Green Synthesis of Luminescent Carbon Nanomaterials from Porphyridium cruentum Microalgae †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of µAlgae-Carbon Nanodots
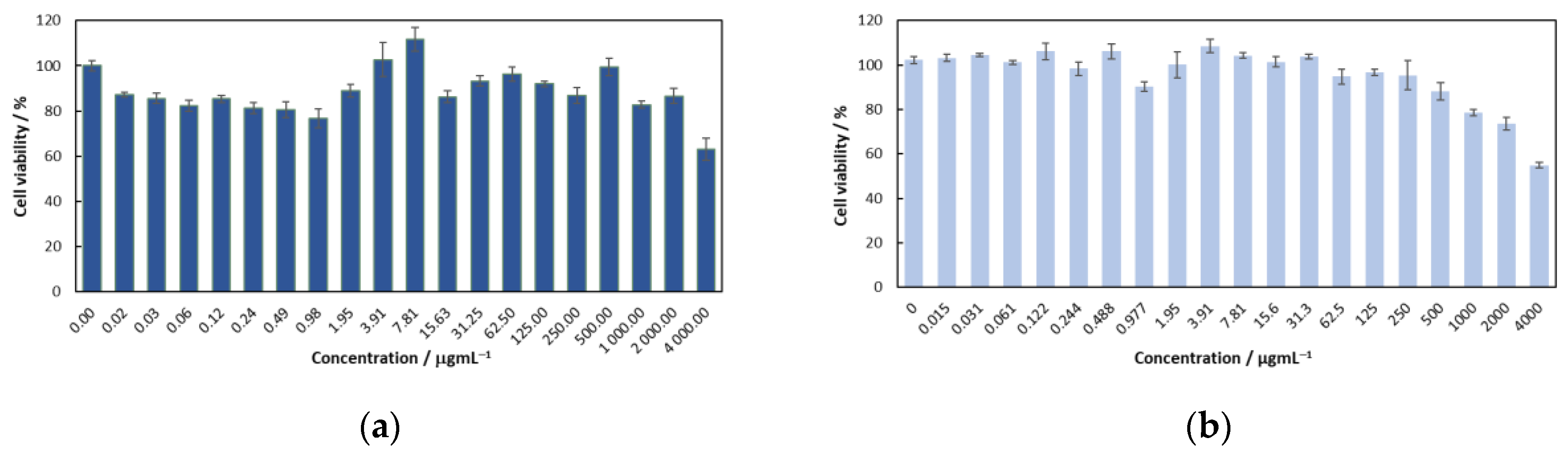
2.2.2. Cytotoxicity Assays
3. Results and Discussion
3.1. Synthesis and Structural Characterization
3.2. Optical Properties
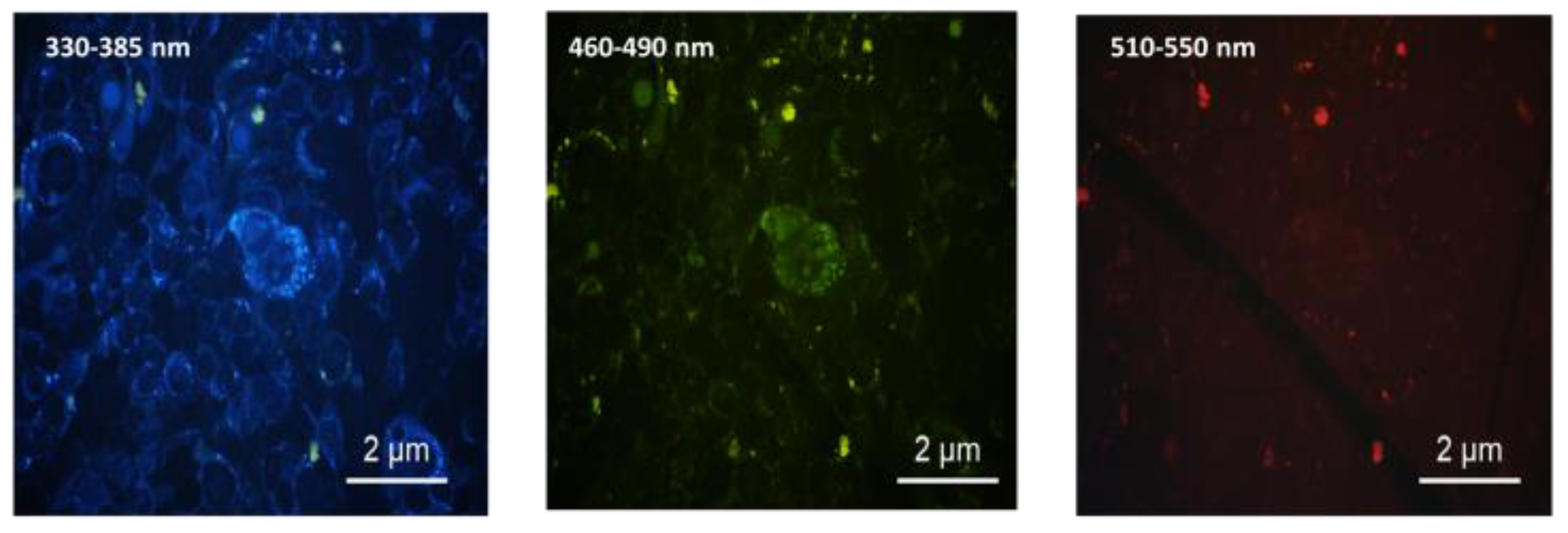
3.3. Cell Viability—Citotoxicity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balasubramaniam, V.; Gunasegavan, R.D.-N.; Mustar, S.; Lee, J.C.; Noh, M.F.M. Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules 2021, 26, 943. [Google Scholar] [CrossRef] [PubMed]
- Bule, M.H.; Ishtiaq, A.; Faheem, M.; Muhammad, B.; Hafiz, M.N.I. Microalgae as a source of high-value bioactive compounds. Front. Biosci. 2018, 10, 197–216. [Google Scholar] [CrossRef]
- Dong, D.; Liu, T.; Liang, D.; Jin, X.; Qi, Z.; Li, A.; Ning, Y. Facile Hydrothermal Synthesis of Chlorella-Derived Environmentally Friendly Fluorescent Carbon Dots for Differentiation of Living and Dead Chlorella. ACS Appl. Bio Mater. 2021, 4, 3697–3705. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, V.; Thiyagarajan, S.K.; Raji, K.; Suresh, R.; Sekar, R.; Ramamurth, P. Outright Green Synthesis of Fluorescent Carbon Dots from Eutrophic Algal Blooms for In Vitro Imaging. ACS Sustain. Chem. Eng. 2016, 4, 4724–4731. [Google Scholar] [CrossRef]
- Anuar, N.K.K.; Tan, H.L.; Lim, Y.P.; So’aib, M.S.; Bakar, N.F.A. A Review on Multifunctional Carbon-Dots Synthesized from Biomass Waste: Design/Fabrication, Characterization and Applications. Front. Energy Res. 2021, 9, 626549. [Google Scholar] [CrossRef]
- Costa, A.I.; Barata, P.D.; Moraes, B.; Prata, J.V. Carbon Dots from Coffee Grounds: Synthesis, Characterization, and Detection of Noxious Nitroanilines. Chemosensors 2022, 10, 113. [Google Scholar] [CrossRef]
- Alexandre, M.R.; Costa, A.I.; Berberan-Santos, M.N.; Prata, J.V. Finding Value in Wastewaters from the Cork Industry: Carbon Dots Synthesis and Fluorescence for Hemeprotein Detection. Molecules 2020, 25, 2320. [Google Scholar] [CrossRef] [PubMed]
- Bello, G.L.; Bartoli, M.; Giorcelli, M.; Rovere, M.; Tagliaferro, A. Review on the Use of Biochar Derived Carbon Quantum Dots Production for Sensing Applications. Chemosensors 2022, 10, 117. [Google Scholar] [CrossRef]
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- A Guide to Recording Fluorescence Quantum Yields, Horiba Scientific; 29. Available online: https://static.horiba.com/fileadmin/Horiba/Application/Materials/Material_Research/Quantum_Dots/quantumyieldstrad.pdf (accessed on 17 September 2023).
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
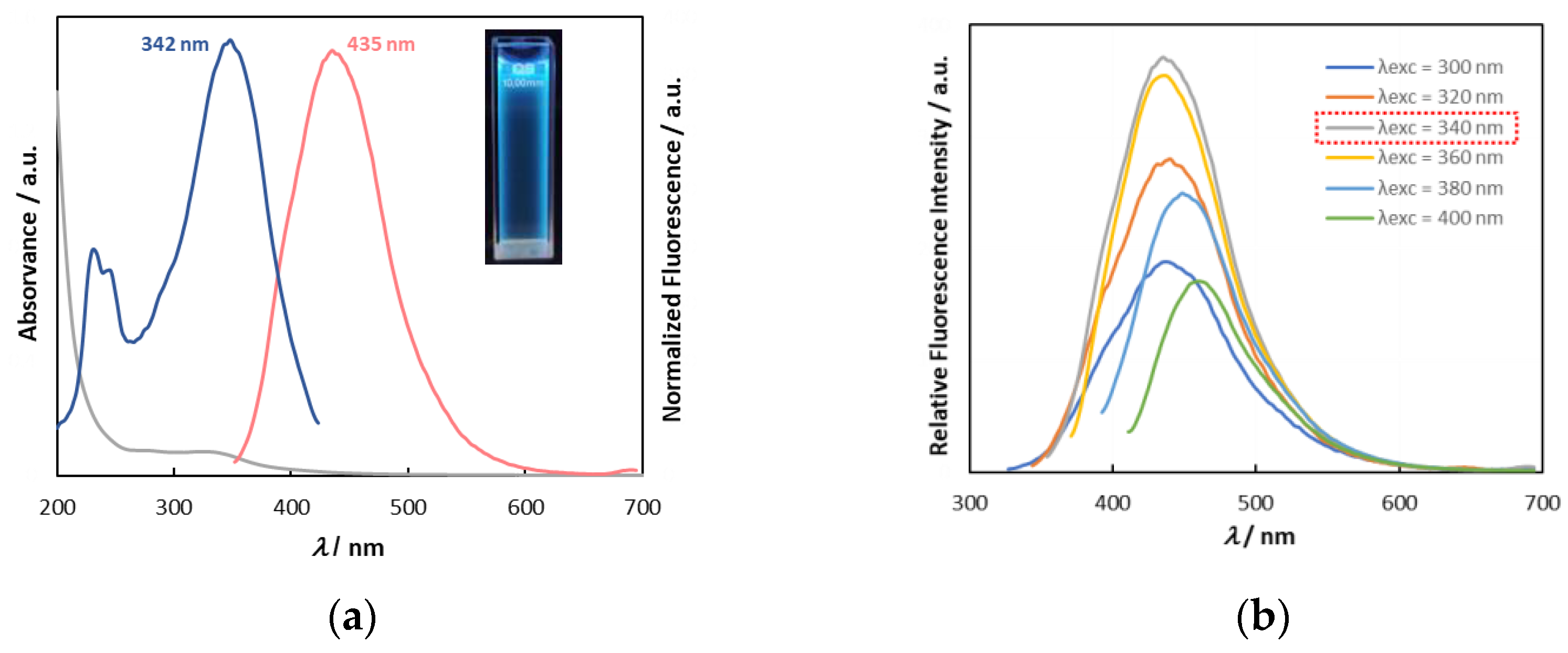
| Entry | ED/Biomass Ratio | ΦF (λ = 340 nm) |
|---|---|---|
| 1 | ___ | 0.058 |
| 2 | 0.16 | 0.124 |
| 3 | 0.32 | 0.152 |
| 4 | 0.64 | 0.171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouzende, I.; Costa, A.I.; Barata, P.D.; Martins, S.; Semedo, M.C.; Cardoso, F.M.H.; Lobo, M.L.; Prata, J.V. Green Synthesis of Luminescent Carbon Nanomaterials from Porphyridium cruentum Microalgae. Med. Sci. Forum 2023, 23, 3. https://doi.org/10.3390/msf2023023003
Chouzende I, Costa AI, Barata PD, Martins S, Semedo MC, Cardoso FMH, Lobo ML, Prata JV. Green Synthesis of Luminescent Carbon Nanomaterials from Porphyridium cruentum Microalgae. Medical Sciences Forum. 2023; 23(1):3. https://doi.org/10.3390/msf2023023003
Chicago/Turabian StyleChouzende, Inês, Alexandra Isabel Costa, Patrícia David Barata, Sónia Martins, Magda Cardoso Semedo, Fernando Manuel Henriques Cardoso, Maria Luísa Lobo, and José Virgílio Prata. 2023. "Green Synthesis of Luminescent Carbon Nanomaterials from Porphyridium cruentum Microalgae" Medical Sciences Forum 23, no. 1: 3. https://doi.org/10.3390/msf2023023003
APA StyleChouzende, I., Costa, A. I., Barata, P. D., Martins, S., Semedo, M. C., Cardoso, F. M. H., Lobo, M. L., & Prata, J. V. (2023). Green Synthesis of Luminescent Carbon Nanomaterials from Porphyridium cruentum Microalgae. Medical Sciences Forum, 23(1), 3. https://doi.org/10.3390/msf2023023003










