1. Introduction
Small-cell lung cancer (SCLC) is the most aggressive form of lung cancer. The 5-year survival rate is about 5%. The treatment of patients with SCLC underwent significant transformation via the introduction of immune checkpoint inhibitors. However, significant progress has still not been made [
1]. At the time of diagnosis, more than 60% of patients have distant metastases [
2,
3], and a significant number of these metastases are found in the brain, liver, and bones [
3]. The presence of distant metastases and their number determine the patient’s life expectancy and prognosis [
4]. Circulating tumor cells (CTCs) are detected before the appearance of other signs of lung cancer, including precancerous conditions [
5]. CTCs and cancer stem cells (CSCs) play a leading role in SCLC metastasis and can be used as diagnostic and prognostic markers [
3,
6,
7] and potential therapeutic targets [
8].
CAR T-cell therapy has been proven to be effective for the treatment of malignant blood disorders. Meanwhile, the creation of CAR T-cells for the treatment of solid tumors, including SCLC, is difficult due to the variability of tumor cells and the problem with the access of modified cells to solid tumor cells [
9]. In addition, when developing cell therapy approaches, special attention should be paid to cell material. For example, the blood of healthy donors or umbilical cord blood are sources of T-cells [
10]. However, allogeneic cell transplantation is associated with graft-versus-host disease. Autologous cells can avoid negative consequences. However, patients’ T-cells are often dysfunctional. After chemotherapy, T-cells are more differentiated and show lower proliferative activity in vivo and ex vivo [
10]. Antigen-specific stimulation leads to the rapid depletion of such cells. Previously, we developed an approach to reprogram mouse T-cells, which increased their survival and targeted CSCs, and thus increased the effectiveness of LLC cell therapy [
8]. MEK and PD-1 inhibitors and the targeted training of CSCs were used for the reprogramming.
In the present study, using an in vitro allogeneic and autologous cell therapy model, we reprogrammed CD8+ T-cells isolated from the blood of healthy volunteers (non-smokers and smokers) and patients with chronic lung diseases (chronic obstructive pulmonary diseases (COPD), SCLC, and asthma in different combinations) and assessed their survival and cytotoxic activity relative to the targeted CSCs of a patient with SCLC and COPD.
2. Materials and Methods
2.1. Design of Investigation
The study included non-smoking (V1) and smoking (V2) volunteers and patients with COPD (P1), COPD and asthma (P2), and SCLC and COPD (P3). The characteristics of the subjects are presented in
Table 1. The study included four stages. In the first stage, mononuclear cells and then CD8
+ T-cells were isolated from the venous blood samples of the subjects. In the second stage, CSCs were isolated from the peripheral blood mononuclear cell fraction of patient P3, and a culture of CSCs was obtained. In the third stage, CD8
+ T-cells of the subjects were reprogrammed using the MEK inhibitor and the PD-1 blocker nivolumab. CSCs from patient P3 were used for T-cell “training”. In the fourth stage, the apoptosis and cytotoxicity of the hrT-cells were assessed in a culture of CSCs isolated from patient P3.
2.2. Isolation of Human CD8+ T-Cells
CD8+ T-cells were isolated from blood mononuclear cells using Lympholyte-H (CEDARLANE, Cedarlane Laboratories, Zierikzee, The Netherlands) and the EasySepTM Human CD8+ T Cell Isolation Kit for magnetic separation.
2.3. Reprogramming of Human CD8+ T-Cells
The reprogramming of human CD8
+ T-cells was carried out as described previously [
8]. For the “training” of T-cells, we used CSCs from patient
P3 (with COPD and SCLC). After reprogramming, the expression of CCR7 hrT-cells was assessed [
11].
2.4. Cultivation and Detection of CSCs Obtained from Blood of Patient P3
The cultivation of CSCs and the evaluation of spheroid formation were carried out according to the protocols described previously [
8]. To confirm the presence of specific markers on tumor spheroids and single cells, cell staining with monoclonal antibodies (CD117 BB515, CD87 BV421, CD274 BV421, Axl BV480, EGF Receptor Alexa Fluor
® 647, all Becton Dickinson, San Jose, CA, USA) was performed. The Cytation 5 instrument was used to obtain images of cells. The image analysis that followed was performed using Gen5™ data-analysis software (BioTek, Instruments, Friedrichshall, Germany).
2.5. Detection of the Cytotoxicity and Apoptosis of Reprogrammed Human CD8+ T-Cells In Vitro
The apoptosis and cytotoxicity of CD8
+ T-cells were studied as described earlier [
11]. The Cytation 5 instrument was used to obtain images of cells. For cell analysis, we used Gen5™ data-analysis software (BioTek, Instruments, Friedrichshall, Germany).
2.6. Statistical Analysis
Statistical analysis was processed with methods of variational statistics using the SPSS Statistics 12.0 (IBM Corp., Armonk, NY, USA) software as described earlier [
8].
3. Results
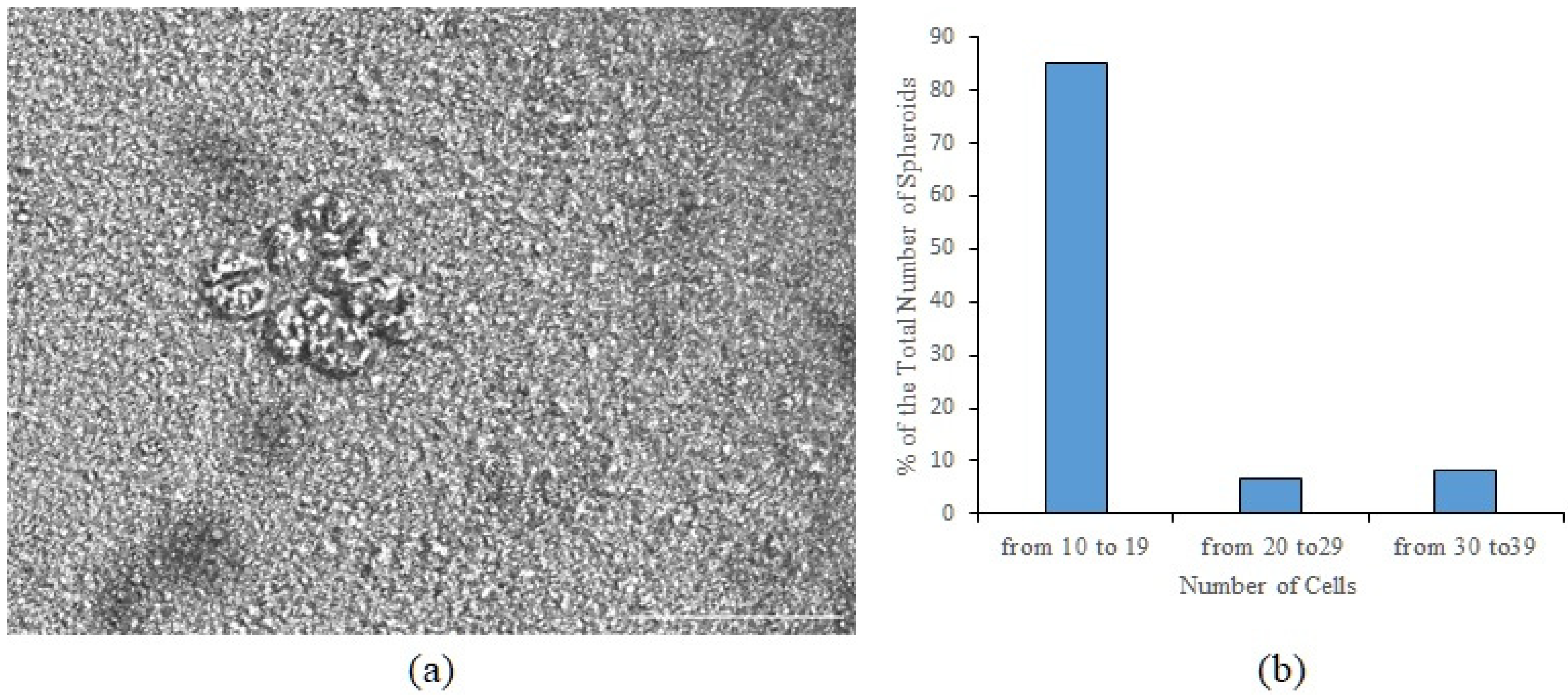
3.1. Tumor Cells Isolated from the Blood of Patient P3 Formed Spheroids In Vitro, Which Included Cells Expressing CD87, CD117, CD274, EGF, and Axl
In a culture of the adherent fraction of mononuclear cells isolated from the blood of patient
P3, we found spheroids (
Figure 1a). Based on the Cytation5 images, a spheroid was defined as a three-dimensional cellular structure. The total number of spheroids was 74 per 200,000 cells. Spheroids were divided into three classes by cellularity: class 1 includes spheroids with the number of cells n = 10–19; class 2—n = 20–29; class 3—n = 30–39 (
Figure 1b).
100% staining of cells in spheroids were stained with dyes in various combinations. The following combinations were used: CFSE/Hoechst/EGF, CD117/CD87/EGF, CD117/Axl/EGF, CD117/CD274(PD-L1)/EGF. Cells stained with 7AAD were not found in the structure of spheroids.
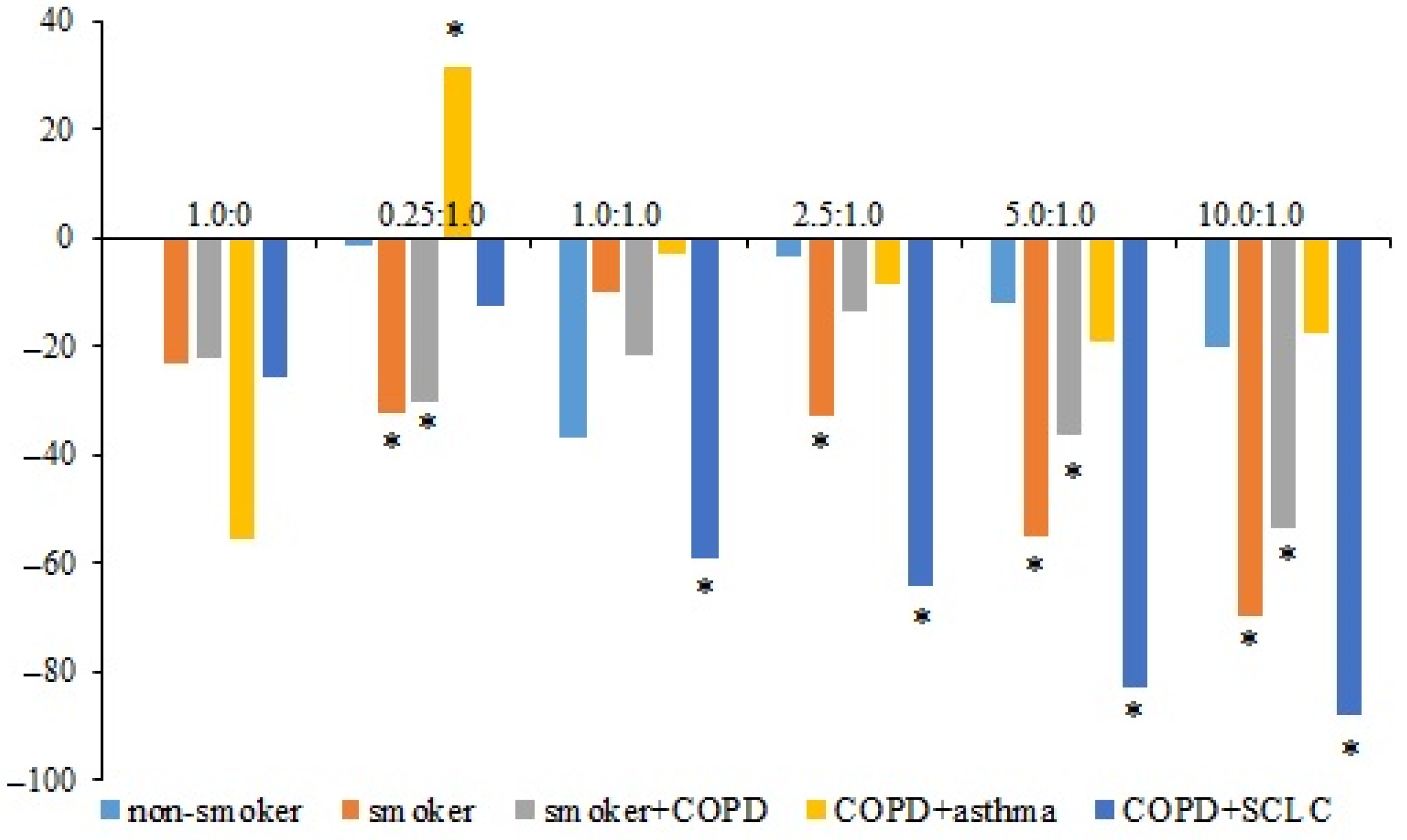
3.2. Apoptosis of hrT-Cells and hnT-Cells of the Subjects in the CSC Culture Isolated from the Blood of Patient P3
In the primary CSC culture isolated from the blood of patient P3 (COPD with SCLC), the apoptosis of hrT-cells from volunteers V1 (healthy volunteer) and V2 (smoker volunteer) and patients P1 (smoker with COPD), P2 (COPD with asthma), and P3 was studied in comparison with the corresponding naive human CD8+ T-cells (hnT-cells). The maximum number of apoptotic cells was observed in the culture of hrT-cells of volunteer V1. The level of apoptosis of hnT-cells of volunteer V2 was higher than in the V1 cell culture. However, hrT-cells of volunteer V2 were more resistant to apoptosis than V1 cells.
The apoptosis of hrT-cells isolated from patient P1 was significantly reduced at ratios of 0.25:1.0, 5.0:1.0, and 10.0:1.0. The most pronounced decrease in apoptosis was observed in hrT-cells isolated from the blood of the patient P3. A decrease in apoptosis in this group was noted at a ratio of 1.0:1.0. At ratios of 2.5:1.0, 5.0:1.0, and 10.0:1.0, the number of apoptotic cells decreased more. The apoptosis of hrT-cells obtained from patient P2 did not change significantly (
Figure 2).
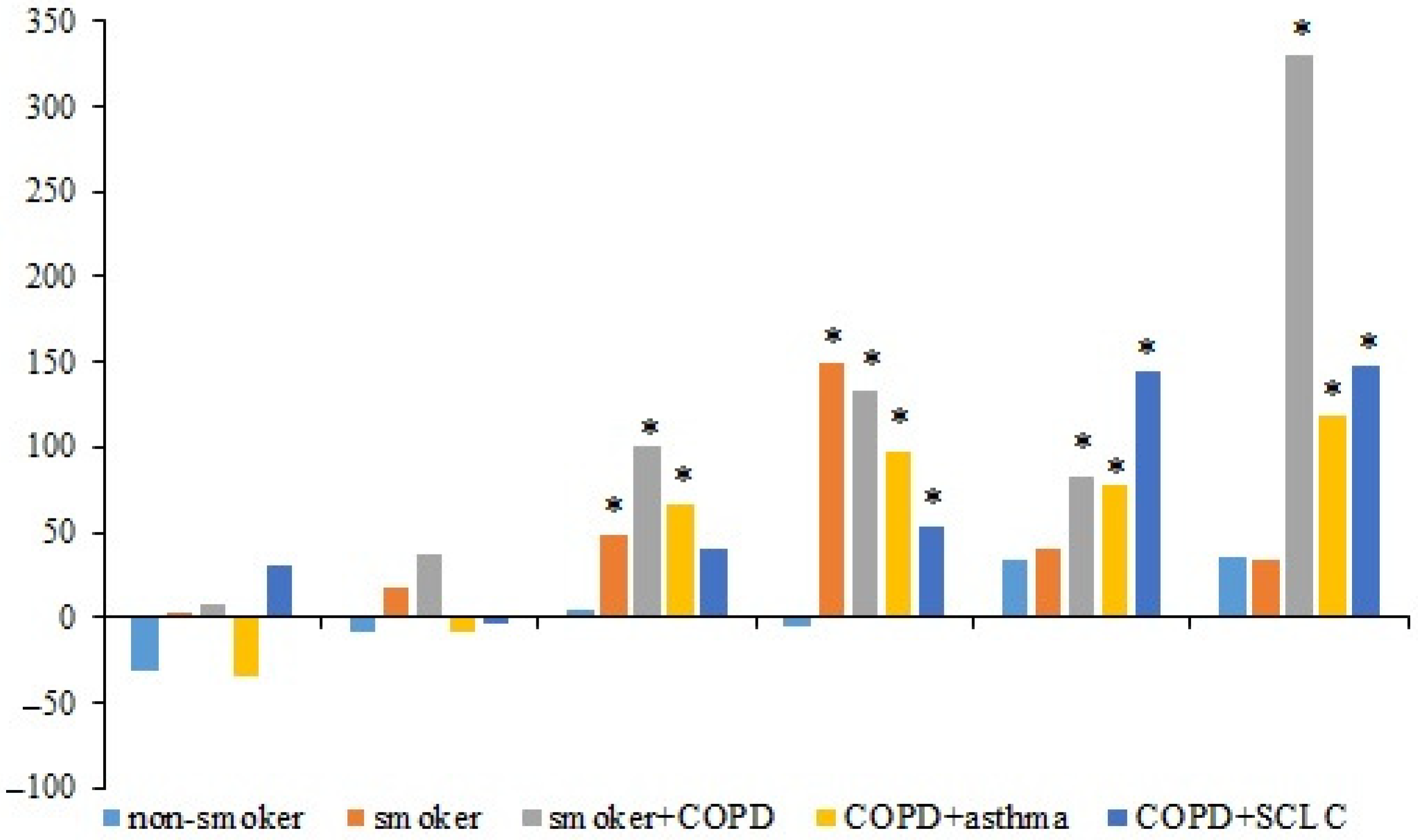
3.3. Cytotoxic Activity of hrT-Cells and hnT-Cells of the Subjects in the Culture of CSCs Isolated from Patient P3
In the primary CSC culture isolated from the blood of patient P3, the cytotoxic activity of hnT-cells and hrT-cells of the subjects was studied. In all groups, the cytotoxicity of hrT-cells increased with an increasing concentration of T-cells in culture (compared to hnT-cells). Cytotoxicity reached the maximum at a T-cells:CSCs ratio of 10.0:1.0. At the same time, a significant increase in the cytotoxicity of hrT-cells in volunteer V2 and patients P1 and P2 was observed at a ratio of 1.0:1.0. The cytotoxicity of hrT-cells in patients P1, P2, and P3 increased more significantly relative to hnT-cells in comparison with volunteers V1 and V2 (
Figure 3).
4. Discussion
Cell therapy with modified immune cells is a promising approach for the treatment of SCLC. An unresolved issue of this approach to therapy is the choice of the optimal cell donor whose modified cells could eliminate the given target of CSCs. This issue can be partially resolved by assessing the cells in vitro. In the present pilot study, we evaluated the activity of allogeneic (from volunteers V1 and V2 and patients P1 and P2) and autologous (from patient P3) hrT-cells on a culture of CSCs isolated from the blood of patient P3 (COPD+SCLC). In a culture of CSCs, hrT-cells from volunteers V1 and V2 and patients P1, P2, and P3 showed significantly greater cytotoxicity and less apoptosis than the corresponding nrT-cells. The most pronounced increase in cytotoxicity was observed in the hrT-cells of patients with lung disease than of volunteers. In addition, the number of apoptotic hrT-cells in a culture obtained from patients with pulmonary diseases was lower. The exception was T-cells isolated from the blood of a patient with COPD and asthma. Reprogramming did not have a significant effect on the change in this indicator.
Thus, T-cells’ reprogramming using the MEK inhibitor, the PD-1 blocker, and personalized “training” with CSCs are effective methods for allogeneic T-cells of volunteers and patients with lung diseases. We demonstrated the efficacy of autologous hrT-cells in SCLC in vitro. The use of autologous cells can significantly reduce the risk of negative immune reactions.
We understand that the study had limitations: the sample of patients was limited, and no follow-up study was conducted. We are currently recruiting additional patients through this project. Further studies will confirm the effectiveness of cellular reprogramming and provide additional data required for the improvement of the personalization of cell therapy.
Author Contributions
Conceptualization, E.G.S.; methodology, M.Z. and N.E.; software, L.K.; validation, E.G.S. and M.Z.; formal analysis, N.E.; investigation, M.Z. and N.E.; resources, A.D.; data curation, E.G.S.; writing—original draft preparation, E.G.S. and M.Z.; writing—review and editing, E.G.S. and A.D.; visualization, M.Z. and N.E.; supervision, E.G.S.; project administration, E.G.S.; funding acquisition, E.G.S. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Ministry of Science and Higher Education Russian Federation, grant number 075-15-2020-773.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Cancer Research Institute Tomsk National Research Medical Center of the Russian Academy of Sciences (protocol No. 8 from 15 June 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pavan, A.; Attili, I.; Pasello, G.; Guarneri, V.; Conte, P.F.; Bonanno, L. Immunotherapy in small-cell lung cancer: From molecular promises to clinical challenges. J. Immunother. Cancer 2019, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Kurishima, K.; Tamura, T.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in small cell lung cancer. Oncol. Lett. 2012, 4, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Winslow, M.M.; Sage, J. Mechanisms of small cell lung cancer metastasis. EMBO Mol. Med. 2021, 13, e13122. [Google Scholar] [CrossRef] [PubMed]
- Megyesfalvi, Z.; Tallosy, B.; Pipek, O.; Fillinger, J.; Lang, C.; Klikovits, T.; Schwendenwein, A.; Hoda, M.A.; Renyi-Vamos, F.; Laszlo, V.; et al. The landscape of small cell lung cancer metastases: Organ specificity and timing. Thorac. Cancer 2021, 12, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Hofman, V.; Long-Mira, E.; Selva, E.; Vignaud, J.M.; Padovani, B.; Mouroux, J.; Marquette, C.H.; Hofman, P. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE 2014, 9, e111597. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, M.; Groen, H.J.M. Circulating tumor cells are prognostic in SCLC, but still lack clinical application. Ann. Oncol. 2019, 30, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.Y.; Heigener, D.; Reck, M.; Califano, R. Immune checkpoint blockade in small cell lung cancer. Lung Cancer 2019, 137, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Skurikhin, E.G.; Pershina, O.; Ermakova, N.; Pakhomova, A.; Widera, D.; Zhukova, M.; Pan, E.; Sandrikina, L.; Kogai, L.; Kushlinskii, N.; et al. Reprogrammed CD8+ T-Lymphocytes Isolated from Bone Marrow Have Anticancer Potential in Lung Cancer. Biomedicines 2022, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Kandra, P.; Nandigama, R.; Eul, B.; Huber, M.; Kobold, S.; Seeger, W.; Grimminger, F.; Savai, R. Utility and Drawbacks of Chimeric Antigen Receptor T Cell (CAR-T) Therapy in Lung Cancer. Front. Immunol. 2022, 13, 903562. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.C.Y.; Xu, N.; Nordon, R.; Haber, M.; Micklethwaite, K.; Dolnikov, A. Donor T cells for CAR T cell therapy. Biomark. Res. 2022, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Skurikhin, E.G.; Pershina, O.; Ermakova, N.; Pakhomova, A.; Zhukova, M.; Pan, E.; Sandrikina, L.; Widera, D.; Kogai, L.; Kushlinskii, N.; et al. Cell Therapy with Human Reprogrammed CD8+ T-Cells Has Antimetastatic Effects on Lewis Lung Carcinoma in C57BL/6 Mice. Int. J. Mol. Sci. 2022, 23, 15780. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).