Polyoxovanadates Contribution to Pharmacological, Antimicrobial and Toxicological Actions of Vanadium †
Abstract
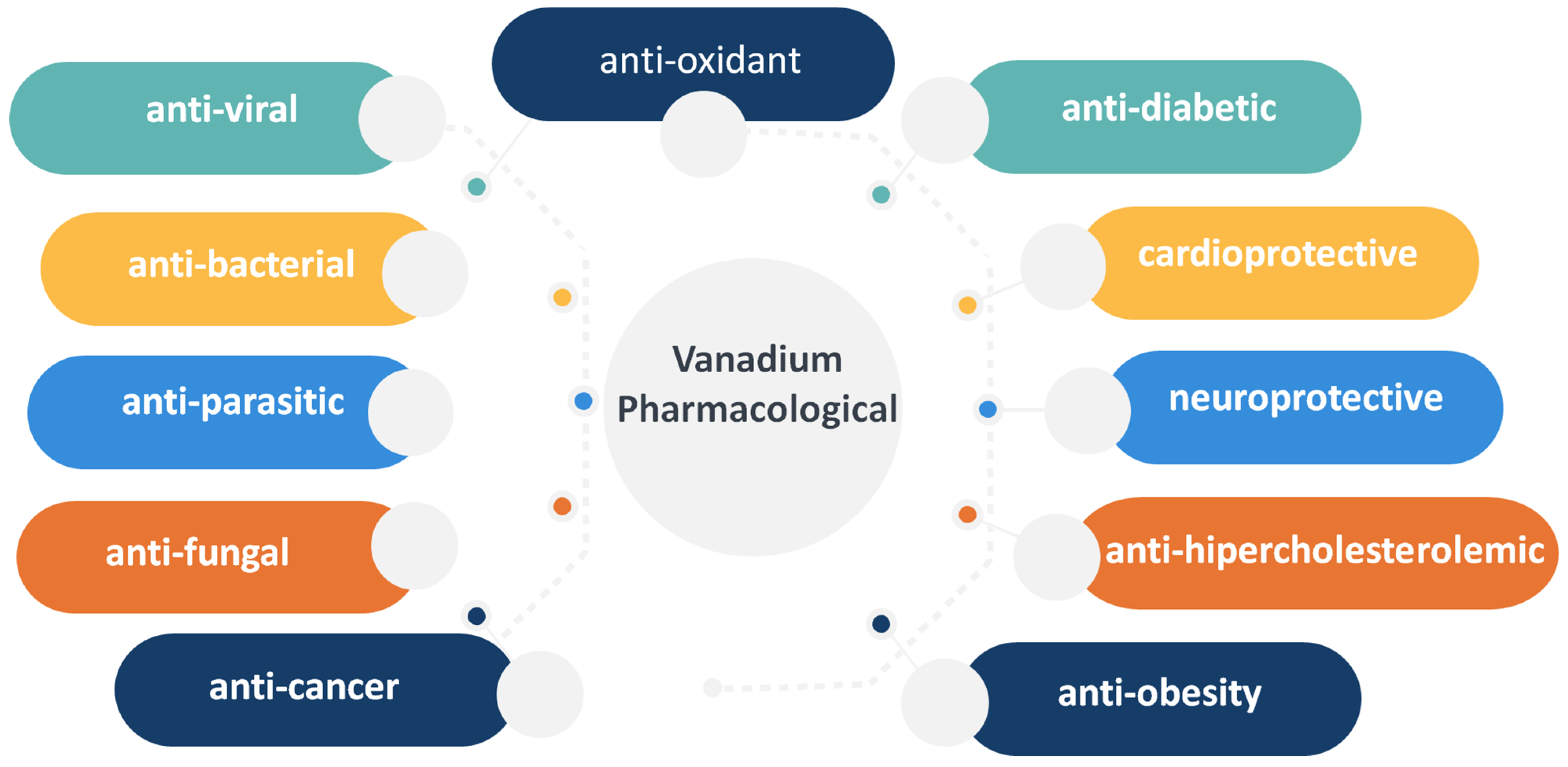
:1. Introduction
2. Vanadium in Biology, Toxicology and Pharmacology
3. POVs’ Contribution to Antimicrobial Activities of Vanadium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elements Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef] [PubMed]
- Harland, B.F.; Harden-Williams, B.A. Is vanadium of human nutritional importance yet? J. Am. Diet. Assoc. 1994, 94, 891–894. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Garribba, E.; Santos, M.F.A.; Santos-Silva, T. Vanadium and proteins: Uptake, transport, structure, activity and function. Coord. Chem. Rev. 2015, 301–302, 49–86. [Google Scholar] [CrossRef]
- Zaporowska, H.; Ścibior, A. Vanadium and its significance in animal cell metabolism. In Vanadium in the Environment. Part 2: Health Effects; Nriagu, J.O., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 1998; pp. 121–133. [Google Scholar]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2021, 454, 214344. [Google Scholar] [CrossRef]
- Aureliano, M.; Marques-da-Silva, D.; Serrano, A.; Martins, J.; Faleiro, L.; Fonseca, C.; Fraqueza, G.; Lagoa, R. Chapter 7, Polyoxometalates with anticancer, antibacterial and antiviral activities. In Polyoxometalates: Advances, Properties, and Applications, 1st ed.; Rubio, L.R., Artetxe, B., Gutiérrez-Zorrilla, J.M., Vilas, J.L., Eds.; Jenny Stanford Publishing: Singapore, 2022; ISBN 9781003277446/9789814968140. [Google Scholar]
- Aureliano, M. The Future Is Bright for Polyoxometalates. BioChem 2022, 2, 8–26. [Google Scholar] [CrossRef]
- Amante, C.; De Sousa-Coelho, A.L.; Aureliano, M. Vanadium and Melanoma: A Systematic Review. Metals 2021, 11, 828. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Element Res. 2018, 188, 68–98. [Google Scholar] [CrossRef]
- Montes, M.R.; Spiaggi, A.J.; Monti, J.L.; Cornelius, F.; Olesen, C.; Garrahan, P.J.; Rossi, R.C. Rb+ occlusion stabilized by vanadate in gastric H+/K+-ATPase at 25 °C. Biochim. et Biophys. Acta (BBA) Biomembr. 2011, 1808, 316–322. [Google Scholar] [CrossRef]
- Aureliano, M. Decavanadate Toxicology and Pharmacological Activities: V10or V1, Both or None? Oxidative Med. Cell. Longev. 2016, 2016, 6103457. [Google Scholar] [CrossRef]
- Fraqueza, G.; Fuentes, J.; Krivosudský, L.; Dutta, S.; Mal, S.S.; Roller, A.; Giester, G.; Rompel, A.; Aureliano, M. Inhibition of Na+/K+- and Ca2+-ATPase activities by phosphotetradecavanadate. J. Inorg. Biochem. 2019, 197, 110700. [Google Scholar] [CrossRef]
- Kita, D.H.; de Andrade, G.A.; Missina, J.M.; Postal, K.; Boell, V.K.; Santana, F.S.; Zattoni, I.F.; Zanzarini, I.D.S.; Moure, V.R.; Rego, F.G.D.M.; et al. Polyoxovanadates as new P-glycoprotein inhibitors: Insights into the mechanism of inhibition. FEBS Lett. 2022, 596, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Mitochondria as a target for decavanadate toxicity in Sparus aurata heart. Aquat. Toxicol. 2007, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Samart, N.; Arhouma, Z.; Kumar, S.; Murakami, H.A.; Crick, D.C.; Crans, D.C. Decavanadate Inhibits Mycobacterial Growth More Potently Than Other Oxovanadates. Front. Chem. 2018, 6, 519. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. Vanadium. Its role in humans. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 139–167. [Google Scholar]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef]
- Rehder, D. Vanadium in health issues. ChemTexts 2018, 4, 20. [Google Scholar] [CrossRef]
- Pessoa, J.C. Thirty years through vanadium chemistry. J. Inorg. Biochem. 2015, 147, 4–24. [Google Scholar] [CrossRef]
- Turner, T.L.; Nguyen, V.H.; McLauchlan, C.C.; Dymon, Z.; Dorsey, B.M.; Hooker, J.D.; Jones, M.A. Inhibitory effects of decavanadate on several enzymes and Leishmania tarentolae In Vitro. J. Inorg. Biochem. 2012, 108, 96–104. [Google Scholar] [CrossRef]
- Mendez, R.S.; Dorsey, B.M.; Mclauchlan, C.C.; Beio, M.; Turner, T.; Nguyen, V.H.; Su, A.; Beynon, W.; Friesen, J.A.; Jones, M. Vanadium Complexes Are in vitro Inhibitors of Leishmania Secreted Acid Phosphatases. Int. J. Chem. 2013, 6, 35. [Google Scholar] [CrossRef]
- Adriazola, I.O.; Amaral, A.E.D.; Amorim, J.C.; Correia, B.L.; Petkowicz, C.L.O.; Mercê, A.L.R.; Noleto, G.R. Macrophage activation and leishmanicidal activity by galactomannan and its oxovanadium (IV/V) complex in vitro. J. Inorg. Biochem. 2014, 132, 45–51. [Google Scholar] [CrossRef]
- Scalese, G.; Machado, I.; Salinas, G.; Pérez-Díaz, L.; Gambino, D. Heteroleptic Oxidovanadium(V) Complexes with Activity against Infective and Non-Infective Stages of Trypanosoma cruzi. Molecules 2021, 26, 5375. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Sumrra, S.H.; Youssoufi, M.H.; Hadda, T.B. Metal based biologically active compounds: Design, synthesis, and antibacterial/antifungal/cytotoxic properties of triazole-derived Schiff bases and their oxovanadium(IV) complexes. Eur. J. Med. Chem. 2010, 45, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Pahontu, E.; Julea, F.; Rosu, T.; Purcarea, V.; Chumakov, Y.; Petrenco, P.; Gulea, A. Antibacterial, antifungal and in vitro antileukaemia activity of metal complexes with thiosemicarbazones. J. Cell. Mol. Med. 2015, 19, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, P.; Akila, E.; Rani, M.U.; Rajavel, R. Synthesis, spectral characterization, electrochemical, anti-microbial, DNA binding and cleavage studies of new binuclear Schiff base metal(II) complexes derived from o-hydroxyacetophenone. J. Saudi Chem. Soc. 2016, 20, 625–634. [Google Scholar] [CrossRef]
- Li, H.; Gong, H.; Qi, Y.; Li, J.; Ji, X.; Sun, J.; Tian, R.; Bao, H.; Song, X.; Chen, Q.; et al. In vitro and in vivo antifungal activities and mechanism of heteropolytungstates against Candida species. Sci. Rep. 2017, 7, 16942. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yang, W.; Zhao, M.; Tian, R.; Zhang, B.; Qi, Y. In Vitro Anticandidal Activity and Mechanism of a Polyoxovanadate Functionalized by Zn-Fluconazole Complexes. Molecules 2018, 23, 1122. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, L.; Marques, A.; Martins, J.; Jordão, L.; Nogueira, I.; Gumerova, N.I.; Rompel, A.; Aureliano, M. The Preyssler-Type Polyoxotungstate Exhibits Anti-Quorum Sensing, Antibiofilm, and Antiviral Activities. Biology 2022, 11, 994. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraqueza, G.; Aureliano, M. Polyoxovanadates Contribution to Pharmacological, Antimicrobial and Toxicological Actions of Vanadium. Med. Sci. Forum 2022, 11, 8. https://doi.org/10.3390/BiTaP-12844
Fraqueza G, Aureliano M. Polyoxovanadates Contribution to Pharmacological, Antimicrobial and Toxicological Actions of Vanadium. Medical Sciences Forum. 2022; 11(1):8. https://doi.org/10.3390/BiTaP-12844
Chicago/Turabian StyleFraqueza, Gil, and Manuel Aureliano. 2022. "Polyoxovanadates Contribution to Pharmacological, Antimicrobial and Toxicological Actions of Vanadium" Medical Sciences Forum 11, no. 1: 8. https://doi.org/10.3390/BiTaP-12844
APA StyleFraqueza, G., & Aureliano, M. (2022). Polyoxovanadates Contribution to Pharmacological, Antimicrobial and Toxicological Actions of Vanadium. Medical Sciences Forum, 11(1), 8. https://doi.org/10.3390/BiTaP-12844






