-medical use, diversion, and accidental exposure, particularly within household settings. Therefore, identifying the key factors potentially contributing to the risk of misuse and abuse is essential.
Table 1 presents a comparative overview of fentanyl dosage forms authorized in Portugal, summarizing their pharmacokinetic profiles, route of administration, and associated abuse deterrence and potential.
Currently, fentanyl ADFs are unavailable in Portugal. Among the existing options, transdermal patches—despite containing high total amounts of fentanyl (up to 16.8 mg per unit)—exhibit a slow onset and prolonged duration of action, which, in combination with partial tamper-resistance in matrix-based systems, contributes to a comparatively lower risk of misuse under normal therapeutic conditions. While not formally classified as ADFs, matrix patches offer some deterrence, since drug extraction is significantly more difficult than using reservoir-type systems. However, methods of tampering (drug extraction and intravenous or inhalation use) and misuse after disposal (oral, transmucosal, rectal use of whole patch) have been reported [
11]. These risks underline the need for improved safeguards in fentanyl delivery systems to reduce diversion and non-medical use.
In contrast, rapid-onset formulations—such as sublingual tablets, buccal films, oromucosal lozenges, and nasal sprays—have fast systemic absorption and short durations, which are characteristics that increase their appeal for non-medical use. These formulations lack abuse-deterrent features and are often prescribed in outpatient settings, intensifying the risk. Injectable forms, although restricted to hospital use, have immediate bioavailability and high potency. While their accessibility is limited, they present a significant risk if diverted due to the high potential for overdose.
Overall, the formulation characteristics—particularly route of administration, onset of action, and presence or absence of abuse-deterrent features—play a critical role in determining the misuse liability of fentanyl products. These insights reinforce the importance of monitoring not only total consumption, but also the distribution by dosage form, identifying those incorporating strategies to mitigate opioid-related harms.
Although limited due to the lack of data on hospital use and the inability to directly distinguish misuse from legitimate prescribing, the growing outpatient use of fentanyl is noted. This aspect, in the absence of ADFs, reveals a critical gap in Portugal’s pharmaceutical and regulatory strategies, which may facilitate non-medical use or unintentional misuse. To address this, several targeted measures should be considered. First, the inclusion of fentanyl ADFs in national formularies to promote safer prescribing. Improved surveillance systems that integrate prescription data with real-world usage are also essential for detecting emerging misuse patterns. Education programs for healthcare professionals and patients should emphasize safe storage, proper disposal, and early identification of misuse. Also, regulatory bodies should encourage or mandate pharmaceutical manufacturers to adopt abuse-deterrent packaging and delivery technologies.
Fentanyl use also poses broader risks across the One Health spectrum. Improper disposal of fentanyl products may result in accidental exposure to animals and environmental contamination, while veterinary use—although limited—still presents diversion risks. Coordinated action across sectors is vital: prescription monitoring, environmental surveillance, and secure veterinary practices must work in concert. Cross-sectoral collaboration is essential to mitigate these interconnected risks and ensure the sustainable and responsible use of fentanyl.