Plant-Based Nanoantibiotics: An Effective Strategy to Overcoming Antibiotic Resistance †
Abstract
:1. Introduction
2. Plant-Based Antimicrobials
2.1. Phenols and Polyphenols
2.2. Essential Oils
3. Nanoencapsulation of Plant-Based Antimicrobials
3.1. Antimicrobial Nanoemulsions
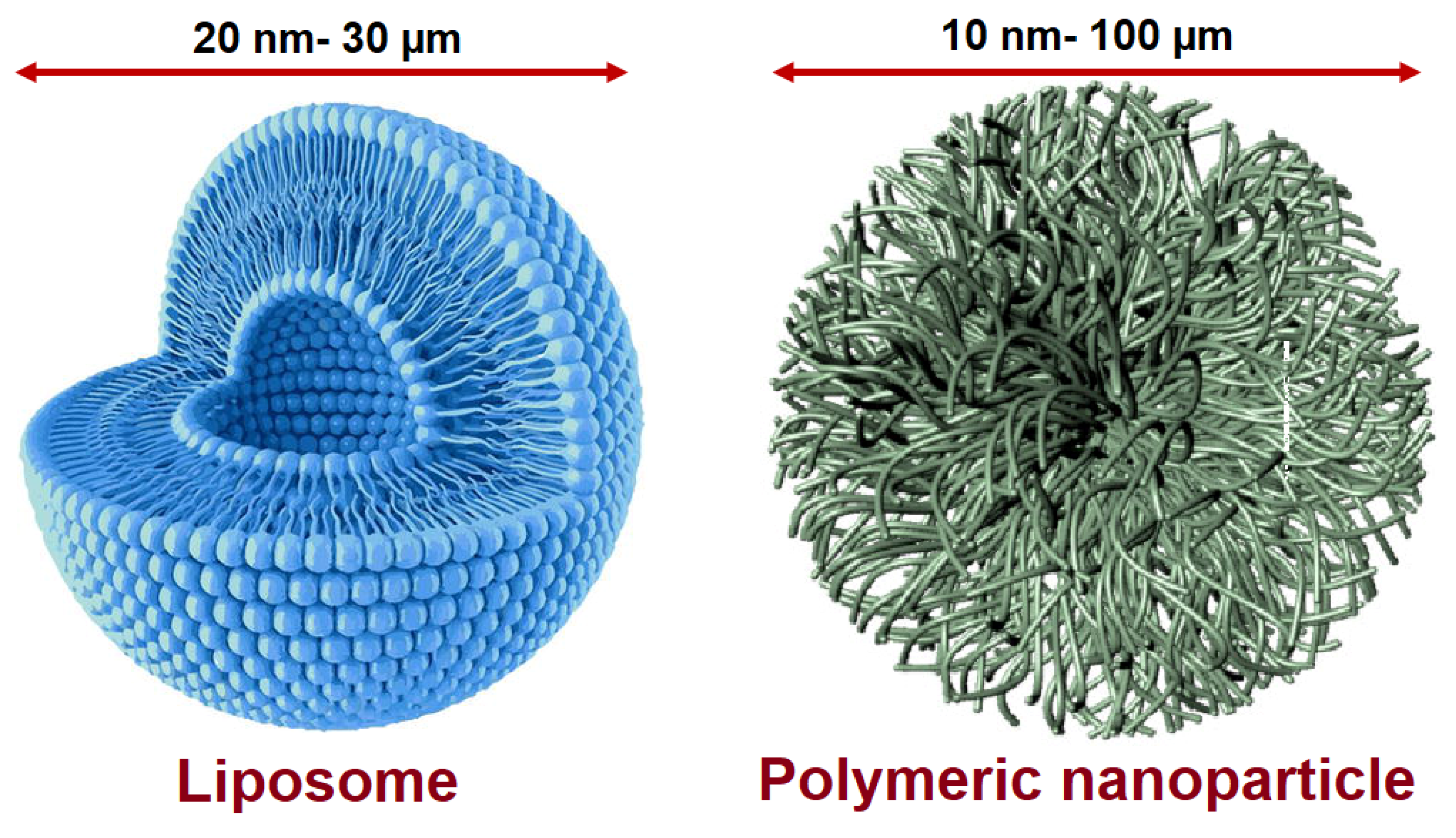
3.2. Liposome-Based Nanoantibiotics
3.3. Polymer-Based Nanoantibiotics
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, W.; Zhang, Q.; Li, W.; Yuan, M.; Zhou, J.; Hua, L.; Chen, Y.; Ye, C.; Ma, Y. Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J. Control. Release 2020, 317, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Omolo, C.A.; Kalhapure, R.S.; Agrawal, N.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. A hybrid of mPEG-b-PCL and G1-PEA dendrimer for enhancing delivery of antibiotics. J. Control. Release 2018, 290, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Amato, D.N.; Amato, D.V.; Mavrodi, O.V.; Braasch, D.A.; Walley, S.E.; Douglas, J.R.; Mavrodi, D.V.; Patton, D.L. Destruction of Opportunistic Pathogens via Polymer Nanoparticle-Mediated Release of Plant-Based Antimicrobial Payloads. Adv. Healthc. Mater. 2016, 5, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Deepika; Dubey, N.K. Eugenol loaded chitosan nanoemulsion for food protection and inhibition of Aflatoxin B1 synthesizing genes based on molecular docking. Carbohydr. Polym. 2021, 255, 117339. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Das, A.K.; Dhar, P.; Nanda, P.K.; Chatterjee, N. Nanoemulsion-Based Technologies for Delivering Natural Plant-Based Antimicrobials in Foods. Front. Sustain. Food Syst. 2021, 5, 643208. [Google Scholar] [CrossRef]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Nanda, P.K.; Chowdhury, N.R.; Dandapat, P.; Gagaoua, M.; Chauhan, P.; Pateiro, M.; Lorenzo, J.M. Application of pomegranate by-products in muscle foods: Oxidative indices, colour stability, shelf life and health benefits. Molecules 2021, 26, 467. [Google Scholar] [CrossRef] [PubMed]
- Coppo, E.; Marchese, A. Antibacterial Activity of Polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, H.; Pour Nikfardjam, M.S. Influence of phenolic compounds and tannins on wine-related microorganisms. In Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin/Heidelberg, Germany, 2017; pp. 421–454. [Google Scholar] [CrossRef]
- Fadli, M.; Chevalier, J.; Saad, A.; Mezrioui, N.E.; Hassani, L.; Pages, J.M. Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2011, 38, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Kon, K. FIGHTING MULTIDRUG RESISTANCE WITH HERBAL EXTRACTS, ESSENTIAL OILS AND THEIR COMPONENTS; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123985392. [Google Scholar]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Saad, A.M.; Salem, H.M.; Ashry, N.M.; Abo Ghanima, M.M.; Shukry, M.; Swelum, A.A.; Taha, A.E.; El-Tahan, A.M.; et al. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: A comprehensive review. Poult. Sci. 2022, 101, 101584. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Carpena, M.; Kowalska-Góralska, M.; Garcia-Perez, P.; Sunita, K.; Bontempi, E.; Dey, A.; Prieto, M.A.; Proćków, J.; Simal-Gandara, J. Safer plant-based nanoparticles for combating antibiotic resistance in bacteria: A comprehensive review on its potential applications, recent advances, and future perspective. Sci. Total Environ. 2022, 821, 153472. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Martin-Belloso, O.; Julian McClements, D. Excipient nanoemulsions for improving oral bioavailability of bioactives. Nanomaterials 2016, 6, 17. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Cheow, W.S. Nano-antibiotics in chronic lung infection therapy against Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2014, 116, 772–785. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, S.S.; Carpena, M.; Echave, J.; Chamorro, F.; Pereira, A.G.; Barral-Martinez, M.; Soria-Lopez, A.; Cassani, L.; Silva, A.; Xiao, J.; et al. Plant-Based Nanoantibiotics: An Effective Strategy to Overcoming Antibiotic Resistance. Med. Sci. Forum 2022, 12, 12. https://doi.org/10.3390/eca2022-12727
Mansour SS, Carpena M, Echave J, Chamorro F, Pereira AG, Barral-Martinez M, Soria-Lopez A, Cassani L, Silva A, Xiao J, et al. Plant-Based Nanoantibiotics: An Effective Strategy to Overcoming Antibiotic Resistance. Medical Sciences Forum. 2022; 12(1):12. https://doi.org/10.3390/eca2022-12727
Chicago/Turabian StyleMansour, Sepidar Seyyedi, Maria Carpena, Javier Echave, Franklin Chamorro, Antia G. Pereira, Marta Barral-Martinez, Anton Soria-Lopez, Lucia Cassani, Aurora Silva, Jianbo Xiao, and et al. 2022. "Plant-Based Nanoantibiotics: An Effective Strategy to Overcoming Antibiotic Resistance" Medical Sciences Forum 12, no. 1: 12. https://doi.org/10.3390/eca2022-12727
APA StyleMansour, S. S., Carpena, M., Echave, J., Chamorro, F., Pereira, A. G., Barral-Martinez, M., Soria-Lopez, A., Cassani, L., Silva, A., Xiao, J., Simal-Gandara, J., & Prieto, M. A. (2022). Plant-Based Nanoantibiotics: An Effective Strategy to Overcoming Antibiotic Resistance. Medical Sciences Forum, 12(1), 12. https://doi.org/10.3390/eca2022-12727














