Epidemiological Study of the Antimicrobial Resistance Pattern of a Suspected Urinary Tract Infection in a Super Surgical, Super Specialty Hospital in Northern India †
Abstract
:1. Background
2. Methods
2.1. Study Population
2.2. Sample Collection
2.3. Sample Processing
2.4. Identification and Sensitivity
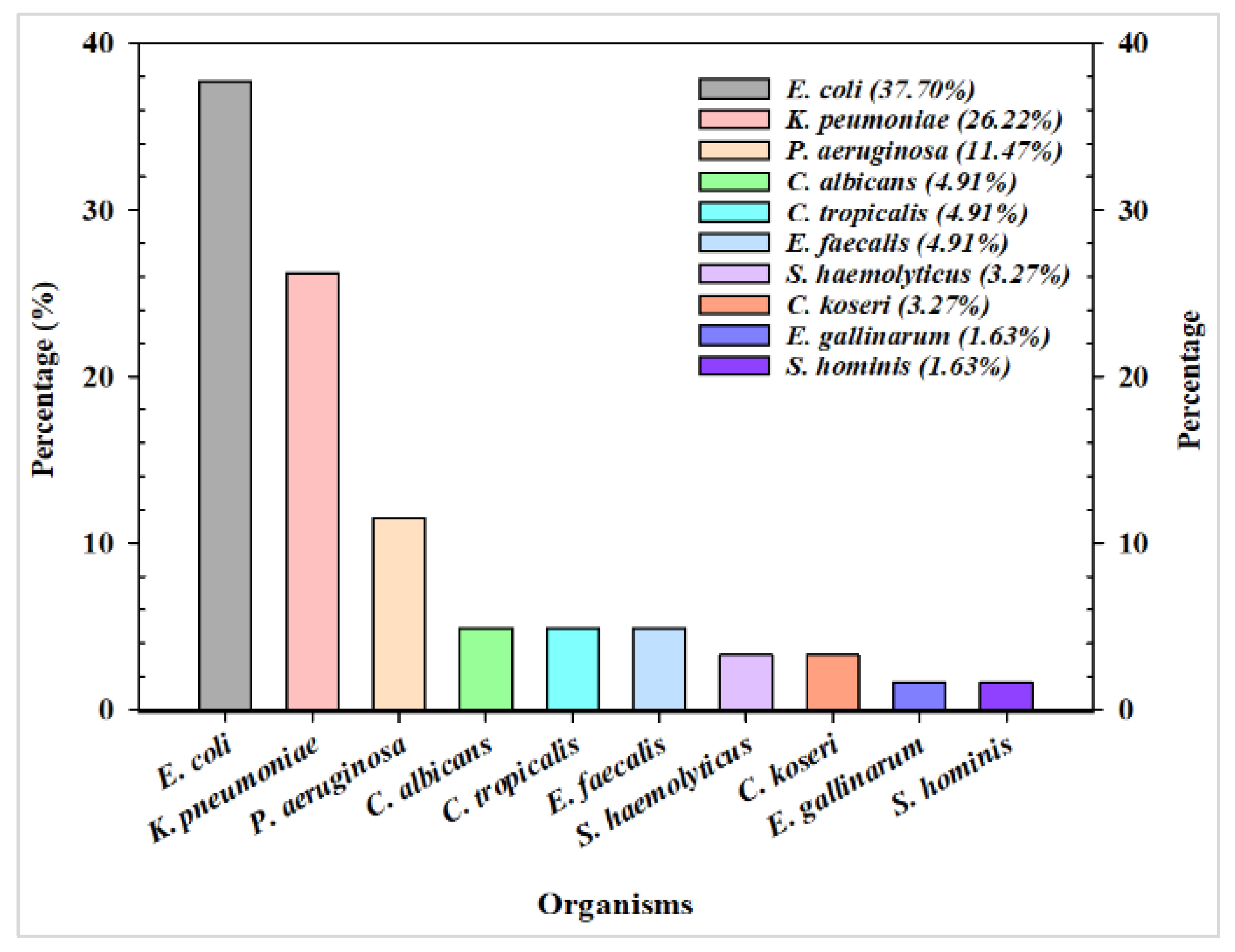
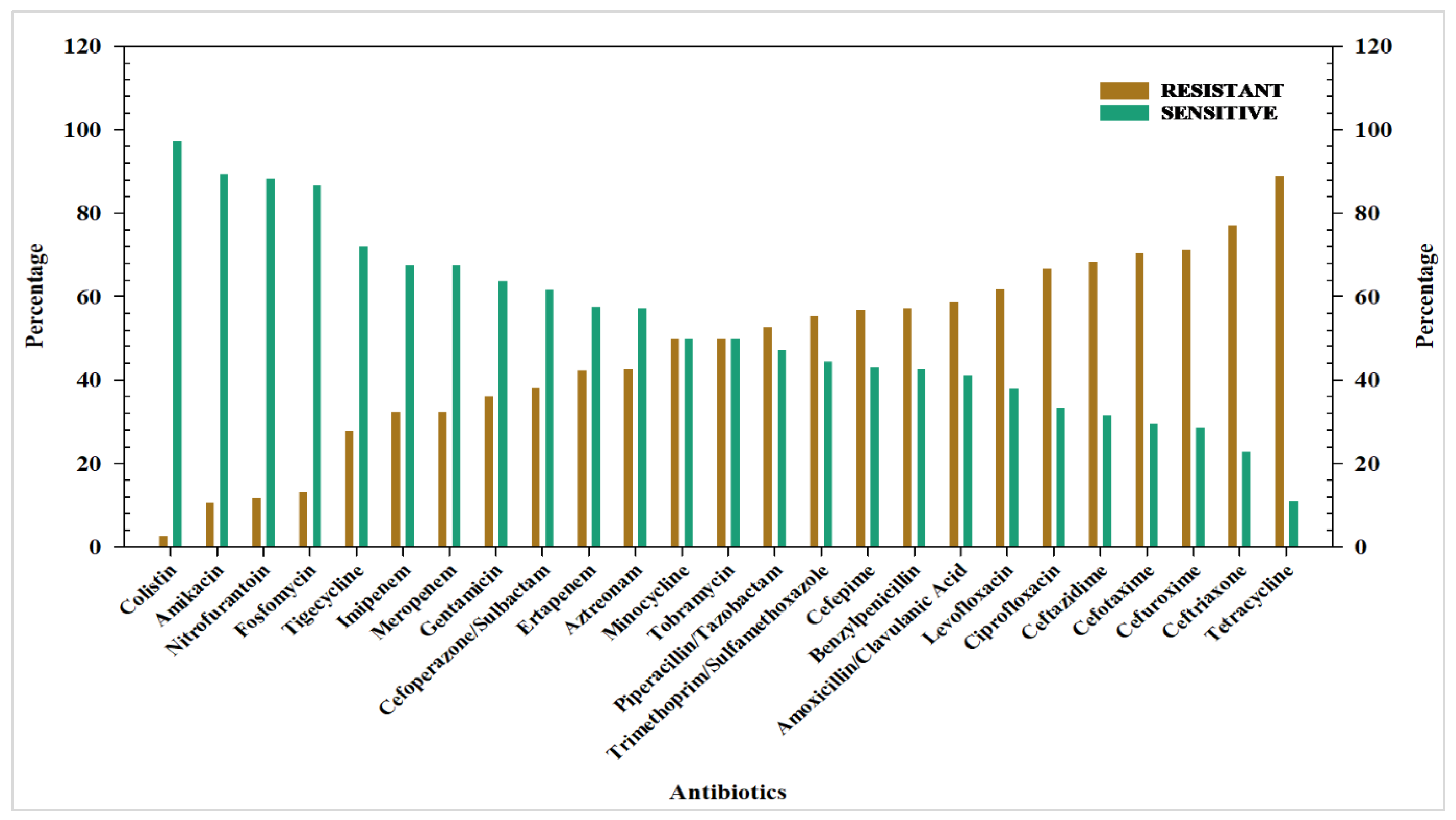
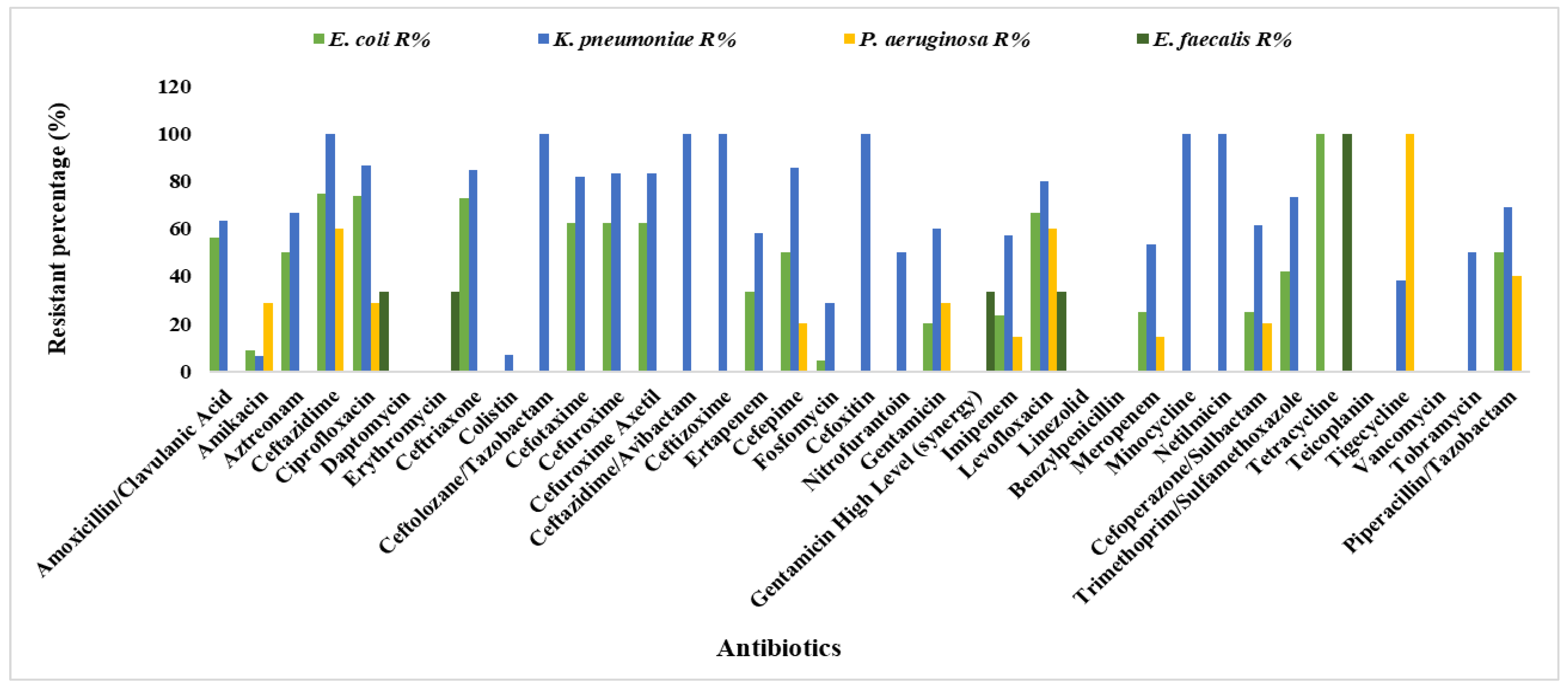
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez, C.M.; Schaeffer, A.J. Treatment of urinary tract infection: What’s old, what’s new, and what works. World J. Urol. 1999, 17, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, S.; Han, L.; Guo, X.; Chen, M.; Ni, Y.; Zhang, Y.; Cui, Z.; He, P. Drug resistance and virulence of uropathogenic Escherichia coli from Shanghai, China. J. Antibiot. 2014, 67, 799–805. [Google Scholar] [CrossRef]
- Schito, G.C.; Naber, K.G.; Botto, H.; Palou, J.; Mazzei, T.; Gualco, L.; Marchese, A. The ARESC study: An international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int. J. Antimicrob. Agents 2009, 34, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Al-Naqshbandi, A.A.; Chawsheen, M.A.; Abdulqader, H.H. Prevalence and antimicrobial susceptibility of bacterial pathogens isolated from urine specimens received in rizgary hospital—Erbil. J. Infect. Public Health 2018, 12, 330–336. [Google Scholar] [CrossRef]
- Faraz, M.A.A.; Mendem, S.; Swamy, M.V.; Patil, S. Prevalence of urinary tract infections and related antimicrobial resistance in india: A systematic review and meta-analysis. Int. J. Pharm. Sci. Res. 2021, 12, 4314–4321. [Google Scholar] [CrossRef]
- Shaifali, I.; Gupta, U.; Mahmood, S.E.; Ahmed, J. Antibiotic susceptibility patterns of urinary pathogens in female outpa-tients. N. Am. J. Med. Sci. 2012, 4, 163. [Google Scholar]
- Medina-Bombardó, D.; Seguí-Díaz, M.; Roca-Fusalba, C.; Llobera, J. What is the predictive value of urinary symptoms for diagnosing urinary tract infection in women? Fam. Pract. 2003, 20, 103–107. [Google Scholar] [CrossRef]
- Emiru, T.; Beyene, G.; Tsegaye, W.; Melaku, S. Associated risk factors of urinary tract infection among pregnant women at Felege Hiwot Referral Hospital, Bahir Dar, North West Ethiopia. BMC Res. Notes 2013, 6, 292–296. [Google Scholar] [CrossRef]
- Parker, C.; Muston, D.; Melia, J.; Moss, S.; Dearnaley, D. A model of the natural history of screen-detected prostate cancer, and the effect of radical treatment on overall survival. Br. J. Cancer 2006, 94, 1361–1368. [Google Scholar] [CrossRef]
- Tan, C.W.; Chlebicki, M.P. Urinary tract infections in adults. Singap. Med. J. 2016, 57, 485. [Google Scholar] [CrossRef]
- Mitiku, E.; Amsalu, A.; Tadesse, B.T. Pediatric urinary tract infection as a cause of outpatient clinic visits in southern Ethiopia: A cross sectional study. Ethiop. J. Health Sci. 2018, 28, 187–196. [Google Scholar] [CrossRef]
- Smelov, V.; Naber, K.; Johansen, T.E.B. Improved Classification of Urinary Tract Infection: Future Considerations. Eur. Urol. Suppl. 2016, 15, 71–80. [Google Scholar] [CrossRef]
- Freedman, A.L.; Urologic Diseases in America Project. Urologic diseases in north america project: Trends in resource utilizationo for urinary tract infections in children. J. Urol. 2005, 173, 949–954. [Google Scholar] [CrossRef]
- Patel, H.; Soni, S.; Bhagyalaxmi, A.; Patel, N. Causative agents of urinary tract infections and their antimicrobial susceptibility patterns at a referral center in Western India: An audit to help clinicians prevent antibiotic misuse. J. Fam. Med. Prim. Care 2019, 8, 154. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Mcgoldrick, M. Urine Specimen Collection and Transport. Home Health Now. 2015, 33, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Saxena, R.S. Distribution and Antimicrobial Susceptibility Pattern of Bacterial Pathogens Causing Urinary Tract Infection in Urban Community of Meerut City, India. ISRN Microbiol. 2013, 2013, 749629. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Alnour, T.M.S.; Shakurfo, O.M.; Aburass, M.M. Prevalence and antimicrobial resistance pattern of bacterial strains isolated from patients with urinary tract infection in Messalata Central Hospital, Libya. Asian Pac. J. Trop. Med. 2016, 9, 771–776. [Google Scholar] [CrossRef]
- Chooramani, G.; Jain, B.; Chauhan, P.S. Prevalence and antimicrobial sensitivity pattern of bacteria causing urinary tract infection; study of a tertiary care hospital in North India. Clin. Epidemiol. Glob. Health 2020, 8, 890–893. [Google Scholar] [CrossRef]
- Manandhar, R.; Raghubanshi, B.R.; Mahato, M.; Neupane, S.; Lama, R. Bacteriological Profile and Antimicrobial Susceptibility Patterns of Urine Culture Isolates from Patients in a Tertiary Care Centre in Lalitpur. Birat J. Health Sci. 2020, 5, 881–885. [Google Scholar] [CrossRef]
- Adugna, B.; Sharew, B.; Jemal, M. Bacterial Profile, Antimicrobial Susceptibility Pattern, and Associated Factors of Community- and Hospital-Acquired Urinary Tract Infection at Dessie Referral Hospital, Dessie, Northeast Ethiopia. Int. J. Microbiol. 2021, 2021, 5553356. [Google Scholar] [CrossRef] [PubMed]
- Kengne, M.; Dounia, A.T.; Nwobegahay, J.M. Bacteriological profile and antimicrobial susceptibility patterns of urine culture isolates from patients in Ndjamena, Chad. Pan Afr. Med. J. 2017, 28, 258. [Google Scholar] [CrossRef] [PubMed]
- Sundvall, P.D.; Elm, M.; Gunnarsson, R.; Mölstad, S.; Rodhe, N.; Jonsson, L.; Ulleryd, P. Antimicrobial resistance in urinary pathogens among Swedish nursing home residents remains low: A cross-sectional study comparing antimicrobial resistance from 2003 to 2012. BMC Geriatr. 2014, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Mandal, S.; Georgalas, A.; Gilani, S.A.D. A Pattern of Antibiotic Resistance in Gram-Negative Rods Causing Urinary Tract Infection in Adults. Cureus 2021, 13, e12977. [Google Scholar] [CrossRef] [PubMed]
- Seifu, W.D.; Gebissa, A.D. Prevalence and antibiotic susceptibility of Uropathogens from cases of urinary tract infections (UTI) in Shashemene referral hospital, Ethiopia. BMC Infect. Dis. 2018, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Odoki, M.; Aliero, A.A.; Tibyangye, J.; Maniga, J.N.; Wampande, E.; Kato, C.D.; Agwu, E.; Bazira, J. Prevalence of Bacterial Urinary Tract Infections and Associated Factors among Patients Attending Hospitals in Bushenyi District, Uganda. Int. J. Microbiol. 2019, 2019, 4246780. [Google Scholar] [CrossRef]
- Sultana, M.; Toaha, S.M. Study of uropathogens in patients with Urinary Tract Infection (UTI) and their antibiotic sensitivity profile. Aust. J. Sci. Technol. 2021, 5, 403–409. Available online: https://www.aujst.com/vol-5-1/1.pdf (accessed on 30 March 2021).
- Bitew, A.; Molalign, T.; Chanie, M. Species distribution and antibiotic susceptibility profile of bacterial uropathogens among patients complaining urinary tract infections. BMC Infect. Dis. 2017, 17, 654. [Google Scholar] [CrossRef]
- Das, S.K.; Baral, P.; Jain, S.; Panigrahy, R. Childhood urinary tract infection: Prevalence and resistance pattern of uropathogens in a tertiary care hospital. Int. J. Curr. Res. Rev. 2021, 13, 59. [Google Scholar] [CrossRef]
- Farjana, N.E.; Islam, A.; Zerin, T.; Begum, M.A. Bacterial association in urinary tract infection and their drug resistance among patients in Rajshahi, Bangladesh. Int. J. Community Med. Public Health 2021, 8, 2144–2149. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113, 5S–13S. [Google Scholar] [CrossRef]
- Dielubanza, E.J.; Schaeffer, A.J. Urinary tract infections in women. Med. Clin. N. Am. 2011, 95, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Tandan, M.; Sloane, P.D.; Ward, K.; Weber, D.J.; Vellinga, A.; Kistler, C.E.; Zimmerman, S. Antimicrobial resistance patterns of urine cul-ture specimens from 27 nursing homes: Impact of a two-year antimicrobial stewardship intervention. Infect. Control Hosp. Epidemiol. 2019, 40, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Anto, N.; Bhardwaj, M.; Prendiville, A.; Elangovan, R.; Bachmann, T.T.; Chanda, D.D.; Bhattacharjee, A. Antimicrobial resistance in patients with suspected urinary tract infections in primary care in Assam, India. JAC-Antimicrob. Resist. 2021, 3, dlab164. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.; Wei, X.; Hann, K.; Soe, K.T.; Satyanarayana, S.; Siwakoti, B.; Bastakoti, S.; Mulmi, R.; Rana, K.; Lamichhane, N. Bacterial Profile and Antibiotic Resistance among Cancer Patients with Urinary Tract Infection in a National Tertiary Cancer Hospital of Nepal. Trop. Med. Infect. Dis. 2021, 6, 49. [Google Scholar] [CrossRef]
- Sari, E.; Yazılıtaş, F.; Oztek, F.; Akcaboy, M.; Akisoglu, O.; Senel, S. Antibiotic drug resistance pattern of uropathogens seen in the first episode of community-acquired pediatric urinary tract infections at a tertiary care hospital. Turk. J. Pediatr. Dis. 2022, 16, 138–143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahirwar, N.; Singha, T.K.; Srivastava, M.; Pal, M. Epidemiological Study of the Antimicrobial Resistance Pattern of a Suspected Urinary Tract Infection in a Super Surgical, Super Specialty Hospital in Northern India. Med. Sci. Forum 2024, 24, 16. https://doi.org/10.3390/ECA2023-16468
Ahirwar N, Singha TK, Srivastava M, Pal M. Epidemiological Study of the Antimicrobial Resistance Pattern of a Suspected Urinary Tract Infection in a Super Surgical, Super Specialty Hospital in Northern India. Medical Sciences Forum. 2024; 24(1):16. https://doi.org/10.3390/ECA2023-16468
Chicago/Turabian StyleAhirwar, Narayan, Tapan Kumar Singha, Malvika Srivastava, and Manisha Pal. 2024. "Epidemiological Study of the Antimicrobial Resistance Pattern of a Suspected Urinary Tract Infection in a Super Surgical, Super Specialty Hospital in Northern India" Medical Sciences Forum 24, no. 1: 16. https://doi.org/10.3390/ECA2023-16468
APA StyleAhirwar, N., Singha, T. K., Srivastava, M., & Pal, M. (2024). Epidemiological Study of the Antimicrobial Resistance Pattern of a Suspected Urinary Tract Infection in a Super Surgical, Super Specialty Hospital in Northern India. Medical Sciences Forum, 24(1), 16. https://doi.org/10.3390/ECA2023-16468




