Protein Extraction from Arthrospira platensis for Use in Food Processing †
Abstract
1. Introduction
2. Materials and Methods
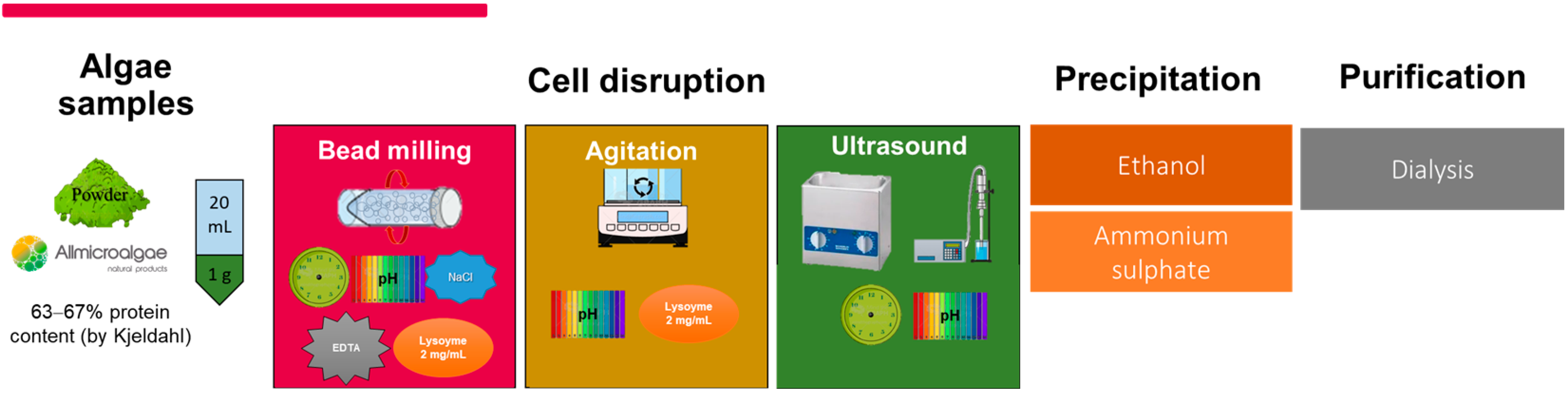
2.1. Sample and Protein Extraction
2.2. Amino Acid Quantification
2.3. Protein Characterization
2.4. Statistical Analysis
3. Results
3.1. Optimization of the Protein Extraction Method
3.2. Quantification of Amino Acid in Extracted Proteins Using the Optimized Method
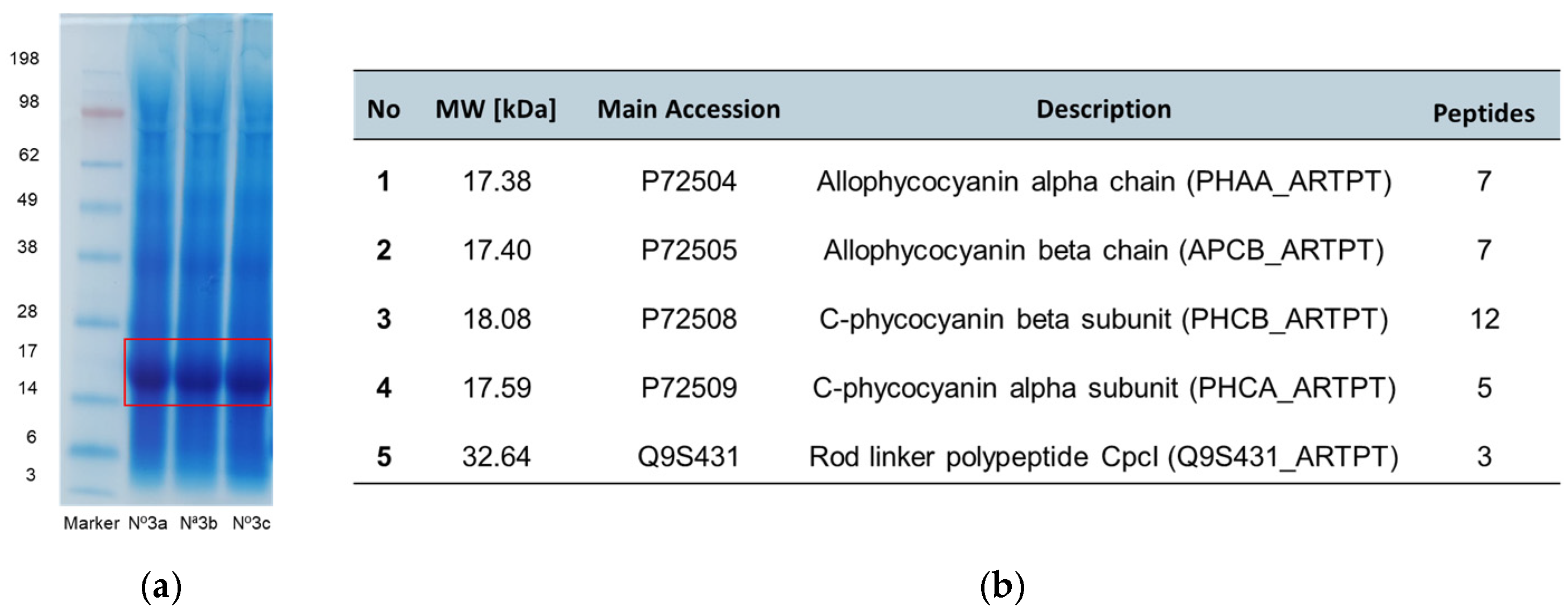
3.3. Protein Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pelizer, L.H.; De Carvalho, J.C.M.; De Oliveira Moraes, I. Protein Production by Arthrospira (Spirulina) Platensis in Solid State Cultivation Using Sugarcane Bagasse as Support. Biotechnol. Rep. 2015, 5, 70–76. [Google Scholar] [CrossRef][Green Version]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.-W.; Huang, C.-Y.; Chang, J.-S. Microalgae as Sustainable Food and Feed Sources for Animals and Humans—Biotechnological and Environmental Aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef] [PubMed]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of PH on the Functional Properties of Arthrospira (Spirulina) Platensis Protein Isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Balboa, E.M.; Parada, M.; Falqué, E. Algal Proteins, Peptides and Amino Acids. In Functional Ingredients from Algae for Foods and Neutracceuticls; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–180. [Google Scholar] [CrossRef]
- Teuling, E.; Wierenga, P.A.; Schrama, J.W.; Gruppen, H. Comparison of Protein Extracts from Various Unicellular Green Sources. J. Agric. Food Chem. 2017, 65, 7989–8002. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous Extraction of Proteins from Microalgae: Effect of Different Cell Disruption Methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef]
- Vernès, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of Ultrasound for Green Extraction of Proteins from Spirulina. Mechanism, Optimization, Modeling, and Industrial Prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Herbert, P.; Santos, L.; Alves, A. Simultaneous Quantification of Primary, Secondary Amino Acids, and Biogenic Amines in Musts and Wines Using OPA/3-MPA/FMOC-CI Fluorescent Derivatives. J. Food Sci. 2001, 66, 1319–1325. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-Gel Digestion for Mass Spectrometric Characterization of Proteins and Proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, E.; Ribeiro, M.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Protein Extraction from Arthrospira platensis for Use in Food Processing. Med. Sci. Forum 2023, 23, 8. https://doi.org/10.3390/msf2023023008
Costa E, Ribeiro M, Filipe-Ribeiro L, Cosme F, Nunes FM. Protein Extraction from Arthrospira platensis for Use in Food Processing. Medical Sciences Forum. 2023; 23(1):8. https://doi.org/10.3390/msf2023023008
Chicago/Turabian StyleCosta, Elisa, Miguel Ribeiro, Luís Filipe-Ribeiro, Fernanda Cosme, and Fernando M. Nunes. 2023. "Protein Extraction from Arthrospira platensis for Use in Food Processing" Medical Sciences Forum 23, no. 1: 8. https://doi.org/10.3390/msf2023023008
APA StyleCosta, E., Ribeiro, M., Filipe-Ribeiro, L., Cosme, F., & Nunes, F. M. (2023). Protein Extraction from Arthrospira platensis for Use in Food Processing. Medical Sciences Forum, 23(1), 8. https://doi.org/10.3390/msf2023023008








