Bioaccessibility of Phenolic Compounds from Cocoa Shell Subjected to In Vitro Digestion and Its Antioxidant Activity in Intestinal and Hepatic Cells †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Cocoa Shell Flour and Aqueous Extract Preparation
2.3. In Vitro Simulated Digestion
2.4. Extraction and Analysis of Total Phenolic Compounds and Antioxidant Capacity
2.4.1. Extraction of Free and Bound Phenolic Compounds
2.4.2. Total Phenolic Compounds
2.4.3. In Vitro Antioxidant Capacity
2.5. Cell Culture
2.5.1. Cell Viability
2.5.2. Determination of Reactive Oxygen Species (ROS)
2.6. Statistical Analysis
3. Results
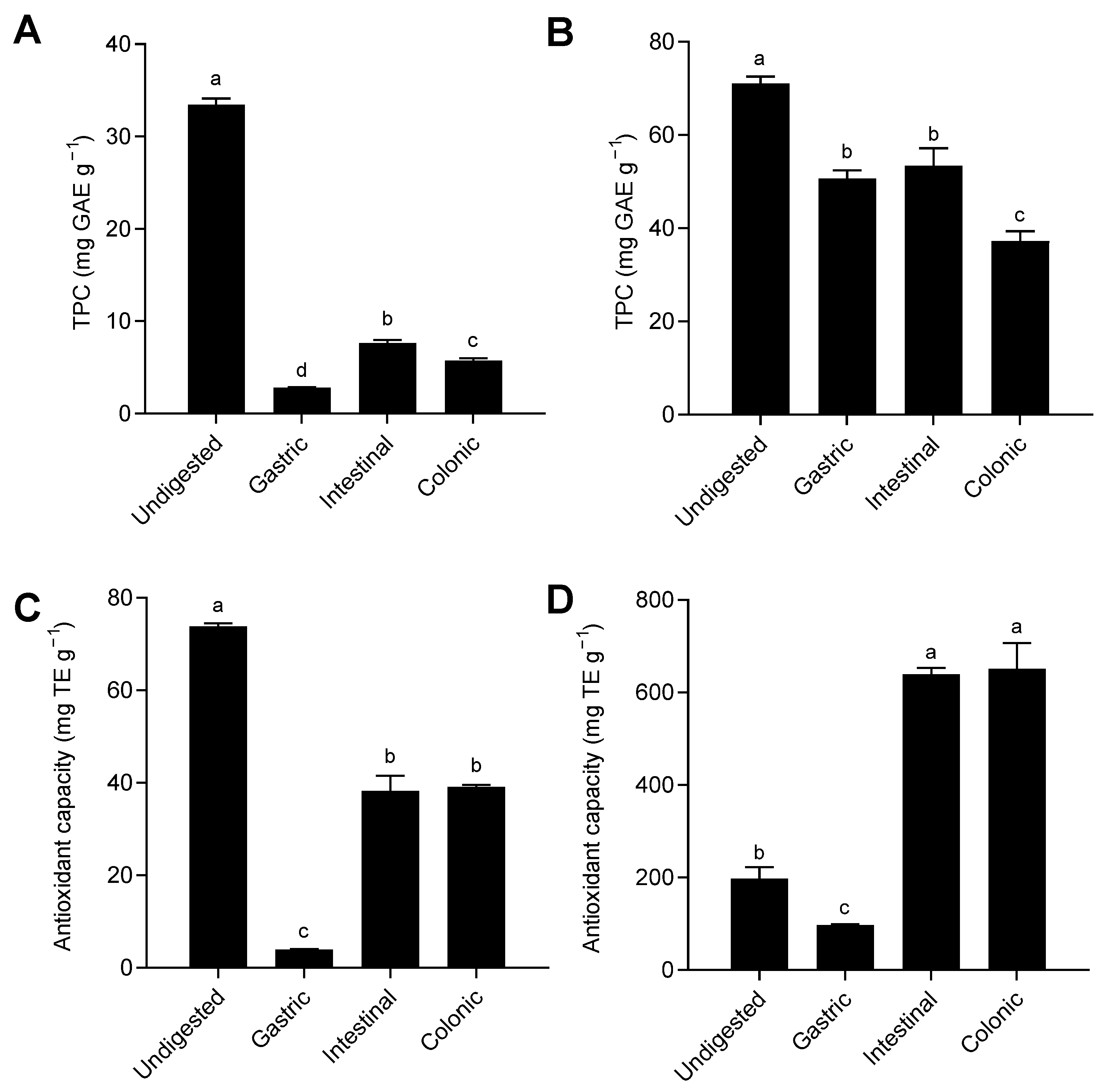
3.1. Bioaccessibility of Total Phenolic Compounds and Antioxidant Activity
3.2. Evaluation of CSF and CSE Cytotoxicity
3.3. Effect of CSF and CSE on ROS Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- International Cocoa Organization ICCO. May 2021 Quarterly Bulletin of Cocoa Statistics. 2021, Volume XLVII. Available online: https://www.icco.org/may-2021-quarterly-bulletin-of-cocoa-statistics/ (accessed on 12 July 2021).
- Azizah, A.H.; Ruslawati, N.N.; Tee, T.S. Extraction and characterization of antioxidant from cocoa by-products. Food Chem. 1999, 64, 199–202. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Aguilera, Y.; Benitez, V.; Gila-Díaz, A.; Rodriguez-Rodriguez, P.; Cobeta, I.M.; de Pablo, A.L.L.; Gonzalez, M.C.; Arribas, S.M. Validation of Cocoa Shell as a Novel Antioxidant Dietary Fiber Food Ingredient: Nutritional Value, Functional Properties, and Safety. Curr. Dev. Nutr. 2020, 4, 773. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Bartolomé, B.; Aguilera, Y.; Martin-Cabrejas, M.A. Extraction of phenolic compounds from cocoa shell: Modeling using response surface methodology and artificial neural networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa bean shell—A by-product with nutritional properties and biofunctional potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martín-Cabrejas, M.A. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019, 10, 4739–4750. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gokmen, V.; Fogliano, V. Release of antioxidant capacity from five plant foods during a multistep enzymatic digestion protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Herrera, T.; Cayuelas, L.T.; Dueñas, M.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Arribas, S.M.; Martín-Cabrejas, M.A. Bioavailability of melatonin from lentil sprouts and its role in the plasmatic antioxidant status in rats. Foods 2020, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Rebollo-Hernanz, M.; Fernández-Gómez, B.; Herrero, M.; Aguilera, Y.; Martín-Cabrejas, M.A.; Uribarri, J.; Del Castillo, M.D. Inhibition of the Maillard reaction by phytochemicals composing an aqueous coffee silverskin extract via a mixed mechanism of action. Foods 2019, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Panak Balentić, J.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.A.; Ramos, S.; Mateos, R.; Granado Serrano, A.B.; Izquierdo-Pulido, M.; Bravo, L.; Goya, L. Protection of human HepG2 cells against oxidative stress by cocoa phenolic extract. J. Agric. Food Chem. 2008, 56, 7765–7772. [Google Scholar] [CrossRef] [PubMed]
- Redgwell, R.; Trovato, V.; Merinat, S.; Curti, D.; Hediger, S.; Manez, A. Dietary fibre in cocoa shell: Characterisation of component polysaccharides. Food Chem. 2003, 81, 103–112. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation between Macrophages and Adipocytes In Vitro. Mol. Nutr. Food Res. 2019, 63, 1801413. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañas, S.; Rebollo-Hernanz, M.; Aguilera, Y.; Benítez, V.; Braojos, C.; Arribas, S.M.; Martín-Cabrejas, M.A. Bioaccessibility of Phenolic Compounds from Cocoa Shell Subjected to In Vitro Digestion and Its Antioxidant Activity in Intestinal and Hepatic Cells. Med. Sci. Forum 2021, 2, 5. https://doi.org/10.3390/CAHD2020-08612
Cañas S, Rebollo-Hernanz M, Aguilera Y, Benítez V, Braojos C, Arribas SM, Martín-Cabrejas MA. Bioaccessibility of Phenolic Compounds from Cocoa Shell Subjected to In Vitro Digestion and Its Antioxidant Activity in Intestinal and Hepatic Cells. Medical Sciences Forum. 2021; 2(1):5. https://doi.org/10.3390/CAHD2020-08612
Chicago/Turabian StyleCañas, Silvia, Miguel Rebollo-Hernanz, Yolanda Aguilera, Vanesa Benítez, Cheyenne Braojos, Silvia M. Arribas, and María A. Martín-Cabrejas. 2021. "Bioaccessibility of Phenolic Compounds from Cocoa Shell Subjected to In Vitro Digestion and Its Antioxidant Activity in Intestinal and Hepatic Cells" Medical Sciences Forum 2, no. 1: 5. https://doi.org/10.3390/CAHD2020-08612
APA StyleCañas, S., Rebollo-Hernanz, M., Aguilera, Y., Benítez, V., Braojos, C., Arribas, S. M., & Martín-Cabrejas, M. A. (2021). Bioaccessibility of Phenolic Compounds from Cocoa Shell Subjected to In Vitro Digestion and Its Antioxidant Activity in Intestinal and Hepatic Cells. Medical Sciences Forum, 2(1), 5. https://doi.org/10.3390/CAHD2020-08612











