Abstract
The manufacture of medicines on demand for a particular patient, at the point of care, may be achieved via 3D printing, improving therapeutic outcomes, medication adherence and patient safety. Tablets are often printed using a combination of hot-melt extrusion and fused deposition modelling. In this work, paracetamol-loaded hydroxypropylcellulose filaments were produced; their extrudability and printability were aided by a plasticizer and a lubricant. For printability, 11% humidity was the ideal storage condition for filaments up to 6 months. The tablets produced complied with the uniformity of mass and content requirements, and showed delayed drug release; these characteristics were maintained after in-use stability testing for 30 days.
1. Introduction
The patient-centric design and manufacture of medicines may be achieved via 3D printing (3DP), an emergent technology that allows the digital customization of the dose and/or the design of the dosage form to tailor drug release [1]. Likewise, the on-demand manufacture of dosage units containing several drugs, according to a distinct dose and prescription regimen, simplifies life and increases compliance and the safety of polymedicated individuals.
Due to its simplicity, cost-effectiveness and versatility, fused deposition modelling (FDM) is one of the most popular 3DP techniques, comprising the successive layering of a molten drug-loaded thermoplastic polymer. FDM has been extensively used in the preparation of simple or complex dosage forms [2], with a variety of doses, shapes and release kinetics. However, besides being inadequate for heat-sensitive drugs [1], printers have to be fed with filaments, which are commercially unavailable. As such, these have to be produced beforehand using hot-melt extrusion (HME), increasing the duration and complexity of the manufacture. Depending on the formulation and processing parameters used, the integration of HME and FDM may not be straightforward [1], and may require evaluation and tuning. For compounding, it is also critical to expedite the process.
Since FDM printers are economical, compact and easy to operate, the decentralization of production from large pharmaceutical industries to community pharmacies and local hospitals is possible [3], being viable even in remote locations and when supply chains are disrupted. In addition to the potential of digital compounding to meet individual medical/clinical needs, the technology will also add value to the role of the pharmacist.
The production of medicines on demand at the dispensing site would benefit from stocking filaments (pre-produced in-house or industrially from a range of polymeric matrices and different drugs/drug loads for dose flexibility) that would be fed into the printer when and as required, according to the needs of a particular patient. The stability of filaments is a major concern, particularly of those produced using cellulosic matrices, which are hygroscopic and lose printability due to the plasticizing effect of water [2].
Therefore, the aim of this work was to establish formulation and processing parameters, as well as storage conditions for filaments, to maximize shelf-life while maintaining printability for the production of tablets, tailored to a specific patient, at the point of care. The stability of filaments was evaluated over time under different moisture conditions; the in-use stability of the 3D-printed tablets was also evaluated up to 30 days in defined relative humidity (RH) environmental settings.
2. Materials and Methods
Paracetamol (PCT; AcoFarma, Madrid, Spain) was used as a model drug, and hydroxypropylcellulose (HPC; Klucel LF®, Ashland, Wilmington, DE, USA), Soluplus® (SLP; BASF, Ludwigshafen, Germany) and magnesium stearate (MgS; Roic Farma, Barcelona, Spain) were used as excipients. Lithium chloride (11% RH), magnesium nitrate (53% RH), sodium chloride (75% RH), potassium nitrate (93% RH), silica gel (below 40% RH) and HPLC-grade methanol were supplied by Sigma Aldrich (Darmstadt, Germany).
Six different drug-loaded filaments (F1–F6; Table 1 for composition) were produced by HME using a single-screw extruder (Noztek Pro, Noztek, Shoreham, UK; nozzle die diameter of 1.5 mm, screw speed of 20 rpm, extruding temperature of 100 ± 2 °C and 65 ± 2 °C).

Table 1.
Composition (% w/w) of the formulations used to produce filaments (F) and tablets (T).
Tablets were printed from the corresponding PCT-loaded filaments using a commercial FDM 3D printer (DeltaWASP 2040 Turbo2, Massa Lombarda, Italy). Disk-shaped tablets with a mean diameter of 6.0 mm and a thickness of 3.00 mm were digitally designed (3D Sprint Software v2.11, 3D Systems, Rock Hill, SC, USA) and the .stl file generated was imported into Cura (Ultimaker B.V., v15.04.2, Utrecht, The Netherlands) for slicing. Printing parameters were set to the following: 180 °C and 220 °C extrusion temperature (respectively, for 30 and 50% PCT); 90 mm/s extrusion speed; 150 mm/s traveling speed; 2 shells, 100% infill; and 0.20 mm layer thickness. Batches of 10 or 20 tablets from each filament were prepared.
The sensitivity of the filaments to moisture was evaluated via storage at 25 °C under controlled RH environments provided by desiccators containing saturated salt solutions. Filaments were collected at day 0, 7, 14, 21, 30 and 180. The in-use stability of tablets was evaluated over a period of 30 days in sealed flasks at 11% RH/25 °C and ambient RH/25 °C in the presence and absence of silica gel.
The mean mass of tablets was determined by individually weighing 10 units, using a calibrated analytical balance (Sartorius). The mean diameter and thickness were measured (n = 10) in two different parallels using a digital caliper (Toolland, 150mm/6’’, Shanghai, China). Likewise, filaments were measured in ten different positions for diameter estimation.
PCT was quantified in individual tablets, or pieces of filaments using HPLC [4]. The drug content was calculated as the amount of drug per tablet, or mass unit in filaments.
In vitro drug release studies of the 3DP tablets were performed using a paddle apparatus (AT7 Sotax AG, Aesch, Switzerland; 50 rpm, 900 mL of phosphate buffer, pH 6.8 as the dissolution media, at 37 ± 0.5 °C). Samples (5.0 mL) were withdrawn at time intervals and quantified using HPLC (HP Agilent 1100, Waldbronn, Germany; λ max 247 nm). From the dissolution profiles, the time required for 50% drug release (t50), the dissolution rate (DR) and the similarity (f2) factor were calculated.
3. Results and Discussion
Compatibility between HME and FDM is critical for the success of 3DP, and a good extrusion behavior does not guarantee accurate and reliable printing. Thus, initial experiments considered the adjustment of the formulation and processing parameters for both techniques. HPC and PCT were used as a model cellulose-based matrix and drug, respectively. To ease the extrusion process and printability, SLP as a solubilizer/plasticizer, and MgS as a hydrophobic lubricant were also introduced in the formulations (Table 1).
3.1. Production of Drug-Loaded Filaments and Effect of Moisture on Printability
Filaments with two drug loads were produced (Table 1) in order to offer dose flexibility. All the formulations were easily hot-melt extruded under the conditions tested, and PCT-loaded filaments F1–F6 were obtained. These were off-white, smooth and flexible, with a mean diameter of ≈1.75 mm, in accordance with the printer manufacturer tolerance. The compliance of dimensions is vital for adequate printer feeding. The drug content of the filaments was assayed, at different locations, before the printing process used to evaluate the effectiveness of the components’ blending and to discard drug loss during extrusion was performed. All the filaments showed drug loadings close to the theoretical value (between 99.90–102.08%).
Printability was ascertained via the ability to print tablets with simple and more complex geometries. The temperature was as low as possible to allow constant flow out of the printer’s nozzle and below the degradation temperature of PCT (240 °C). To maintain extrudability and printability, the drug fraction could not exceed 50%; equally, filaments containing 50% PCT (F4 and F5) required a higher printing temperature (220 °C).
Immediately after production, all the filaments were consistently squeezed by the printer’s gears and ruptured, precluding printing to finish. Hence, the filaments were left to settle for 7 days under different controlled RH values to evaluate the influence of moisture on printability. Under high RH (55, 75 and 95%), the filaments became sticky and lost shape due to water uptake, which plasticized the matrix and modified its mechanical behavior. Appropriate apparent elasticity–brittleness was obtained after storage at 11% RH for filaments F1–F5; filament F6, which had a slightly smaller diameter, could not be fed into the printer.
Filaments F1–F5 retained their dimensions, drug content, and adequate rheology for printing up to 6 months under the same storage (11% RH) and printing conditions.
3.2. Characterization and In-Use Stability of Tablets
Filaments F1–F5 could be printed into tablets (T1–T5), whose average mass, dimensions, and drug content are shown in Table 2. Besides being uniform in their dimensions and shape (in agreement with the digital template), the tablets complied with the Eur. Pharm. [4] regarding the intra-batch uniformity of mass, regardless of the batch size produced. The drug content of the tablets was within the range 100 ± 5% of the theoretical value, suggesting no drug loss.

Table 2.
Properties of tablets 3D-printed from filaments stored at 11% RH for 7 days, immediately after production.
Considering that tablets are produced on demand and in small batches as a result of personalized prescriptions, stability testing was performed for 30 days in a desiccator (11% RH) and under conditions that mimicked tablet usage by the patient. After this time, the tablets maintained identical masses, dimensions, and amounts of PCT, regardless of the storage conditions, suggesting the good and desirable stability of both the matrix and drug.
The results of the in vitro dissolution testing of tablets, immediately after printing and after 30 days storage in silica gel, are presented in Figure 1A,B. The use of large—molecular-weight polymers, such as HPC, resulted in extended, but complete, drug release patterns. Due to the swelling of HPC, the matrix expanded in the dissolution media, followed by the steady erosion of the polymer, leading to a somewhat complex release behavior.
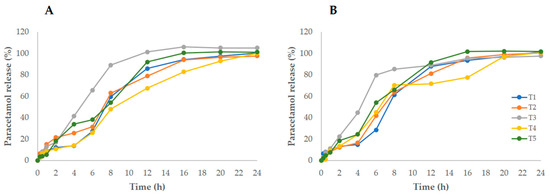
Figure 1.
PCT release from 3D-printed tablets (T1–T5) immediately after printing (A) and after 30 days of storage in a bottle containing silica gel (B).
The dissolution profiles enabled the kinetic parameters (t50 and DR) and similarity factors (f2) to be calculated, which aided a comparison between the different formulations. As expected, the decrease in HPC (as the PCT content increased) resulted in greater DR (T1–T3 > T4–T5), suggesting that, indeed, the matrix controls drug release; as for t50, the opposite trend could be observed. Formulation T3, with the highest MgS content, stood out for the lowest t50 (≈4.73 h), despite its hydrophobic nature. At day 30, a comparison with the initial corresponding release profile resulted in f2 > 50, indicating similar curves.
These results, although preliminary, contribute to the yet limited knowledge about the stability of both filaments and tablets manufactured via FDM–3D printing. The formulation developed may also be used as a polymeric carrier for drugs other than PCT, provided the necessary adjustments are made.
Author Contributions
Conceptualization, G.G.P., A.I.F. and J.F.P.; methodology, investigation and formal analysis, G.G.P.; writing—review and editing, A.I.F.; resources, project administration and funding acquisition, A.I.F. and J.F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundação para a Ciência e a Tecnologia, grant number PTDC/CTM CTM/30949/2017 (Lisboa 010145 Feder 030949).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is not publicly available.
Acknowledgments
Ashland is acknowledged for providing the Klucel LF® samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D printing by fused deposition modeling of single- and multi-compartment hollow systems for oral delivery—A review. Int. J. Pharm. 2020, 579, 119155. [Google Scholar] [CrossRef] [PubMed]
- Beer, N.; Kaae, S.; Genina, N.; Sporrong, S.K.; Alves, T.L.; Hoebert, J.; De Bruin, M.L.; Hegger, I. Magistral Compounding with 3D Printing: A Promising Way to Achieve Personalized Medicine. Ther. Innov. Regul. Sci. 2023, 57, 26–36. [Google Scholar] [CrossRef] [PubMed]
- EDQM (Ed.) Council of Europe European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2019; ISBN 9789287175250. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).