Effects of RF Currents on Cytokines Production in Human Keratinocytes †
Abstract
1. Introduction
2. Methods
2.1. Cell Culture
2.2. Electric Treatment
2.3. Cell Proliferation Assay
2.4. ELISA Assay
2.5. Statistical Analysis
3. Results and Discussion
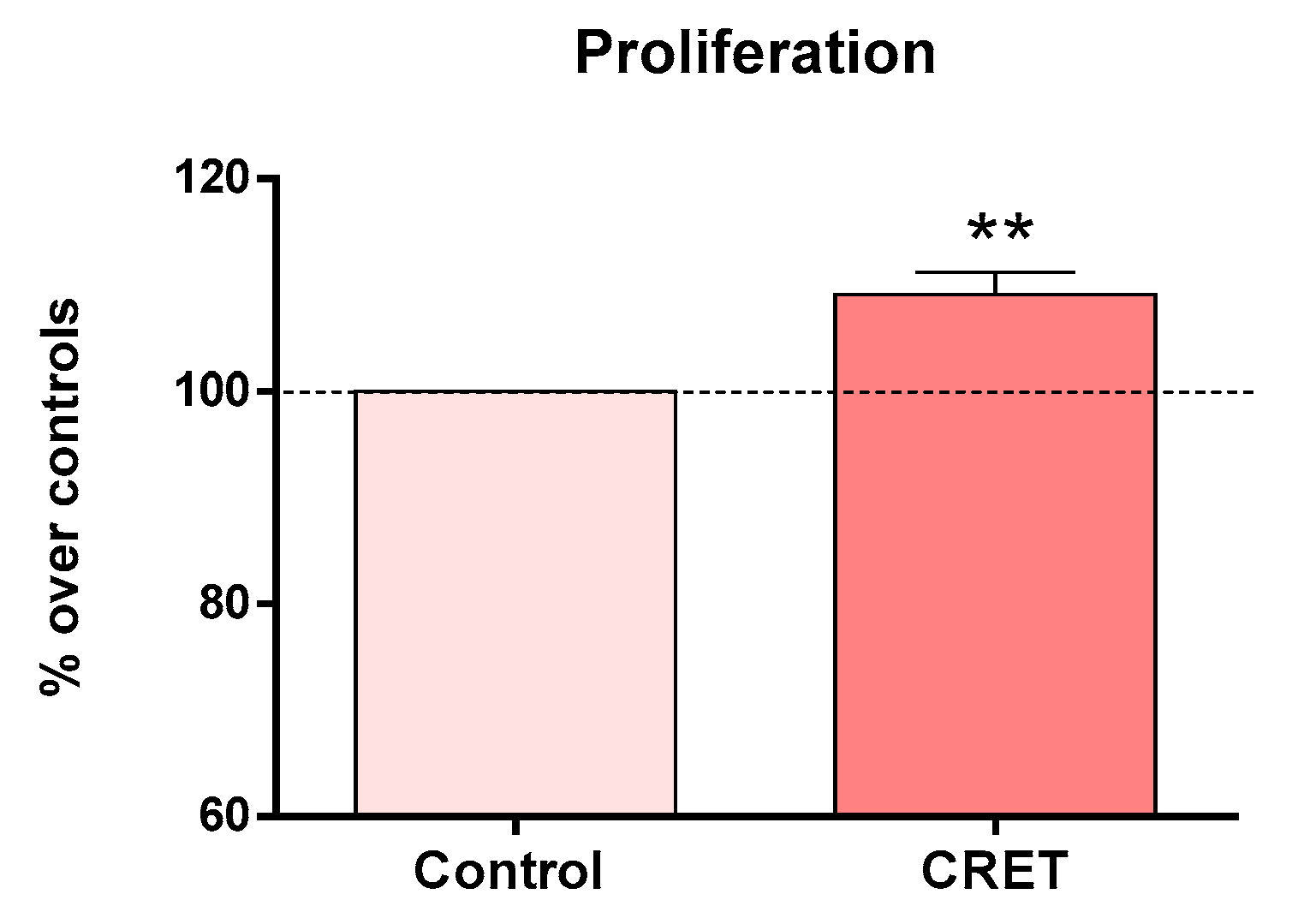
3.1. Cell Proliferation
3.2. Cytokine Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gillitzer, R.; Goebeler, M. Chemokines in Cutaneous Wound Healing. J. Leukoc. Biol. 2001, 69, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Kaszuba-Zwoiñska, J.; Cie, I.; Mach, T. Magnetic Field Anti-Inflammatory Effects in Crohn’s Disease Depends Upon Viability and Cytokine Profile of the Immune Competent Cells. J. Physiol. Pharmacol. 2008, 59, 177–187. [Google Scholar] [PubMed]
- Lushnikov, K.V.; Shumilina, Y.V.; Yakushina, V.S.; Gapeev, A.B.; Sadov, V.B. Effects of Low-Intensity Ultrahigh Frequency Electromagnetic Radiation on inflammatory processes. Bull. Exp. Biol. Med. 2004, 137, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Carralero-Martínez, A.; Muñoz Pérez, M.A.; Kauffmann, S.; Blanco-Ratto, L.; Ramírez-García, I. Efficacy of Capacitive Resistive Monopolar Radiofrequency in the Physiotherapeutic Treatment of Chronic Pelvic Pain Syndrome: A Randomized Controlled Trial. Neurourol. Urodyn. 2022, 41, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Paíno, C.L.; Trillo, M.Á.; Úbeda, A. Electric Stimulation at 448 KHz Promotes Proliferation of Human Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2014, 34, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Toledano-Macías, E.; Naranjo, A.; de Andrés-Zamora, M.; Úbeda, A. In Vitro Stimulation with Radiofrequency Currents Promotes Proliferation and Migration in Human Keratinocytes and Fibroblasts. Electromagn. Biol. Med. 2021, 40, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Trillo, M.A.; Cid, M.A.; Leal, J.; Ubeda, A. In Vitro Exposure to 0.57-MHz Electric Currents Exerts Cytostatic Effects in HepG2 Human Hepatocarcinoma Cells. Int. J. Oncol. 2007, 30, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Rojavin, M.A.; Rogers, T.J.; Ziskin, M.C. Reactions of Keratinocytes to in Vitro Millimeter Wave Exposure. Bioelectromagnetics 2001, 22, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Ferrone, A.; Costantini, E.; Franceschelli, S.; Pesce, M.; Speranza, L.; Amerio, P.; D’Angelo, C.; Felaco, M.; Grilli, A.; et al. Extremely Low-Frequency Electromagnetic Fields Accelerates Wound Healing Modulating MMP-9 and Inflammatory Cytokines. Cell Prolif. 2018, 51, e12432. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Rowland-Jones, S.L. RANTES: A Versatile and Controversial Chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Vianale, G.; Reale, M.; Amerio, P.; Stefanachi, M.; Di Luzio, S.; Muraro, R. Extremely Low Frequency Electromagnetic Field Enhances Human Keratinocyte Cell Growth and Decreases Proinflammatory Chemokine Production. Br. J. Dermatol. 2008, 158, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
| MCP-1 | IL-8 | RANTES | |
|---|---|---|---|
| CRET | 118.564 ± 3.483 * | 88.884 ± 14.555 | 86.439 ± 12.395 |
| Control | 100.0 ± 0.00003 | 100.0 ± 0.00012 | 100.0 ± 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Bule, M.L.; Toledano-Macías, E.; Martínez-Pascual, M.A.; Úbeda, A.; Fernández-Guarino, M. Effects of RF Currents on Cytokines Production in Human Keratinocytes. Med. Sci. Forum 2023, 21, 29. https://doi.org/10.3390/ECB2023-14096
Hernández-Bule ML, Toledano-Macías E, Martínez-Pascual MA, Úbeda A, Fernández-Guarino M. Effects of RF Currents on Cytokines Production in Human Keratinocytes. Medical Sciences Forum. 2023; 21(1):29. https://doi.org/10.3390/ECB2023-14096
Chicago/Turabian StyleHernández-Bule, María Luisa, Elena Toledano-Macías, María Antonia Martínez-Pascual, Alejandro Úbeda, and Montserrat Fernández-Guarino. 2023. "Effects of RF Currents on Cytokines Production in Human Keratinocytes" Medical Sciences Forum 21, no. 1: 29. https://doi.org/10.3390/ECB2023-14096
APA StyleHernández-Bule, M. L., Toledano-Macías, E., Martínez-Pascual, M. A., Úbeda, A., & Fernández-Guarino, M. (2023). Effects of RF Currents on Cytokines Production in Human Keratinocytes. Medical Sciences Forum, 21(1), 29. https://doi.org/10.3390/ECB2023-14096






