Total Antioxidant Capacity and Phenolic Content of 17 Mediterranean Functional Herbs and Wild Green Extracts from North Aegean, Greece †
Abstract
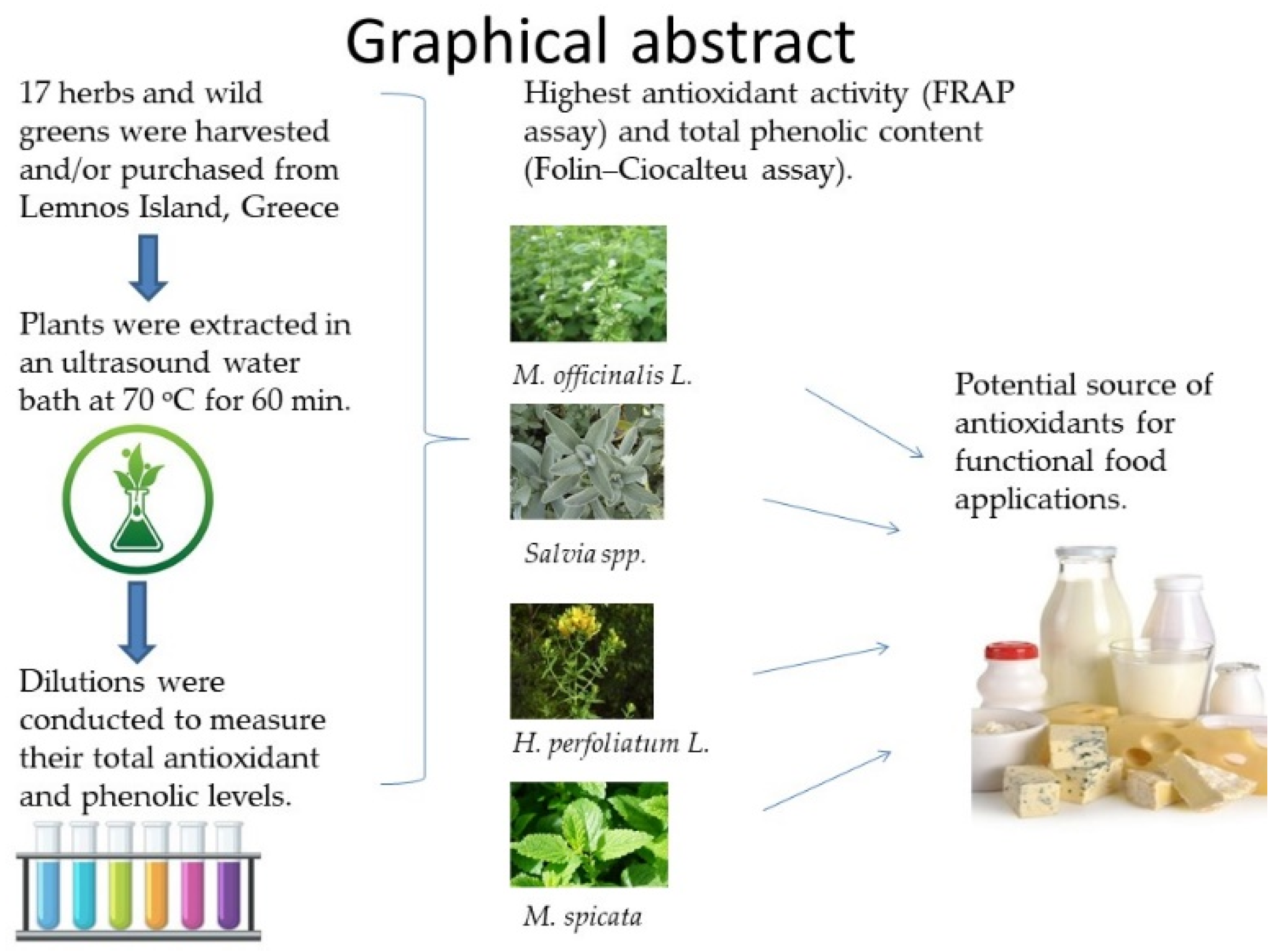
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallo, M. Novel foods: Algae. In Reference Module in Food Science; Ferranti, P., Berry, E., Jock, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 300–306. [Google Scholar]
- Nethravathy, M.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Brown, L.; Caligiuri, S.P.; Brown, D.; Pierce, G.N. Clinical trials using functional foods provide unique challenges. J. Funct. Foods 2018, 45, 233–238. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. S. Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Koutelidakis, A.; Dimou, C. The effects of functional food and bioactive compounds on biomarkers of cardiovascular diseases. In Functional Foods Text Book, 1st ed.; Martirosyan, D., Ed.; Functional Food Center: Dallas, TX, USA, 2017; pp. 89–117. [Google Scholar]
- Martirosyan, D.; Miller, E. Bioactive Compounds: The Key to Functional Foods. Bioact. Compd. Health Dis. 2018, 1, 36. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.S.; Coelho, M.S.; de las Mercedes Salas-Mellado, M. Bioactive Compounds as Ingredients of Functional Foods: Polyphenols, Carotenoids, Peptides from Animal and Plant Sources New. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 129–142. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Nyakudya, T.T.; Tshabalala, T.; Dangarembizi, R.; Erlwanger, K.H.; Ndhlala, A.R. The potential therapeutic value of medicinal plants in the management of metabolic disorders. Molecules 2020, 25, 2669. [Google Scholar] [CrossRef]
- Carlsen, Μ.; Halvorsen, Β.; Siv, K.; Bøhn, K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Eur. Food Res. Technol. 2011, 233, 3–376. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The “QUENCHER” approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Tarakci, Z.; Temiz, H. A review of the chemical, biochemical and antimicrobial aspects of Turkish Otlu (herby) cheese. Int. J. Dairy Technol. 2009, 62, 354–360. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [Green Version]
- Spanos, G.A.; Wrolstad, R.E. Influence of Processing and Storage on the Phenolic Composition of Thompson Seedless Grape Juice. J. Agric. Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef]
- Kapsokefalou, M.; Zhu, L.; Miller, D.D. Adding iron to green tea may decrease its antioxidant capacity in rats after an oral dose of the mixture. Nutr. Res. 2006, 26, 480–485. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from Oregano: Occurrence, biological activity and health benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P. Evaluation of antioxidant activity, toxicity, and phenolic profile of aqueous extracts of chamomile (Matricaria chamomilla L.) and sage (Salvia ocinalis L.) prepared at different temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef] [Green Version]
- Boneza, M.M.; Niemeyer, E.D. Cultivar affects the phenolic composition and antioxidant properties of commercially available lemon balm (Melissa officinalis L.) varieties. Ind. Crops Prod. 2018, 112, 783–789. [Google Scholar] [CrossRef]
- Saija, A.; Speciale, A.; Trombetta, D.; Leto, C.; Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Bonsangue, G.; Gennaro, M.C.; et al. Phytochemical, Ecological and Antioxidant Evaluation of Wild Sicilian Thyme: Thymbra capitata (L.) Cav. Chem. Biodivers. 2016, 13, 1641–1655. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Koutelidakis, A.E.; Rallidis, L.; Koniari, K.; Panagiotakos, D.; Komaitis, M.; Zampelas, A.; Anastasiou-Nana, M.; Kapsokefalou, M. Effect of green tea on postprandial antioxidant capacity, serum lipids, C-reactive protein and glucose levels in patients with coronary artery disease. Eur. J. Nutr. 2014, 53, 479–486. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Ulewicz-Magulska, B.; Wesolowski, M. Total Phenolic Contents and Antioxidant Potential of Herbs Used for Medical and Culinary Purposes. Plant Foods Hum. Nutr. 2019, 74, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shori, A.B. Inclusion of phenolic compounds from different medicinal plants to increase α-amylase inhibition activity and antioxidants in yogurt. J. Taibah Univ. Sci. 2020, 14, 1000–1008. [Google Scholar] [CrossRef]
| Plant Sample | Total Phenol (mg GA/g of Sample) | Total Antioxidant (mmol Fe2+/L) |
|---|---|---|
| Ocimum basilicum L. | 16.51 ± 3.13 | 7.42 ± 0.71 |
| Matricaria chamomilla L. | 9.57 ± 3.12 | 5.31 ± 0.81 |
| Crataegus azarolus L. | 1.32 ± 0.52 | 0.57 ± 0.08 |
| Thymbra capitata L. | 36.68 ± 8.64 | 12.29 * ± 2.07 |
| Rosmarinus officinalis L. | 10.78 ± 2.86 | 4.52 ± 1.47 |
| Melissa officinalis L. | 61.70 ± 15.31 | 30.58 * ± 5.15 |
| Mentha spicata | 39.61 ± 31.83 | 26.97 * ± 11.28 |
| Pistacia lentiscus var. chia | 0.18 ± 0.05 | 0.06 ± 0.04 |
| Origanum sp. | 32.09 ± 12.72 | 12.11 ± 3.97 |
| Thymus sp. | 18.42 ± 2.65 | 4.40 ± 2.22 |
| Pancratium maritimum | 1.09 ± 0.31 | 0.18 ± 0.03 |
| Otanthus maritimus | 1.33 ± 0.38 | 0.47 ± 0.06 |
| Crithmum maritimum L. | 27.09 ± 13.82 | 0.28 ± 0.18 |
| Salicornia europaea L. | 21.32 ± 6.39 | 0.12 ± 0.05 |
| Salvia spp. | 946.91 * ± 126.21 | 4.72 ± 0.61 |
| Hypericum perfoliatum L. and Hypericum perforatum L. | 2282.80 * ± 453.64 | 8.76 ± 1.88 |
| Sideritis sp. | 819.03 * ± 247.64 | 3.96 ± 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaloteraki, C.; Almpounioti, K.; Potsaki, P.; Bousdouni, P.; Kandyliari, A.; Koutelidakis, A.E. Total Antioxidant Capacity and Phenolic Content of 17 Mediterranean Functional Herbs and Wild Green Extracts from North Aegean, Greece. Biol. Life Sci. Forum 2021, 6, 43. https://doi.org/10.3390/Foods2021-11003
Kaloteraki C, Almpounioti K, Potsaki P, Bousdouni P, Kandyliari A, Koutelidakis AE. Total Antioxidant Capacity and Phenolic Content of 17 Mediterranean Functional Herbs and Wild Green Extracts from North Aegean, Greece. Biology and Life Sciences Forum. 2021; 6(1):43. https://doi.org/10.3390/Foods2021-11003
Chicago/Turabian StyleKaloteraki, Chrysoula, Kalliopi Almpounioti, Panagiota Potsaki, Panoraia Bousdouni, Aikaterini Kandyliari, and Antonios E. Koutelidakis. 2021. "Total Antioxidant Capacity and Phenolic Content of 17 Mediterranean Functional Herbs and Wild Green Extracts from North Aegean, Greece" Biology and Life Sciences Forum 6, no. 1: 43. https://doi.org/10.3390/Foods2021-11003
APA StyleKaloteraki, C., Almpounioti, K., Potsaki, P., Bousdouni, P., Kandyliari, A., & Koutelidakis, A. E. (2021). Total Antioxidant Capacity and Phenolic Content of 17 Mediterranean Functional Herbs and Wild Green Extracts from North Aegean, Greece. Biology and Life Sciences Forum, 6(1), 43. https://doi.org/10.3390/Foods2021-11003







