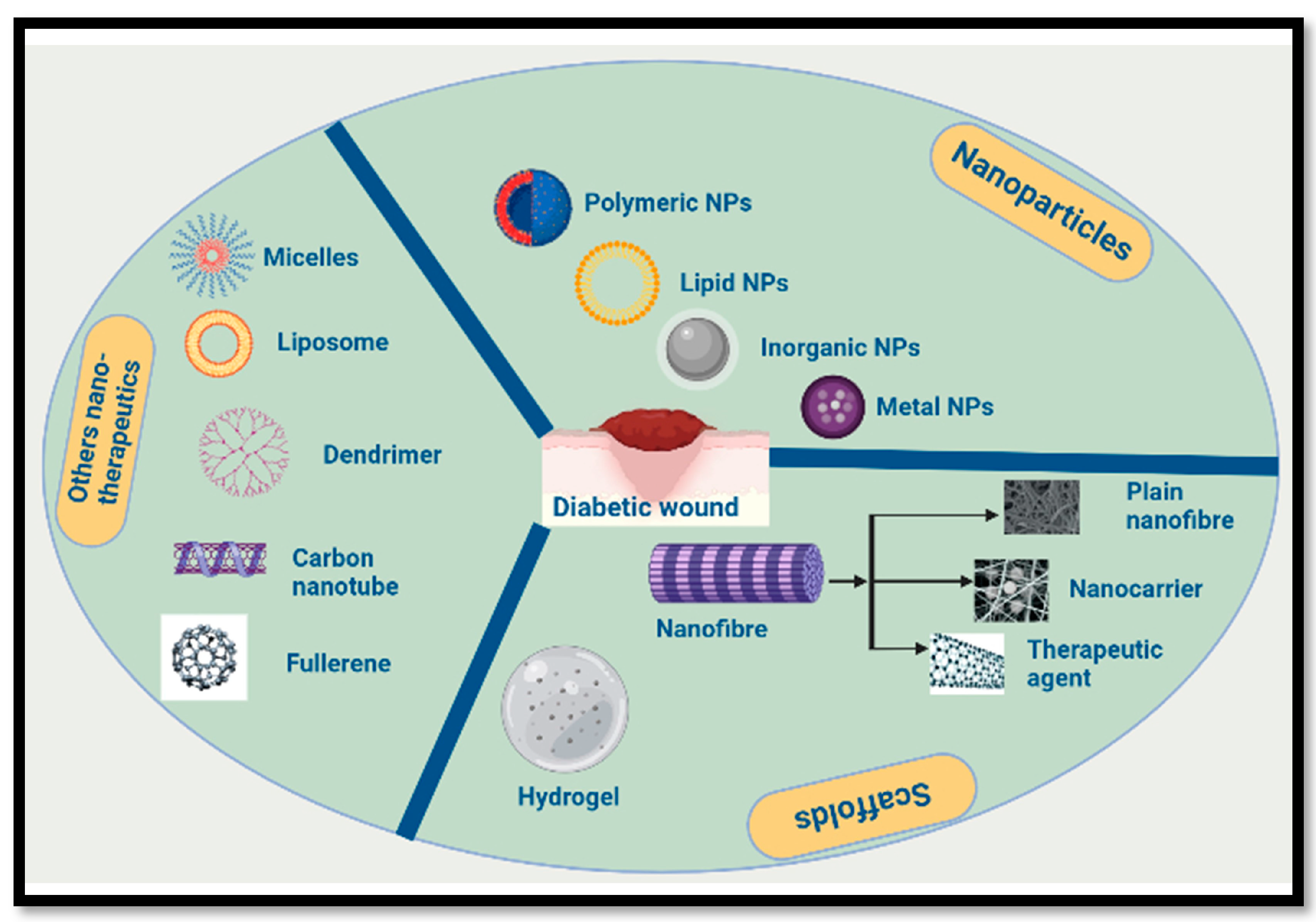
1. Introduction
Diabetes mellitus, more commonly referred to by its medical name, diabetes, is a widespread condition that affects millions of people worldwide. Diabetes mellitus develops when the body either develops resistance to insulin or is unable to produce enough insulin [
1]. The genesis of this phenomenon is mostly attributable to a conjunction of two primary factors. The first cause is a malfunction in the production of insulin by beta cells in the pancreas. The second factor is that insulin-sensitive cells do not respond appropriately to insulin [
2]. Prevalence estimates from the International Diabetes Federation (IDF) indicated that there would be 537 million individuals (20–79) with DM in the world in 2021, with that figure rising to 643 million by 2030 and 783 million by 2045. One out of every five people lost their life to diabetes in 2021 [
3]. The rise in health care costs attributable to diabetes has been significant worldwide, rising from approximately USD 232 billion in 2007 to approximately USD 966 billion in 2021 for people aged 20–79 years. This reflects a growth rate of 316% over the course of the last 15 years [
3].
Our skin is an extremely versatile and adaptive organ that has developed over the course of millennia to serve as a barrier against the chemical, mechanical, and ultraviolet radiation hazards that we are exposed to on a daily basis [
4,
5]. Our skin’s highly evolved compensatory processes allow it to recover fast and effectively from the harsh external environment. Many cellular components of the damage response can become attenuated, preventing the lesion from closing [
6].
Diabetes decreases metabolic activity, which increases infection risk and delays wound healing. The main reason for this is disturbed blood glucose management. Type 2 diabetic patients often need to have limbs amputated or otherwise become handicapped as a significant secondary consequence due to their poor wound-healing ability [
7]. Tissue repair involves blood coagulation, swelling, proliferation, and reorganisation. Hyperglycaemia disrupts the natural transition between these wound healing stages, keeping the site inflamed and preventing epithelialization. Indeed, having diabetes increases the likelihood that a wound may become chronic (one that takes more than 12 weeks to completely heal) [
8].
Nanotechnology is already transforming our lives. The biological sciences are using it creatively, leading to new frameworks, technologies, and systems that can potentially change illness, treatment, and diagnosis. On the other hand, conventional medicines often include treatments that are both expensive and time-consuming, in contrast to medications based on nanotechnology [
9]. In comparison to traditional methods, nanotechnology-based drug delivery systems hold more promise because of their controlled release and targeted activity [
10]. In this review article, we focus on the implications of advancements in nanotechnology and nanoformulations in accelerating diabetes associated wound healing.
2. Nanotechnology in Wound Healing
In regard to skin regeneration, nanotechnology is a promising new area of study. Nano-based therapeutics such as nanofibers, nanoemulsions, etc., have attracted considerable interest for application as key components in skin regeneration because of their structural resemblance to the extracellular matrix [
11]. Many different forms of polymeric nanofibers have been developed and trialled as scaffolds for tissue restoration, after years of intensive study and testing. A number of nanoscale drug delivery scaffolds, including nanomaterials, nanoemulsions, nanocapsules, and liposomes, have been shown to hasten wound healing. Furthermore, nanofibrous materials can be modified in terms of shape, biodegradability, and other qualities to optimise wound healing in a range of contexts [
11,
12,
13] (
Figure 1).
2.1. Nanoparticles
Nanoparticles (NPs) with a size of 1–100 nm have received a great deal of attention from researchers in the biomedical and tissue regeneration disciplines. There are two primary types of NPs used for wound healing: those with inherent qualities that promote healing and those used as medication delivery systems. The main benefits include an increased medication half-life, bioavailability, and regulated and sustained release. Polymeric nanoparticles and metallic nanoparticles are the two primary categories that can be used to categorise nanoparticles [
14,
15]. Biocompatible polymeric nanoparticles for sustained drug delivery to chronic wounds have been developed in recent years. These nanoparticles include substances such as Poly Lactic-co-Glycolic Acid (PLGA), alginate, gelatine (GEL), and other polycaprolactones (PCLs), as well as PEG [
16]. However, for hundreds of years, metals such as silver have been utilised to cure a wide range of disorders. However, modern medicine has primarily relied on silver salts and compounds, such as silver nitrate and silver sulphadiazine, due to their antibacterial properties, particularly in the treatment of wounds [
17]. Previous research published in the scientific literature reported that formulations loaded into the silver nanoparticle showed inhibitory activity and the low bactericidal concentration against drug-resistant multidrug-resistant bacteria and standard reference cultures. Gram-negative bacteria were found to be easier to eradicate using the nano formulation than Gram-positive bacteria [
18].
Gold nanoparticles (AuNPs) are the focus of extensive investigation for wound healing because to their novel electrical, optoelectronic (subatomic size effect), biochemical, and magnetic capabilities [
19]. The antioxidant and antimicrobial properties of AuNPs have been demonstrated, and their roles in wound healing have been found to be critical [
20,
21]. Photobiomodulation treatment (PBMT), more often known as Low-Level Laser Therapy (LLLT), has recently brought attention to the role of AuNPs in wound healing. When applied to wounds, AuNPs considerably accelerated repair, decreased pain and inflammation, and promoted angiogenesis more effectively throughout the early stages of wound healing [
22].
Zinc nanoparticles are another important nanoparticle with a lot of applications in wound healing. A novel sustained release wound dressing was developed by incorporating zinc oxide nanoparticles (ZnO NPs) coated with gentamicin into a chitosan gel matrix [
23].
2.2. Nanoemulsion
Due to their benefits, nanoemulsions have been explored as tissue repair medication delivery mediums. Their advantages include a small droplet size, a large surface area, a greater solubilisation efficiency, a long shelf life, and a simple formulation procedure [
24]. Compared to normal Neomycin ointment, eucalyptus oil nanoemulsion accelerates the healing process of wounds in Wistar rats [
25]. In an in vitro experiment, a bio-active nanoemulsion system was formed of Nigella sativa (NS) oil, Calendula officinalis (CO) extract, and lipoic-acid-capped AuNPs (AuNP-LA). The augmented NS nanoemulsion demonstrated significantly higher antioxidant and anti-thrombotic activities compared to the unenhanced NS emulsion [
26]. Several nanoformulations based on nanoemulsions have demonstrated efficacy in promoting diabetic wound healing [
27,
28]. Researchers also created and tested a nanoemulsion (NE) of curcumin (Cur) to improve transdermal medication delivery. They discovered anti-inflammatory and wound-healing properties of the Cur NE, showing its promise as a nanoformulation for non-invasive transdermal administration [
29].
2.3. Nanohydrogel
When treating wounds, nanohydrogel is frequently seen to be the best formulation, as its porous three-dimensional structure can help to prevent wounds from drying out and foster a wet environment that promotes healing [
30]. Nanohydrogel’s calming texture provides a pleasant therapeutic experience, and its nonadherent nature safeguards the insertion site while still enabling the oxygen penetration necessary for healing [
31]. Nanohydrogel can encapsulate several different drugs for skin regeneration without reducing their efficacy or diminishing their compatibility. Baicalin was combined with a gellan-cholesterol to accelerate the recovery process [
32]. Xi Loh et al. [
33] found that bacterial nanocrystal cellulose/acrylic acid nanogels rapidly attached to fibroblasts, maintained the interaction and morphological characteristics of skin fibroblasts, slowed the metabolism of cells, accelerated the proliferation of cells, and modulated the expression of nine genes involved in wound healing (including IL-6, IL-10, GM-CSF, TGF-β, MMP-2).
2.4. Carbon-Based Nanotherapeutics
Carbon nanomaterials such as fullerenes, carbon nanohorns, and carbon nanotubes, in addition to graphene, have gained attention in the field of biomedicine as a result of the potential applications that they have in advanced organogenesis and the transport of drugs or genes [
34]. Fullerenes and carbon nanotubes both displayed excellent performance in the wound healing process. This was accomplished by changing the immunological and regenerative stages of the wound. Due to the powerful antioxidant characteristics of fullerenes, reactive oxygen species (ROS) and reactive nitrogen species (RNS) can be neutralised and detoxified by fullerenes, hence reducing their harmful effects [
35]. In order to cure wounds, scientists have developed and introduced CBNs-TES-PAMAM-G3-collagen scaffolds. Improved mechanical qualities, higher cell viability, and faster wound healing were all observed in the collagen scaffold. According to the findings, the CNT-TES-PAMAM-G3-collagen scaffolds show great promise as a material for use in tissue engineering and wound healing [
36].
2.5. Nanocomposite
As previously established, several nanotechnologies can be utilised in the production of a wide variety of wound dressings. It is feasible that by integrating these numerous nanotechnologies, a new method of wound healing that is more efficient could be developed. Chen et al. produced a konjac glucomannan (KGM)/AgNP composite with powerful antibacterial activity with the intention of facilitating the healing process following an injury [
37]. In the treatment of chronic wounds, Giuseppina Sandri and colleagues created a nanocomposite that was composed of halloysite nanotubes (HNTs) and chitosan oligosaccharides. This nanocomposite was intended to be used as a pour powder to accelerate the healing process [
38]. A curcumin nanocomposite was produced for use as a wound dressing by G. Devanand Venkatasubbu and his colleagues. The findings of their research showed that the nanocomposite had a high degree of efficacy for the healing of wounds, in addition to possessing antibacterial properties [
39]. Kokabi et al. [
40] prepared a nanocomposite hydrogel wound dressing from a mixture of polyvinyl alcohol hydrogel and organoclay. The experimental results demonstrated that the nanocomposite hydrogels have the necessary qualities for a suitable wound dressing, including adequate swelling, an appreciable vapour transmission rate, effective barrierity against microbial penetration, and satisfactory mechanical properties [
40].
2.6. Others
Several different kinds of nanotherapeutics based on nanofiber have been developed [
41]. For wound healing, in particular, nanostructured lipid carriers and peptide nanoformulations have been developed [
38]. The exciting developments in nanotechnology-based nanoformulation can enable the simple production of biocompatible nanomaterials (NMs) and open the door to a new method of treating wounds [
42].
3. Conclusions
Injuries to living tissues, such as wounds, render them particularly delicate. Many factors contribute to successful wound healing. Wound healing agents come in a wide range of types, and the current formulations possess their own sets of requirements for application and performance. Wound healing is greatly influenced by elements such as cellular growth, biocompatibility, cell adhesion, and anti-microbial activity. Since many nanotechnologies, such as NPs, hydrogels, and nanocomposites, possess these qualities, they make for effective wound healing materials. In this summary, the benefits of utilising nanomaterials in the wound healing process were presented. Because they have antibacterial and anti-inflammatory actions, as well as proangiogenic and proliferative qualities, nanoformulations have the ability to change each phase of the wound healing process. It is possible for nanoformulations to correct the expression level of many essential proteins and signal molecules, which will accelerate the healing process. Therefore, nanoformulations may become effective enough to overcome the majority of the obstacles that currently exist in the management of wound care.
Author Contributions
D.M.: investigation, formal analysis, methodology, writing—original draft, writing—review and editing. J.G.: writing—review and editing, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research receives no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas 9th Edition 2019, Global Estimates for the Prevalence of Diabetes for 2019, 2030 and 2045. Available online: http://www.diabetesatlas.org/ (accessed on 30 January 2023).
- Lyman, M. The Remarkable Life of the Skin: An Intimate Journey across Our Surface; Random House: New York, NY, USA, 2019. [Google Scholar]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Buffo, A.; Rolando, C.; Ceruti, S. Astrocytes in the damaged brain: Molecular and cellular insights into their reactive response and healing potential. Biochem. Pharmacol. 2010, 79, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Syafril, S. Pathophysiology diabetic foot ulcer. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 125, p. 012161. [Google Scholar]
- Den Dekker, A.; Davis, F.M.; Kunkel, S.L.; Gallagher, K.A. Targeting epigenetic mechanisms in diabetic wound healing. Transl. Res. 2019, 204, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Tocco, I.; Zavan, B.; Bassetto, F.; Vindigni, V. Nanotechnology-based therapies for skin wound regeneration. J. Nanomater. 2012, 2012, 4. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.F.; Gunaseelan, S.; Kiellani, M.H.H.; Thottempudi, V.V.K.; Neuenschwander, P.; Nie, H. A review of injectable and implantable biomaterials for treatment and repair of soft tissues in wound healing. J. Nanotechnol. 2017, 2017, 6341710. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Nanotechnology approaches for skin wound regeneration using drug-delivery systems. In Nanobiomaterials in Soft Tissue Engineering; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 31–55. [Google Scholar]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology approaches in chronic wound healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Jahromi, M.A.M.; Zangabad, P.S.; Basri, S.M.M.; Zangabad, K.S.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Ghosh, B.; Mukhopadhyay, M. Development of nanotechnology for advancement and application in wound healing: A review. IET Nanobiotechnol. 2019, 13, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Leu, J.G.; Chen, S.A.; Chen, H.M.; Wu, W.M.; Hung, C.F.; Yao, Y.D.; Tu, C.S.; Liang, Y.J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Muthuvel, A.; Adavallan, K.; Balamurugan, K.; Krishnakumar, N. Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties. Biomed. Prev. Nutr. 2014, 4, 325–332. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.; Prabu Poorani, G.; Jegatheeswaran, S.; Balakumar, C.; Gurumallesh Prabu, H.; Anand, K.; Marimuthu Prabhu, N.; Jeyakanthan, J.; Saravanan, M. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int. J. Nanomed. 2020, 15, 7553–7568. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef]
- Vasile, B.S.; Oprea, O.; Voicu, G.; Ficai, A.; Andronescu, E.; Teodorescu, A.; Holban, A. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin–chitosan composite with potential applications in wounds care. Int. J. Pharm. 2014, 463, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Ansari, M.J.; Anwer, M.K.; Raish, M.; Kamal, Y.K.; Shakeel, F. Wound healing effects of nanoemulsion containing clove essential oil. Artif. Cells Nanomed. Biotechnol. 2017, 45, 591–597. [Google Scholar] [CrossRef]
- Sugumar, S.; Ghosh, V.; Nirmala, M.J.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: Antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason. Sonochem. 2014, 21, 1044–1049. [Google Scholar] [CrossRef]
- Guler, E.; Barlas, F.B.; Yavuz, M.; Demir, B.; Gumus, Z.P.; Baspinar, Y.; Coskunol, H.; Timur, S. Bio-active nanoemulsions enriched with gold nanoparticle, marigold extracts and lipoic acid: In vitro investigations. Colloids Surf. B Biointerfaces 2014, 121, 299–306. [Google Scholar] [CrossRef]
- Alam, P.; Shakeel, F.; Anwer, M.K.; Foudah, A.I.; Alqarni, M.H. Wound healing study of eucalyptus essential oil containing nanoemulsion in rat model. J. Oleo Sci. 2018, 67, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.R.; Kuisma, S.W.; Kanninen, L.; Kanninen, L.; Lille, M.; Corlu, A.; GuGuen-Guillouzo, C.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Anumolu, S.S.; Menjoge, A.R.; Deshmukh, M.; Gerecke, D.; Stein, S.; Laskin, J.; Sinko, P.J. Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials 2011, 32, 1204–1217. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Cencetti, C.; di Meo, C.; Zoratto, N.; Nacher, A.; Fadda, A.M.; Matricardi, P. Preparation of gellan-cholesterol nanohydrogels embedding baicalin and evaluation of their wound healing activity. Eur. J. Pharm. Biopharm. 2018, 127, 244–249. [Google Scholar] [CrossRef]
- Xi Loh, E.Y.; Fauzi, M.B.; Ng, M.H.; Ng, P.Y.; Ng, S.F.; Ariffin, H.; Mohd Amin, M.C.I. Cellular and molecular interaction of human dermal fibroblasts with bacterial nanocellulose composite hydrogel for tissue regeneration. ACS Appl. Mater. Interfaces 2018, 10, 39532–39543. [Google Scholar] [CrossRef]
- Zhang, Y.; Petibone, D.; Xu, Y.; Mahmood, M.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A.S. Toxicity and efficacy of carbon nanotubes and graphene: The utility of carbon-based nanoparticles in nanomedicine. Drug Metab. Rev. 2014, 46, 232–246. [Google Scholar] [CrossRef]
- Zhou, Z. Liposome formulation of fullerene-based molecular diagnostic and therapeutic agents. Pharmaceutics 2013, 5, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Vedhanayagam, M.; Nair, B.U.; Sreeram, K.J. Dimension effect: Dendrimer functionalized carbon based nanomaterial mediated collagen scaffold for wound healing application. Materialia 2019, 7, 100354. [Google Scholar] [CrossRef]
- Chen, H.; Lan, G.; Ran, L.; Xiao, Y.; Yu, K.; Lu, B.; Dai, F.; Wu, D.; Lu, F. A novel wound dressing based on a Konjac glucomannan/silver nanoparticle composite sponge effectively kills bacteria and accelerates wound healing. Carbohydr. Polym. 2018, 183, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbu, G.D.; Anusuya, T. Investigation on Curcumin nanocomposite for wound dressing. Int. J. Biol. Macromol. 2017, 98, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA–clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Reddy, V.J.; Radhakrishnan, S.; Ravichandran, R.; Mukherjee, S.; Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen. 2013, 21, 1–16. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Kim, B.S. Nanomaterial-Based Therapy for Wound Healing. Nanomaterials 2022, 12, 618. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).