The Promising Shadow of Nanohybrid Liposomal Cerasomes towards the Treatment of Diabetes Mellitus †
Abstract
:1. Introduction
2. Physicochemical Properties of Cerasomes
3. Cerasomes in the Drug Delivery System
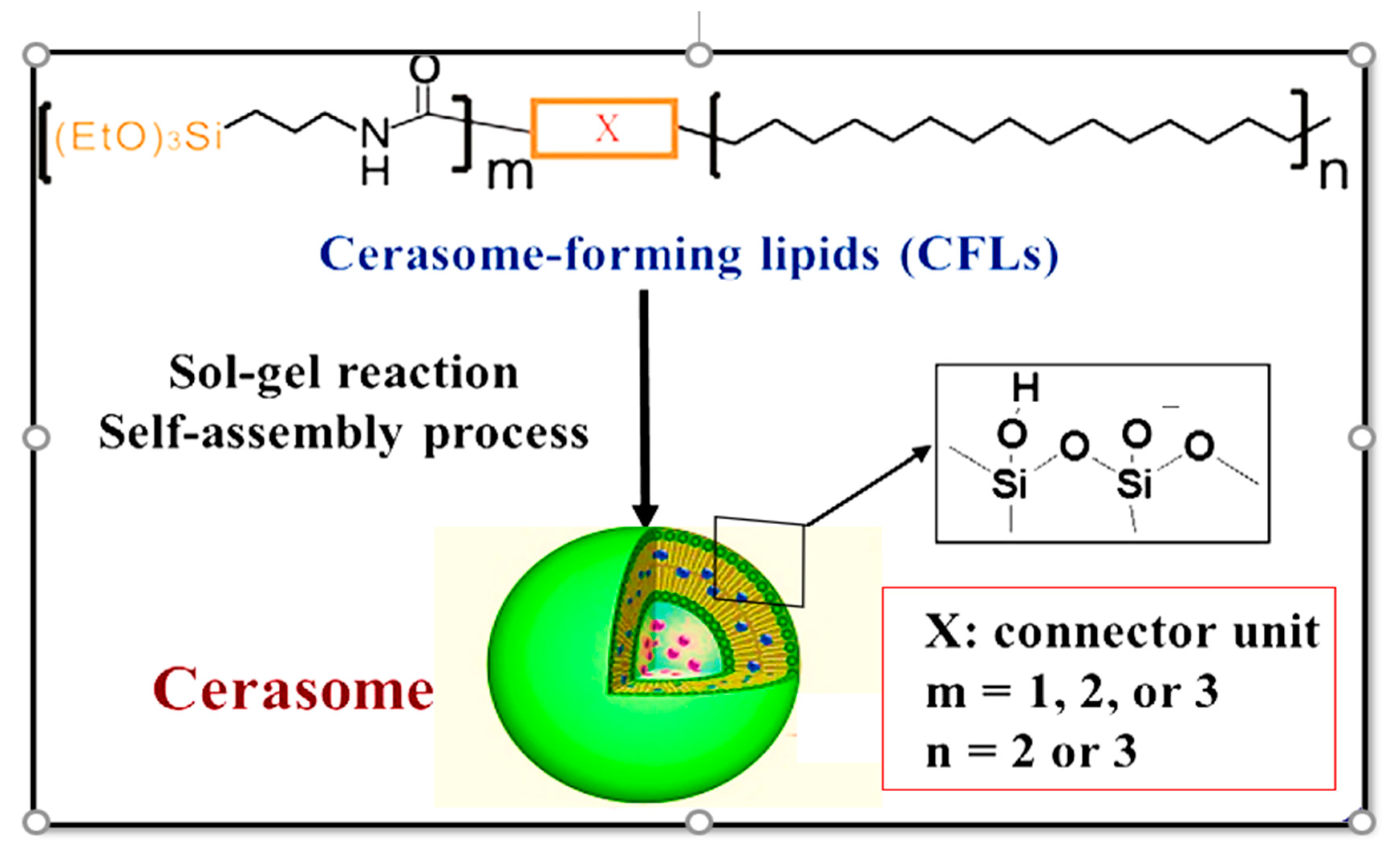
3.1. Design and Formulation of Cerasomes
3.2. Characterization of Cerasomes Concerning Their Morphology
- Morphology of the aggregate
- b.
- Surface siloxane network
- c.
- Phase separation and phase transition behavior
3.3. Cerasomes in Drug Delivery System
- a.
- Cerasomes as potent drug carriers
- b.
- Molecular devices for processing of information
3.4. Cerasomes towards the Treatment of Diabetes
4. Upcoming Challenges and Future Prospects of Cerasomes in Diabetes Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karamanou, M.; Protogerou, A.; Tsoucalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, K.; Mishra, V.; Hemani, V.; Verma, A.; Jain, A.; Jain, S.; Charyulu, R.N. Reverse pharmacology of phytoconstituents of food and plant in the management of diabetes: Current status and perspectives. Trends Food Sci. Technol. 2021, 110, 594–610. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas 9th Edition. Global Fact Sheet. 2019. Available online: https://diabetesatlas.org/upload/resources/material/20201028_180116_Global-factsheet-final.pdf (accessed on 13 July 2021).
- IDF Diabetes Atlas 9th Edition. Demographic and Geographic Outline. 2019. Available online: https://diabetesatlas.org/en/sections/demographic-and-geographic-outline.html (accessed on 13 July 2021).
- He, L.; Sabet, A.; Djedjos, S.; Miller, R.; Sun, X.; Hussain, M.A.; Radovick, S.; Wondisford, F.E. Metformin and Insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 2009, 137, 635–646. [Google Scholar] [CrossRef]
- Lin, S.H.; Cheng, P.C.; Tu, S.T.; Hsu, S.R.; Cheng, Y.C.; Liu, Y.H. Effect of Metformin monotherapy on serum lipid profile in statin-naïve individuals with newly diagnosed type 2 diabetes mellitus: A cohort study. PeerJ 2018, 6, e4578. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Shurrab, N.T.; Arafa, E.-S.A. Metformin: A review of its therapeutic efficacy and adverse effects. Obes. Med. 2020, 17, 100186. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, X.; Luo, C.; He, Z. Emerging nanoparticulate drug delivery systems of metformin. J. Pharm. Investig. 2020, 50, 219–230. [Google Scholar] [CrossRef]
- Proks, P.; Reimann, F.; Green, N.; Gribble, F.; Ashcroft, F. Sulfonylurea stimulation of insulin secretion. Diabetes 2002, 51 (Suppl. 3), S368–S376. [Google Scholar] [CrossRef]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Pan, H.; Dai, Z. Liposomal nanohybrid cerasomes for controlled insulin release. RSC Adv. 2014, 4, 42808–42815. [Google Scholar] [CrossRef]
- Yue, X.; Dai, Z. Recent advances in liposomal nanohybrid cerasomes as promising drug nanocarriers. Adv. Colloid Interface Sci. 2013, 207, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, J.; Yasuhara, K. Microscopy & micro/nano-imaging techniques: TEM. In Supramolecular Chemistry: From Molecules to Nanomaterials, 1st ed.; Steed, J.W., Gale, P.A., Eds.; John Wiley & Sons: Chichester, UK, 2012; Volume 1, pp. 231–250. [Google Scholar]
- Katagiri, K.; Hashizume, M.; Ariga, K.; Terashima, T.; Kikuchi, J. Preparation and characterization of a novel organic-inorganic nanohybrid “cerasome” formed with a liposomal membrane and silicate surface. Chem. Eur. J. 2007, 13, 5272–5281. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kikuchi, J. Supramolecular assemblies formed with synthetic peptide lipids. Functional models of biomembranes and enzymes. In Bioorganic Chemistry Frontiers, 1st ed.; Dugas, H., Ed.; Springer: Berlin, Germany, 1991; Volume 2, pp. 73–113. [Google Scholar]
- Matsui, K.; Sando, S.; Sera, T.; Aoyama, Y.; Sasaki, Y.; Komatsu, T.; Terashima, T.; Kikuchi, J. Cerasome as an infusible, cell-friendly, and serum-compatible transfection agent in a viral size. J. Am. Chem. Soc. 2006, 128, 3114–3115. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Matsui, K.; Aoyama, Y.; Kikuchi, J. Cerasome as an infusible and cell-friendly gene carrier: Synthesis of cerasome-forming lipids and transfection using cerasome. Nat. Protoc. 2006, 1, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Sasaki, Y.; Komatsu, T.; Mukai, M.; Kikuchi, J.; Aoyama, Y. RNAi gene silencing using cerasome as a vial-size siRNA-carrier free from fusion and crosslinking. Bioorg. Med. Chem. Lett. 2007, 17, 3935–3938. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Yamada, M.; Terashima, T.; Wang, J.-F.; Hashizume, M.; Fan, S.-D.; Kikuchi, J. Construction of intermolecular communication system on “cerasome” as an organic-inorganic nanohybrid. Kobunshi Ronbunshu 2004, 61, 541–546. [Google Scholar] [CrossRef]
- Dai, Z.-F.; Tian, W.-J.; Yue, X.-L.; Zheng, Z.-Z.; Qi, J.-J.; Tamai, N.; Kikuchi, J. Efficient fluorescence resonance energy transfer in highly stable liposomal nanohybrid cerasome. Chem. Commun. 2009, 15, 2032–2034. [Google Scholar] [CrossRef]
- Yucel, C.; Karatoprak, G.S.; Aktas, Y. Nanoliposomal resveratrol as a novel approach to treatment of diabetes mellitus. J. Nanosci. Nanotechnol. 2018, 18, 3856–3864. [Google Scholar] [CrossRef]
- Das, R.J.; Baishya, K.; Pathak, K. Recent advancement of lipid drug conjugate as nanoparticulate drug delivery system. Int. Res. J. Pharm. 2013, 4, 73–78. [Google Scholar]
- Roumie, C.L.; Hung, A.M.; Greevy, R.A.; Grijalva, C.G.; Liu, X.; Murff, H.J.; Elasy, T.A.; Griffin, M.R. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: A cohort study. Ann. Intern. Med. 2012, 157, 601–610. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saikia, R.; Pathak, K.; Das, A.; Ahmad, M.Z. The Promising Shadow of Nanohybrid Liposomal Cerasomes towards the Treatment of Diabetes Mellitus. Med. Sci. Forum 2022, 10, 5. https://doi.org/10.3390/IECH2022-12295
Saikia R, Pathak K, Das A, Ahmad MZ. The Promising Shadow of Nanohybrid Liposomal Cerasomes towards the Treatment of Diabetes Mellitus. Medical Sciences Forum. 2022; 10(1):5. https://doi.org/10.3390/IECH2022-12295
Chicago/Turabian StyleSaikia, Riya, Kalyani Pathak, Aparoop Das, and Mohammad Zaki Ahmad. 2022. "The Promising Shadow of Nanohybrid Liposomal Cerasomes towards the Treatment of Diabetes Mellitus" Medical Sciences Forum 10, no. 1: 5. https://doi.org/10.3390/IECH2022-12295
APA StyleSaikia, R., Pathak, K., Das, A., & Ahmad, M. Z. (2022). The Promising Shadow of Nanohybrid Liposomal Cerasomes towards the Treatment of Diabetes Mellitus. Medical Sciences Forum, 10(1), 5. https://doi.org/10.3390/IECH2022-12295







