Abstract
Tramadol (TRA) is a central-acting opioid whose biological activities are achieved by interaction with several bodily receptors such as μ-opioid receptors. Considering that central-acting drugs may promote oxidative stress, which could lead to neurodegeneration, this work reported the investigation of the redox behavior of TRA by electrochemical and semi-empirical quantum chemistry approaches (i.e., voltammetry and extended Hückel method—EHM) in order to study TRA pro-oxidant features. Electrochemical results showed that TRA exhibited two anodic peaks, namely: 1a at Ep1a ≈ +0.03 V and 2a at Ep2a ≈ +0.8 V; and a cathodic peak at Ep1c ≈ −0.01 V, whereas the quantum chemistry model suggested that the highest occupied molecular orbital n = 0 (HOMO-0) was associated with the tertiary amine in the TRA molecule, while HOMO-1 and the lowest unoccupied molecular orbital n = 0 (LUMO-0) were associated with the aromatic benzene ring. The findings were then used to propose an electrooxidation pathway according to the observations and compared to the literature, which further offered hints about TRA’s pro-oxidant nature. In conclusion, the work reported herein shows that voltammetric and semi-empirical quantum chemistry approaches can be correlated to investigate the redox behavior of CNS-acting compounds.
1. Introduction
Tramadol (TRA) is a central-acting opioid whose biological activities are achieved by interaction with several bodily receptors such as μ-opioid receptors [1]. This compound is widely used in medicine to achieve analgesia, and due to its potential for abuse, it is a controlled substance in most countries [2]. Considering that TRA’s main biological targets are in the central nervous system (CNS), several authors have raised concerns about the potential impact of this drug on homeostasis. In this sense, the literature describes TRA as a pro-oxidant whose chronic use could lead to oxidative stress by stimulating reactive oxygen species (ROS) build up [3].
TRA is commercially available as a racemic mixture, and its core structure includes an aromatic benzene ring with 1,3 substitutions (i.e., methoxyl and a 2-((dimethylamino)methyl) cyclohexanol moiety—DMC), as shown in Figure 1.
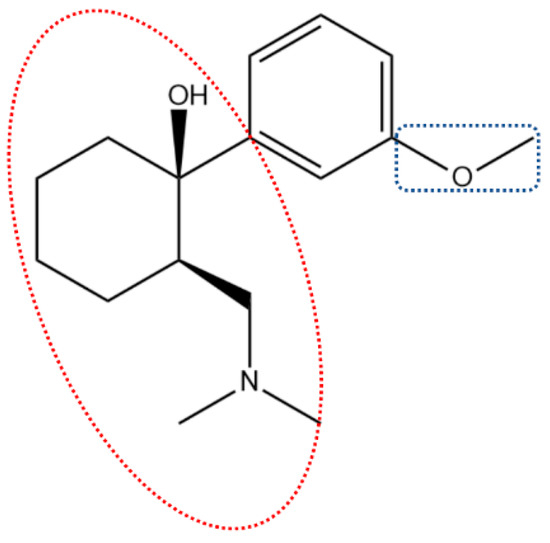
Figure 1.
TRA chemical structure showing noteworthy moieties, namely: methoxyl unit in the blue dotted square and the tertiary amine-bearing DMC moiety in the red dotted ellipse.
The DMC unit has a tertiary amine and a tertiary alcohol, and the richness in C-C σ bonds between sp3 hybridized carbons in this moiety allows some degree of mobility (Figure 1). Owing to the good electron-accepting and donating activities of aromatic compounds [4], as well as the possibility of demethylation and genesis of a phenolic moiety in the TRA molecule upon excitation, some authors describe TRA as having pro-oxidant capacity [1,3]. Nonetheless, long-term use of TRA is often associated with oxidative damage and higher levels of lipid peroxidation biomarkers (i.e., malondialdehyde) [5].
Oxidative stress is a major concern for drugs acting in the CNS due to the susceptibility of neurons to ROS [6,7]. Although the endogenous antioxidant arsenal is highly effective in mopping up highly energetic species, their build up can eventually overcome the activity of catalases, superoxide dismutase and other reductive enzymes, therefore leading to oxidative stress and damage [4,8]. In this sense, the investigation of the pro-oxidant properties of drug candidates intended for CNS-targeted therapies is of utmost relevance in order to aid the development of effective and safe drugs which could also lead to neuroprotection.
Among the several tools which are used to investigate the physicochemical features of compounds are electrochemistry and theoretical chemistry [9,10]. These approaches allow researchers to gather information regarding the thermodynamics of compounds in several energy levels and reaction coordinates, shedding light on the feasibility of chemical transformations such as redox processes [11]. Moreover, reactional kinetics can also be investigated through these tools, thereby allowing a comprehensive study of the physicochemical behavior of compounds.
Nonetheless, several authors employed the electroanalytical approach of voltammetry to study the redox properties of drugs, as well as to correlate the results of ab initio quantum chemistry calculations based on density functional theory [12,13], or semi-empirical approaches consisting of Hartree–Fock formalism such as the extended Hückel method (EHM) [14] to better understand the thermodynamics and kinetics involved in oxidation and reduction reactions, as well as to draw interpretations of how chemical compounds might scavenge ROS or promote their formation.
Therefore, this work aimed at the investigation of TRA redox behavior at a neutral pH (i.e., 7.0) through electrochemical tools and theoretical chemistry calculations in order to better shed light on its reported pro-oxidant properties.
2. Experiments
2.1. Drugs, Solutions and Reagents
TRA chlorhydrate solution (100 mg mL−1); pencil graphite 0.5 mm B; silver filament; potassium phosphate monobasic; potassium phosphate dibasic; potassium ferrocyanide; potassium chloride; potassium chloride solution (3.0 M) saturated with silver chloride; household bleach and distilled water were used. All salts were purchased from Êxodo Científica, Brazil.
2.2. Voltammetric Assays
The voltammetric investigation was performed in a 5.0 mL electrochemical cell containing 900 µL of phosphate-buffered saline (PBS), pH 7.0. TRA solution was diluted to a concentration of 1.0 mg mL−1, after which a volume of 100 µL was added to the electrochemical cell. The experimental system employed a custom potentiostat/galvanostat coupled to a three-electrode arrangement consisting of 0.5 mm B pencil graphite as the working electrode, a AgCl reference electrode and a stainless-steel counter electrode. The pencil graphite had its lateral surface insulated with electrical tape, while the bottom surface was polished with cellulose paper until an even surface was achieved. The reference electrode was constructed upon treatment of a silver filament with bleach until an even gray-colored cover was visible on the wire surface. Thereafter, the treated wire was placed inside a single open-ended glass container, filled with KCl saturated with AgCl solution, and a porous glass frit was added to the end to make the salt bridge.
The voltammetric assay chosen in this work was cyclic voltammetry, due to its fast and reliable execution and optimal performance, to gather information of redox processes taking place at the working electrode surface [15]. The experimental parameters for this assay were: start potential of 0 V, anodic and cathodic scan of 100 mVs−1 between vertex potentials of −0.8 V and +1.2 V and stop potential of 0 V.
2.3. Quantum Chemistry Calculations
In this work, the semi-empirical calculation EHM was employed in parallel to the electrochemical assays in order to aid the discovery of an electro-oxidation pathway for TRA [16]. This method was selected due to its fast and easy execution as well as accessibility when compared to ab initio approaches such as density functional theory. The calculations were performed after steric energy minimization through the force field approach from classical molecular mechanics, i.e., the MM2 method, and also by assisted model building and energy refinement [17,18]. A TRA energy-minimized conformer underwent EHM and subsequent rendering of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO).
3. Results
In order to evaluate TRA redox behavior at the pencil graphite electrode surface, cyclic voltammetry was conducted in triplicate without electrode surface renewal. Results are shown in Figure 2, wherein a blank assay conducted in triplicate with a clean electrode in PBS solution, pH 7.0, is displayed for comparison.
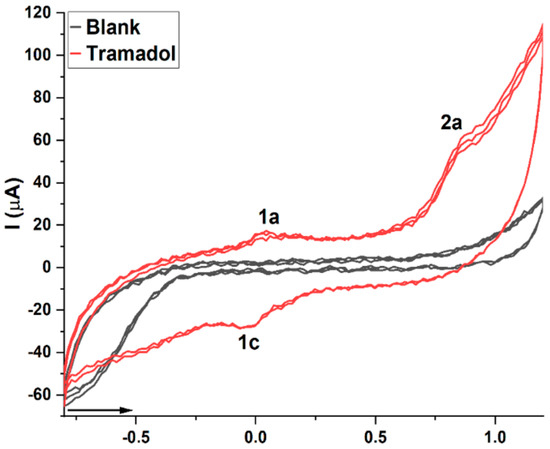
Figure 2.
Cyclic voltammogram of TRA. Anodic peaks 1 and 2a suggest the oxidation of TRA at pencil graphite electrode surface, while cathodic peak 1c suggests reduction. The blank assay was performed in 1 mL PBS solution, pH 7.0.
Through cyclic voltammetry, TRA exhibited two anodic peaks, namely: 1a at Ep1a ≈ +0.03 V and 2a at Ep2a ≈ +0.8 V and a cathodic peak at Ep1c ≈ −0.01 V (Figure 2). The anodic peaks suggest that electroactive moieties in the TRA chemical structure underwent oxidation, while the cathodic peak suggests reduction [19]. Moreover, the faradaic current ratio displayed by peaks 1a and 1c suggests at least some degree of reversibility, hence Ipa/Ipc ≈ 1. Peak 2a, however, did not show reversibility (Figure 2) [19].
After the execution of the electrochemical assay, the TRA molecule underwent steric energy minimization and quantum calculations by EHM. Results of the rendered model are shown in Figure 3 and Table 1.
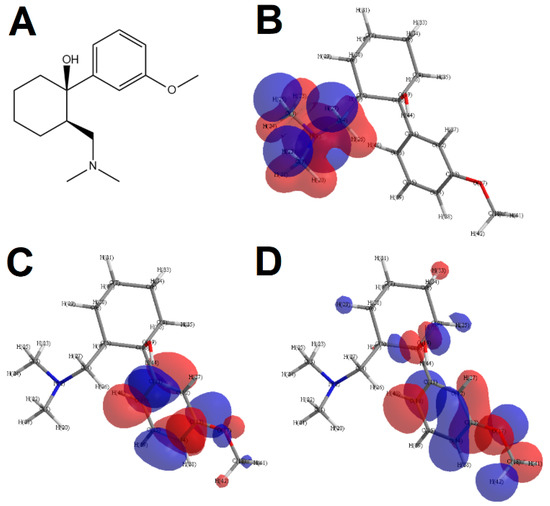
Figure 3.
TRA molecule (A) and graphical rendering of its HOMO-0 (B), LUMO-0 (C) and HOMO-1 (D). Negative charges are rendered in blue while positive charges are rendered in red.

Table 1.
Theoretical energies of HOMO, LUMO and ΔELUMO—HOMO gap for TRA according to EHM.
Results gathered by EHM suggest that HOMO-0 is associated with the tertiary amine in the TRA molecule, while HOMO-1 and LUMO-0 are associated with the aromatic benzene ring (Figure 3B–D). An interesting feature was that in HOMO-1, the rendering of the electric orbital also encompassed the methoxyl moiety (Figure 3D). Considering that smaller energy gaps favor the occurrence redox processes [13], HOMO-n and LUMO-n can be correlated to the thermodynamic feasibility of oxidation or reduction [14]. In this sense, HOMO-0 and HOMO-1 might be associated with oxidative processes undergone by TRA, while LUMO-0 can be correlated to reduction.
After the electrochemical and quantum chemistry investigation of TRA redox processes, the findings of these techniques were compared to allow us to propose an electro-oxidation pathway for this drug. Results are shown in Figure 4.
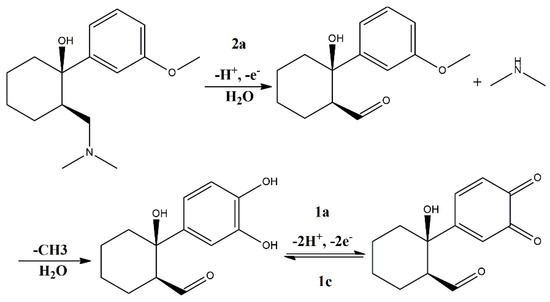
Figure 4.
Proposed electro-oxidation pathway for TRA molecule.
The proposed electro-oxidation pathway shown in Figure 4 shows the irreversible oxidation of the tertiary amine suggested by HOMO-0 graphical rendering, followed by demethylation and the formation of a catechol–quinone system in the aromatic benzene ring, wherein HOMO-1 and LUMO-0 renderings are displayed. Each electro-oxidation step is marked by its particular faradaic signal, i.e., 2a, 1a and 1c.
4. Discussion
The literature reports that electrochemistry is a valuable approach to investigate redox processes in organic compounds, with voltammetry being the most common technique used to characterize electron transfer phenomena on working electrode surfaces [20,21]. In this sense, we employed cyclic voltammetry to gather information of all redox processes undergone by TRA in the selected electric potential interval. Moreover, the use of pencil graphite electrode (a carbon-based electrode matrix) allowed clear peak visualization, which was previously reported in electroanalytical investigations of other central-acting drugs and organic compounds [22].
The anodic and cathodic processes seen in Figure 2 indicate that electroactive moieties in the TRA molecule underwent oxidation and reduction, respectively. Considering that the electric current corresponds to the kinetic parameter of a redox reaction seen in a voltammogram, while the electric potential corresponds to the thermodynamic parameter [20], it can be suggested that more energy is needed to promote the oxidation indicated by peak 2a than to promote the oxidation seen at 1a. In addition, peak 2a was clearly higher, therefore hinting higher kinetics (although capacitive influences cannot be ruled out). Considering that endogenous antioxidants operate at about +0.5 V [7,19], it can be proposed that TRA did not exhibit strong hints of antioxidant capacity, due to its fairly low electrochemical index (Ip1a/Ep1a + Ip2a/Ep2a) [19], while the higher amplitude of peak 1c in comparison to 1a suggests better reduction kinetics of this molecule. Furthermore, since aromatic rings exhibit good acceptor–donor behavior, it can be suggested that this moiety could contribute to TRA reported pro-oxidant behavior [14].
The quantum chemistry calculations by EHM showed that, in the model, HOMO-0 was rendered around the tertiary amine of the DMC moiety, while HOMO-1 and LUMO-0 were rendered in the aromatic benzene ring, with the methoxyl moiety also being encompassed in HOMO-1 rendering (Figure 3). Owing to previous reports, in which the correlations of HOMO and LUMO renderings and energy gaps to possible oxidation sites in molecules are shown [13,14,23], we therefore suggest that the aforementioned regions are the most thermodynamically feasible to undergo redox reactions.
When analyzing the voltammetric and quantum chemistry calculations, it could be suggested that the first oxidation (i.e., HOMO-0) would most likely be in the tertiary amine of the DMC moiety, thereby corresponding to peak 2a. Since the first voltammetric scan starting at E = 0 V did not show strong faradaic output where peak 1a would later appear, we therefore suggest 2a as an oxidation site. Moreover, the oxidation of amines is well reported in the literature regarding voltammetric investigations, with +0.8 V being the most common electric potential value associated with this phenomenon [24,25], which is nonetheless in agreement with our results. Furthermore, considering that the pattern of peaks 1a and 1c suggests a somewhat reversible process, and the HOMO-1 and LUMO-0 were rendered around the aromatic benzene ring, we therefore suggest that the TRA chemical structure undergoes demethylation and then the electrosynthesis of a catechol–quinone system [26]. Taking into account that this system is reported to occur at electric potentials close to the ones depicted here, and also that the proposed electro-oxidation of TRA follows a very similar mechanism to others in the literature [27,28], we therefore showcase how voltammetric and semi-empirical quantum chemistry approaches can be combined to investigate the redox behavior of CNS-acting compounds.
5. Conclusions
This work reported the investigation of the redox behavior of TRA by electrochemical and semi-empirical quantum chemistry approaches (i.e., voltammetry and EHM). Electrochemical results showed that TRA exhibited two anodic peaks and a cathodic peak, whereas the quantum chemistry model suggested that HOMO-0 was associated with the tertiary amine in the TRA molecule, while HOMO-1 and LUMO-0 were associated with the aromatic benzene ring. The findings were then used to propose an electro-oxidation pathway according to the observations and compared to the literature, which further offered hints about TRA’s pro-oxidant nature, therefore showing how voltammetric and semi-empirical quantum chemistry approaches can be correlated to investigate the redox behavior of CNS-acting compounds.
Author Contributions
U.A.C., M.M. and D.V.T. conducted electrochemical experiments, quantum chemistry calculations, analyzed and treated data, as well as wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank CAPES-Brazil for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TRA | tramadol |
| CNS | central nervous system |
| ROS | reactive oxygen species |
| DMC | 2-((dimethylamino)methyl) cyclohexanol |
| EHM | extended Hückel method |
| HOMO | highest occupied molecular orbital |
| LUMO | lowest unoccupied molecular orbital |
References
- Miotto, K.; Cho, A.K.; Khalil, M.A.; Blanco, K.; Sasaki, J.D.; Rawson, R. Trends in Tramadol: Pharmacology, Metabolism, and Misuse. Anesth. Analg. 2017, 124, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Jain, R.; Dhawan, A.; Kaur, A. Assessment of abuse liability of Tramadol among experienced drug users: Double-blind crossover randomized controlled trial. J. Opioid Manag. 2016, 12, 421–430. [Google Scholar] [CrossRef]
- Mohamed, H.M.; Mahmoud, A.M. Chronic exposure to the opioid tramadol induces oxidative damage, inflammation and apoptosis, and alters cerebral monoamine neurotransmitters in rats. Biomed. Pharmacother. 2019, 110, 239–247. [Google Scholar] [CrossRef]
- Thomaz, D.V. Flavonoid chemistry and neuroprotection. Front. Drug Chem. Clin. Res. 2020, 3, 1–3. [Google Scholar] [CrossRef]
- Nagakannan, P.; Shivasharan, B.D.; Thippeswamy, B.S.; Veerapur, V.P. Effect of tramadol on behavioral alterations and lipid peroxidation after transient forebrain ischemia in rats. Toxicol. Mech. Methods 2012, 22, 674–678. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar]
- Thomaz, D.V.; Peixoto, L.F.; de Oliveira, T.S.; Fajemiroye, J.O.; da Silva Neri, H.F.; Xavier, C.H.; Costa, E.A.; Alcantara dos Santos, F.C.; de Souza Gil, E.; Ghedini, P.C. Antioxidant and neuroprotective properties of Eugenia dysenterica leaves. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Thomaz, D.V. The Therapeutic Potential of Phytomedicines from Brazilian Cerrado Herbs against Neurodegenerative Diseases. Am. J. Biomed. Sci. Res. 2020, 7, 374–377. [Google Scholar] [CrossRef]
- Zinola, C.F. Density functional theory. In Electrocatalysis: Computational, Experimental, and Industrial Aspects; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420045451. [Google Scholar]
- Deepa, P.; Kolandaivel, P.; Senthilkumar, K. Theoretical investigation of interaction between psoralen and altretamine with stacked DNA base pairs. Mater. Sci. Eng. C 2012, 32, 423–431. [Google Scholar] [CrossRef]
- Rodrigues, E.S.B.; de Macêdo, I.Y.L.; da Silva Lima, L.L.; Thomaz, D.V.; da Cunha, C.E.P.; Teles de Oliveira, M.; Ballaminut, N.; Alecrim, M.F.; Ferreira de Carvalho, M.; Isecke, B.G.; et al. Electrochemical Characterization of Central Action Tricyclic Drugs by Voltammetric Techniques and Density Functional Theory Calculations. Pharmaceuticals 2019, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- El-Gogary, T.M.; Koehler, G. Interaction of psoralens with DNA-bases (II): An ab initio quantum chemical, density functional theory and second-order MØller-Plesset perturbational study. J. Mol. Struct. Theochem 2009, 895, 57–64. [Google Scholar] [CrossRef]
- Thomaz, D.V.; Santos, P.A. Electrochemical behavior of Methotrexate upon binding to the DNA of different cell lines. In Proceedings of the 1st International Electronic Conference on Cancers: Exploiting Cancer Vulnerability by Targeting the DNA Damage Response, Virtual Event, 1–14 February 2021. [Google Scholar]
- Thomaz, D.V.; de Oliveira, M.G.; Rodrigues, E.S.B.; da Silva, V.B.; Santos, P.A. Dos Physicochemical investigation of psoralen binding to double stranded dna through electroanalytical and cheminformatic approaches. Pharmaceuticals 2020, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Climent, V.; Feliu, J.M. Cyclic voltammetry. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128098943. [Google Scholar]
- Matito, E.; Feixas, F.; Solà, M. Electron delocalization and aromaticity measures within the Hückel molecular orbital method. J. Mol. Struct. Theochem 2007, 811, 3–11. [Google Scholar] [CrossRef]
- Ponder, J.W.; Richards, F.M. An efficient newton-like method for molecular mechanics energy minimization of large molecules. J. Comput. Chem. 1987, 8, 1016–1024. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [Green Version]
- Leite, K.C.D.S.; Garcia, L.F.; Lobón, G.S.; Thomaz, D.V.; Moreno, E.K.G.; Carvalho, M.F.D.; Rocha, M.L.; Santos, W.T.P.D.; Gil, E.D.S. Antioxidant activity evaluation of dried herbal extracts: An electroanalytical approach. Braz. J. Pharmacogn. 2018, 28, 325–332. [Google Scholar] [CrossRef]
- Thomaz, D.V.; Filho, A.M.D.A.; Macedo, I.Y.L.; Rodrigues, E.S.B.; Gil, E.D.S. Predictive Modelling to Study the Electrochemical Behaviour of PdO, TiO2 and Perovskite-Type LaFeO3 Modified Carbon Paste Electrodes. Path Sci. 2019, 5, 4001–4007. [Google Scholar] [CrossRef]
- Mendoza, S.; Bustos, E.; Manríquez, J.; Godínez, L.A. Voltammetric Techniques. In Agricultural and Food Electroanalysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; ISBN 9781118684030. [Google Scholar]
- David, I.G.; Popa, D.E.; Buleandra, M. Pencil graphite electrodes: A versatile tool in electroanalysis. J. Anal. Methods Chem. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabeen, H.; Saleemi, S.; Razzaq, H.; Yaqub, A.; Shakoor, S.; Qureshi, R. Investigating the scavenging of reactive oxygen species by antioxidants via theoretical and experimental methods. J. Photochem. Photobiol. B Biol. 2018, 180, 268–275. [Google Scholar] [CrossRef]
- Thomaz, D.V.; de Oliveira, M.T.; Lobón, G.S.; da Cunha, C.E.P.; Machado, F.B.; Moreno, E.K.G.; Leite, K.C.S.; Ballaminut, N.; Alecrim, M.F.; de Carvalho, M.F.; et al. Development of Laccase-TiO2@carbon paste biosensor for voltammetric determination of paracetamol. Int. J. Electrochem. Sci. 2018, 13. [Google Scholar] [CrossRef]
- Antunes, R.S.; Thomaz, D.V.; Garcia, L.F.; Gil, E.D.S.; Sommerset, V.S.; Lopes, F.M. Determination of Methyldopa and Paracetamol in Pharmaceutical Samples by a Low Cost Genipa americana L. Polyphenol Oxidase Based Biosensor. Adv. Pharm. Bull. 2019, 9, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuVall, S.H.; McCreery, R.L. Control of catechol and hydroquinone electron-transfer kinetics on native and modified glassy carbon electrodes. Anal. Chem. 1999. [Google Scholar] [CrossRef]
- Lütke Eversloh, C.; Schulz, M.; Wagner, M.; Ternes, T.A. Electrochemical oxidation of tramadol in low-salinity reverse osmosis concentrates using boron-doped diamond anodes. Water Res. 2015. [Google Scholar] [CrossRef]
- Zimmermann, S.G.; Schmukat, A.; Schulz, M.; Benner, J.; Gunten, U.V.; Ternes, T.A. Kinetic and mechanistic investigations of the oxidation of tramadol by ferrate and ozone. Environ. Sci. Technol. 2012. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).