Abstract
Fibroblasts play a crucial role in wound healing and skin fibrosis. It is also probably the cell model used to study in vitro photobiomodulation. Our previous in vivo results evidenced a faster recovery in blue-light-treated wounds (410–430 nm). In vitro experiments demonstrated that the lower dose increases cell metabolism, while higher doses (30.9 and 41.2 J/cm2) reduce it. Furthermore, 20.6 J/cm2 affects outward currents and Cytochrome C. Here, we described our preliminary results on the effects of blue LED light on mitochondria and reactive oxygen species production. Globally, our results demonstrated that short-wavelength blue LED light has PBM properties.
1. Introduction
More than 50 years have passed since Endre Mester identified the first medical evidence of low-level laser therapy (LLLT). Experimental observations such as faster hair regrowth in rats and the promotion of wound healing were categorized as “laser biostimulation” [1]. Soon, it became clear that it was not necessary to use lasers and their peculiarities (monochromatic, coherent, etc.) to obtain an effect at a biological level. Non-coherent light-emitting diodes (LEDs) with comparable application parameters could also be exploited with lower production costs and better handling. For these reasons, the term LLLT has been replaced with photobiomodulation (therapy), PBM(T) [2]. Following the debates about the appropriateness of the terms, a nomenclature consensus meeting was organized by the North American Association for Light Therapy and the World Association for Laser Therapy. The term LLLT (cited in the Medical Subject Headings (MeSH)) led to thinking of laser as the only usable source and gathered together terms and techniques significantly different from each other without any distinction. In November 2015, the term “Photobiomodulation Therapy” officially entered into the MeSH. This event represents an advance not only from a technological and methodological point of view but also considering a rigorous organization of the scientific literature and the experimental protocols [1,2].
Today, the mechanism underlying PBM effects is still partially unclear. The most widely accepted theory is based on “hormesis”, a dose–response relationship caused by a biphasic effect. From a biological point of view, it is considered an adaptive response: a very low dose or a treatment applied for a very short time does not induce any effect, while a high dose or a prolonged treatment time induces an inhibitory response.
Our research is focused on the effects of short-wavelength blue LED light (emission range 410–430 nm) in the field of wound healing and skin fibrosis. For these reasons, we dedicated our studies to analyzing the behavior of fibroblasts, both through in vivo and in vitro experiments.
In vitro experiments were performed on several cultures of fibroblasts isolated from keloids and their perilesional tissue, compared to fibroblasts obtained from normal human skin and purchased-cell cultures of adult human dermal fibroblasts (HDFa). Using colorimetric tests, we showed that blue LED light (3.43–6.87–13.75–20.6–30.93–41.2 J/cm2) affects in different ways on fibroblasts with different origins, but the response is always dose-dependent. Electrophysiological recordings in patch-clamp showed that only keloid-derived fibroblasts increased the outward current after applying 20.6 J/cm2. Furthermore, micro-Raman spectroscopy performed on single cells demonstrated that the same dose of blue LED light directly affects Cytochrome C, inducing a transition from an oxidized to a reduced form [3]. A scratch test executed in a co-culture of healthy fibroblasts and human keratinocytes (HaCaT cells) showed that the application of 20.6 J/cm2 of blue light induces an acceleration of the closure of the scratch, compared with unirradiated samples [4].
From in vivo studies performed on superficial wounds induced in CD1 mice, we demonstrated that the application of 20.6 J/cm2 of blue LED light stimulates the activation of fibroblasts into myofibroblasts, without inducing any changes in the cell density. Myofibroblasts were detected using alpha-smooth muscle actin antibody (alpha-SMA, Sigma-Aldrich, Milano, Italy) and revealed by fluorescence microscopy. Furthermore, the distribution and morphology of type I collagen in the wounded and treated tissue appeared to be more similar to a never-injured one when compared to the wounded but untreated tissue [5,6].
Wound healing is an intricate process consisting of three different phases: inflammation, tissue formation and remodeling [7]. Reactive oxygen species (ROS) play a key role in tissue regeneration. Indeed, the regulated production of ROS is crucial to maintaining physiological processes and adaptive responses such as chemotaxis, cytoskeletal remodeling and calcium homeostasis [8,9].
Here, we irradiated human dermal fibroblast cells with three different doses of blue light (5–21–42 J/cm2) to study ROS production and changes in the shape and morphology of mitochondria.
2. Materials and Methods
2.1. Cell Cultures
Adult human dermal fibroblast cells (HDFa, Lot# 2207322) were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and used following the recommendation of the manufacturer. The primary cultures of human dermal fibroblasts were obtained from 7 patients subjected to mole removal, while keloid-derived fibroblasts were obtained from 11 patients subjected to keloid tissue removal. In each case, the samples were fragmented into small pieces (2–4 mm in diameter), collected in a scratched-Petri dish (Greiner Bio-One, GmbH, Kremsmünster, Austria) and kept under laminar flow until adhesion to the plate occurred. Within three weeks from the preparation, fibroblasts migrated out of the tissue. All the cells were cultivated in Dulbecco’s modified Eagle medium (DMEM, 1.5 g/L in glucose) added with 10% of fetal bovine serum (FBS), 1% of glutamine and streptomycin (PAN-Biotech GmbH, Aidenbach, Germany) and detached using trypsin-EDTA solution (Sigma-Aldrich, Milan, Italy) when necessary. DMEM was refreshed every 48 h.
2.2. Irradiation Protocols in Cultured Cells
Cells were counted using a Neubauer chamber (Karl Hecht Assistent GmbH, Sondheim vor der Rhön, Germany), and an appropriate number of cells were seeded in specific support for the analysis to be performed. The following fluences of blue LED light were applied: 5–21 and 42 J/cm2.
2.3. ROS Analysis
Reactive oxygen species were detected using a cell-based, fluorescent kit purchased from AbCam (ab113851, Cambridge, UK) and was used according to the manufacturer’s recommendations. The signal was revealed by confocal microscopy (Leica SP8, Martinsried, Germany).
2.4. Electron Microscopy
Twenty-four hours after irradiation, fibroblasts were detached using trypsin EDTA 0.25% (Pan-React Applichem, Milan, Italy). After 3 centrifuges in phosphate-buffered saline, the pellet was resuspended in an appropriate volume of Karnovsky’s fixative. Electron microscopy was conducted as follows. Briefly, the specimens were washed with cold 0.01 M phosphate-buffered saline, pH 7.4, and were directly fixed in cold 4% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) overnight at 4 °C and post-fixed in cold 1% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4) for 1 h at room temperature. The samples were dehydrated in graded acetone, passed through propylene oxide and embedded in Epon 812. Ultrathin sections were stained with uranyless and alkaline bismuth subnitrate and examined under a JEM 1010 electron microscope (Jeol, Tokyo, Japan) at 80 kV.
3. Results
3.1. Blue LED Light Modulates Reactive Oxygen Species
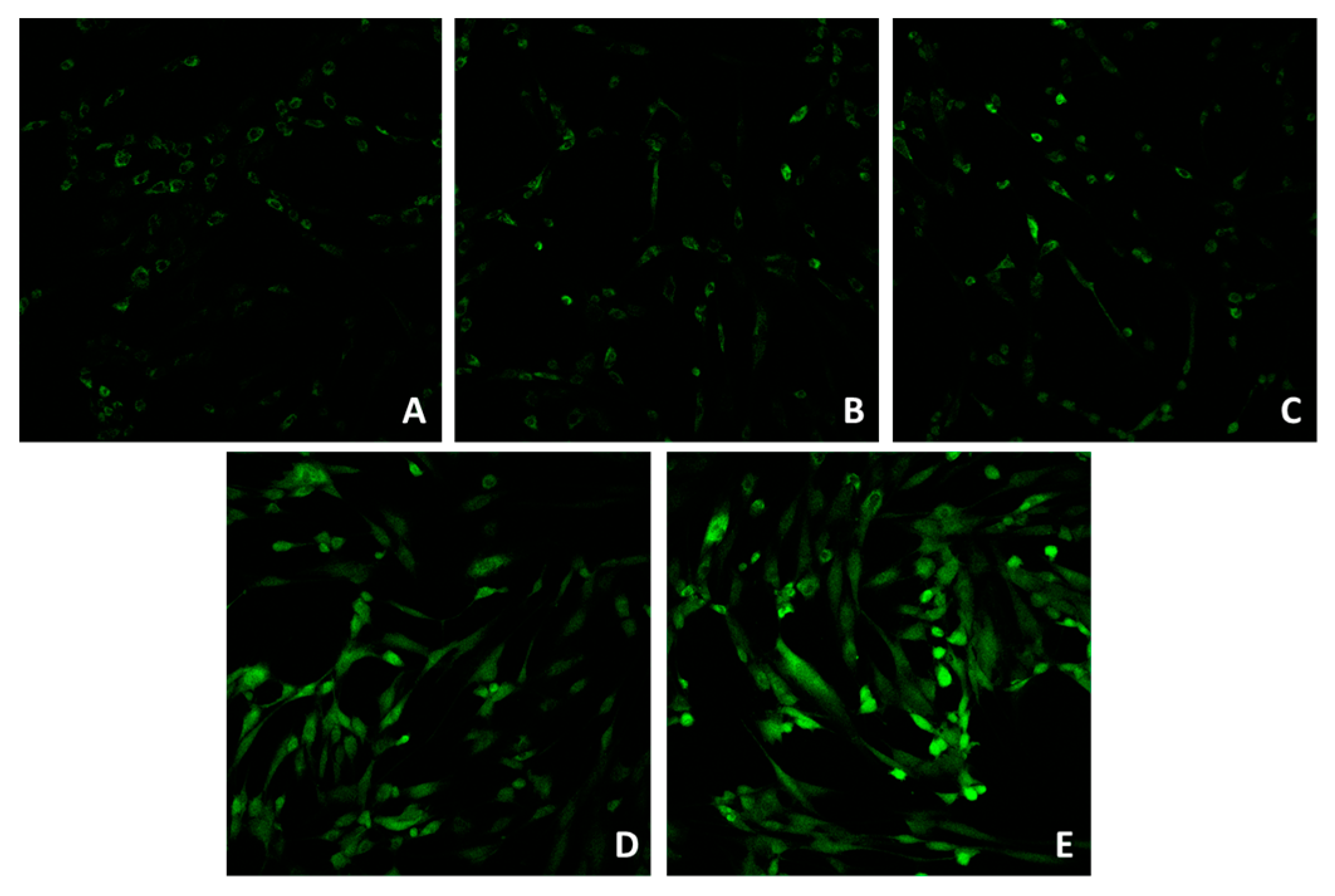
Confocal microscopy (Figure 1A–E) showed that the application of 5 and 21 J/cm2 (Figure 1B,C) did not induce a significant ROS increase, compared to the control (unirradiated cells, Figure 1A). The application of 41 J/cm2 (Figure 1D) induced an increase in the ROS signal. Figure 1E represents the signal after the addition of 100 μM of H2O2.
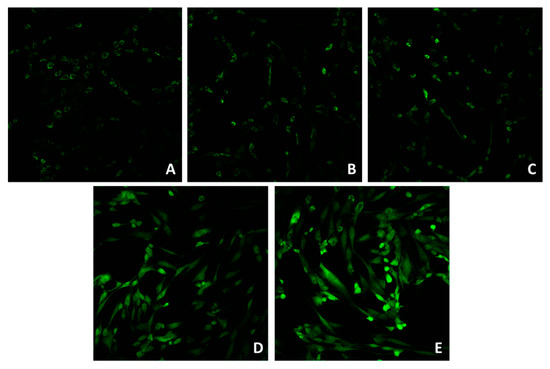
Figure 1.
Confocal microscope imaging of unirradiated cells (A), cells irradiated with 5 (B), 21 (C) and 42 (D) J/cm2. Positive control (E) was obtained by incubating cells in 100 μM of H2O2. Magnification: 20×.
3.2. Blue LED Light Affects Mitochondria in Human Dermal Fibroblasts
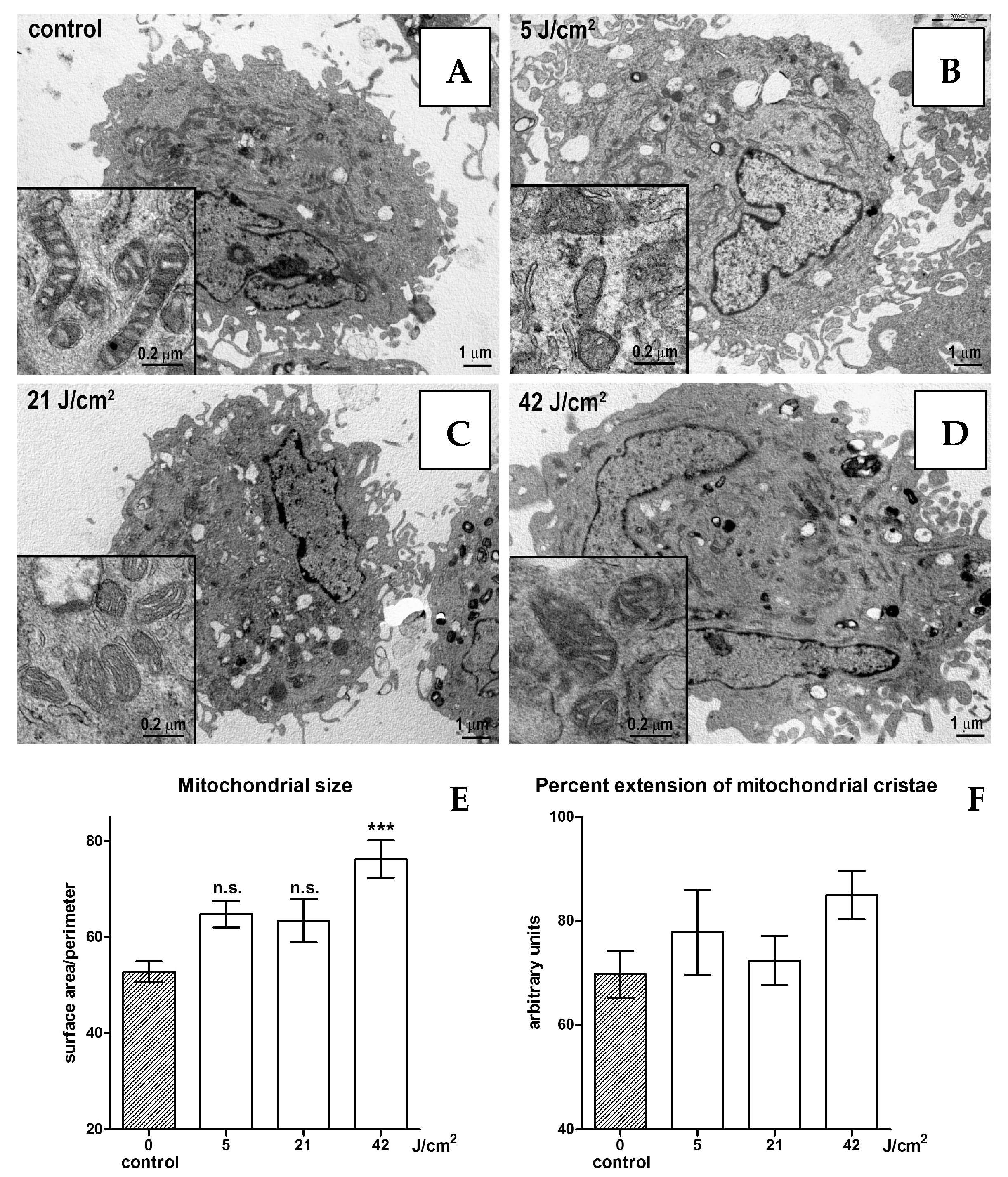
No substantial changes in cell morphology were evidenced by electron microscopy, with any of the blue light doses in the range of 0–21 J/cm2 (Figure 2A–C). The size of the mitochondria increases significantly when 42 J/cm2 is applied (Figure 2D,E). Mitochondrial morphology also slightly changes from rod-shaped in the controls to oval-shaped in the irradiated cells. Mitochondrial cristae show no significant changes in their overall extent (Figure 2F).
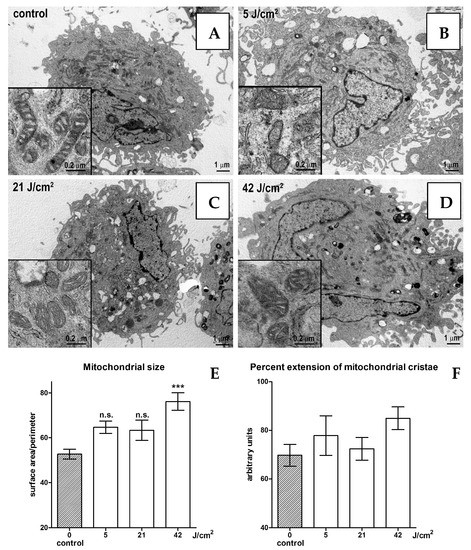
Figure 2.
Electron microscopy of unirradiated cells (A), cells irradiated with 5 (B), 21 (C) and 42 (D) J/cm2. Statistical analyses of mitochondrial size (E) and percent extension of mitochondrial cristae (F). Statistical analyses: n.s. > 0.05; *** p < 0.001. Magnification: 5000×.
4. Discussion
Despite the limitations, in vitro experiments are still helpful in studying some biological processes. Here, we applied three fluences of short wavelengths of blue LED light: a low, a medium and a high dose, in human dermal fibroblasts to investigate the eventual effects on the mitochondria shapes and ROS synthesis. Our preliminary results show that at low and medium fluences (4 and 21 J/cm2, respectively), blue LED light does not stimulate ROS and does not damage the mitochondria, while the higher fluence (42 J/cm2) induces a significant increase in ROS and mitochondrial size. However, mitochondrial cristae show no significant changes in their overall extent, suggesting substantially similar patterns of energy metabolism. Together with our previous observations [3,4,6,10] and clinical evidence [11,12,13], these findings suggest that blue LED light-photobiomodulation (410–430 nm) represents a safe and effective treatment in the management of skin wounds and fibrosis.
Author Contributions
Conceptualization, F.R., F.T., S.B. and G.M.; methodology, A.M.P., F.C., M.B., E.C., G.M., D.G., P.N., M.F. and F.T.; validation, F.C., G.M., D.G., P.N. and M.B.; formal analysis, F.C., G.M. and M.B.; investigation, F.C., G.M. and M.B.; resources, F.R. and S.B.; data curation, F.R., G.M. and S.B.; writing—original draft preparation, G.M.; writing—review and editing, F.R., G.M. and S.B.; supervision, F.R. and S.B.; funding acquisition, F.R. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EmoLED s.r.l, Fondazione Cassa di Risparmio (grant n. 2017/0771) and the University of Florence.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of A.O.U Città della Salute e della Scienza di Torino—A.O. Ordine Mauriziano di Torino—A.S.L. Città di Torino (protocol code 0073787, 26/07/2017) and by the Hospital Ethical Board of A.O.U di Perugia (protocol code 16806/19/AV, 07/17/2019). The animal study protocol was approved by the Institutional Review Board of the Italian Ministry of Health (protocol code 791/2016-PR).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Acknowledgments
The authors wish to thank Daniele Bani and the Imaging Platform, Dept. Experimental and Clinical Medicine of the University of Florence for valuable support in electron microscopy. The authors wish to thank Azienda Ospedaliera Università degli Studi di Perugia (Perugia, Italy).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-Level Light/Laser Therapy Versus Photobiomodulation Therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photobiomodulation or low-level laser therapy. J. Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Banchelli, M.; Cherchi, F.; Coppi, E.; Fraccalvieri, M.; Rossi, M.; Tatini, F.; Pugliese, A.M.; Degl’innocenti, D.R.; Alfieri, D.; et al. Experimental Study on Blue Light Interaction with Human Keloid-Derived Fibroblasts. Biomedicines 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Magni, G.; Tatini, F.; Banchelli, M.; Cherchi, F.; Rossi, M.; Coppi, E.; Pugliese, A.M.; Rossi degl’Innocenti, D.; Alfieri, D.; et al. Photobiomodulation of Human Fibroblasts and Keratinocytes with Blue Light: Implications in Wound Healing. Biomedicines 2021, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Cicchi, R.; Rossi, F.; Alfieri, D.; Bacci, S.; Tatini, F.; De Siena, G.; Paroli, G.; Pini, R.; Pavone, F.S. Observation of an improved healing process in superficial skin wounds after irradiation with a blue-LED haemostatic device. J. Biophotonics 2016, 9, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Tatini, F.; Siena, G.; De Pavone, F.S.; Alfieri, D.; Cicchi, R.; Rossi, M.; Murciano, N.; Paroli, G.; Vannucci, C.; et al. Blue-LED-Light Photobiomodulation of Inflammatory Responses and New Tissue Formation in Mouse-Skin Wounds. Life 2022, 12, 1564. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Recalcati, S.; Cairo, G. Dual Role of ROS as Signal and Stress Agents: Iron Tips the Balance in favor of Toxic Effects. Oxid. Med. Cell. Longev. 2016, 2016, 8629024. [Google Scholar] [CrossRef] [PubMed]
- Szarka, A.; Lőrincz, T.; Hajdinák, P. Friend or Foe: The Relativity of (Anti)oxidative Agents and Pathways. Int. J. Mol. Sci. 2022, 23, 5188. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Tatini, F.; Bacci, S.; Paroli, G.; De Siena, G.; Cicchi, R.; Pavone, F.S.; Pini, R.; Rossi, F. Blue LED light modulates inflammatory infiltrate and improves the healing of superficial wounds. Photodermatol. Photoimmunol. Photomed. 2020, 36, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Fraccalvieri, M.; Amadeo, G.; Bortolotti, P.; Ciliberti, M.; Garrubba, A.; Mosti, G.; Bianco, S.; Mangia, A.; Massa, M.; Hartwig, V.; et al. Effectiveness of Blue light photobiomodulation therapy in the treatment of chronic wounds. Results of the Blue Light for Ulcer Reduction (B.L.U.R.) Study. Ital. J. Dermatol. Venereol. 2022, 157, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, C.; Purpura, V.; Melandri, D. Blue Led Light in Burns: A New Treatment’s Modality Case Report. J. Clin. Investig. Dermatol. 2021, 9, 5. [Google Scholar]
- Mosti, G.; Gasperini, S. Observations made on three patients suffering from ulcers of the lower limbs treated with Blue Light. Chronic Wound Care Manag. Res. 2018, 5, 23–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).