Abstract
The Cachrys genus (Apiaceae) is widely distributed in the Mediterranean Basin. Previous studies have highlighted the photobiological properties of different Cachrys species, such as C. pungens Jan, C. libanotis L. and C. sicula L. Based on these previous promising results, and in order to continue exploring such an interesting genus, the aim of this study was to evaluate the photocytotoxic activity of extracts from Cachrys ferulacea (L.) Calest. Aerial parts were collected in Calabria (southern Italy) and extracted through three different techniques: traditional maceration, supercritical CO2 and pressurized cyclic solid–liquid (PCSL) extraction using Naviglio extractor®. The phytochemical composition was assessed with gas chromatography–mass spectrometry (GC–MS) and the photocytotoxic potential of samples was evaluated on a UVA-irradiated C32 melanoma cell line. The apoptotic responses on treated cells were also assessed. Furthermore, the phenolic and flavonoid contents and the in vitro antioxidant activity were also estimated. Different coumarins were identified and quantified. All the extracts affected cell viability in a concentration-dependent manner after irradiation with UVA light for 1 h at a dose of 1.08 J/cm2. Samples obtained through supercritical CO2 extraction showed the highest activity, with an IC50 value equal to 4.91 μg/mL. This study could provide a starting point for further research focusing on new photosensitizing agents useful in cancer photochemotherapy.
1. Introduction
PUVA-therapy, whose name is an abbreviation of psoralen (linear furanocoumarins) plus ultraviolet-A radiation, is a photochemotherapy approach commonly used in the treatment of psoriasis, vitiligo and other dermatologic diseases. Due to the ability of furanocoumarins to interact with DNA and disrupt its replication, there is great interest in developing anti-cancer therapies based on the action of these compounds against malignant cells [1,2]. PUVA is a first-line option for the treatment of mycosis fungoides, the most common type of cutaneous T-cell lymphoma, which is a heterogenous group of non-Hodgkin lymphomas arising in the skin [3,4].
Furanocoumarins are coumarins derivatives whose structure is based on a furan ring attached to the coumarin backbone. These compounds are divided into two groups according to the attachment place of the furan ring: linear (C6/C7) and angular (C7/C8) types. The first type is the most common, frequently found in plants belonging to the Apiaceae and Rutaceae families, whereas the occurrence of the C7/C8 derivatives is limited to Apiaceae and Fabaceae [5,6].
The Cachrys genus (Apiaceae) is widely distributed in the Mediterranean Basin. We previously investigated the photobiological properties of different Cachrys species, such as C. pungens Jan [7], C. libanotis L. and C. sicula L. [8].
Based on promising results obtained previously, we aimed to investigate another species belonging to the same interesting genus: C. ferulacea (L.) Calest. The aerial parts were extracted using three different techniques: traditional maceration, pressurized cyclic solid–liquid extraction using Naviglio extractor® and supercritical CO2. The phytochemical composition was verified with gas chromatography–mass spectrometry (GC–MS) and the photobiological properties were assessed in vitro on the melanoma C32 cell line.
2. Materials and Methods
C. ferulacea aerial parts were collected in Calabria (southern Italy) and extracted with methanol (plant to-solvent ratio, 1:10 g/mL) through traditional maceration (TM) and pressurized cyclic solid–liquid (PCSL) extraction with a Naviglio extractor® (Atlas Filtri SRL, Limena, Italy). A further extract was obtained through supercritical CO2 (S-CO2) extraction.
The phytochemical profile was investigated with gas chromatography–mass spectrometry (GC–MS) [8]. Total phenolics were determined spectrophotometrically using the Folin–Ciocalteu method, and flavonoid contents were assessed by the formation of a complex with aluminum chloride after acid hydrolysis [9]. The DPPH and the β-carotene bleaching assays were used to verify the antioxidant potential of the extracts [10].
The photobiological properties of C. ferulacea samples were assessed in vitro on the C32 cell line (human melanoma cancer cells, ATCC no. CRL-1585). Cells were cultured in RPMI-1640 medium supplemented with 1% penicillin/streptomycin, 1% l-glutamine and 10% fetal bovine serum. Cells were then trypsinized, placed in 96-well plates (3.8 × 104 cells/well) and incubated to allow for cell attachment. Twenty-four hours later, the medium was removed and samples at different concentrations in Hanks’ balanced salt solution (100 µL) were added. After 30 min, microtiter plates were irradiated at 365 nm for 1 h at a dose of 1.08 J/cm2 [8]. After irradiation, sample solutions were replaced by fresh medium and the cytotoxicity was evaluated 48 h later using the 3-[4,5-dimethyl-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay [11]. Experiments were performed in quadruplicates and the well-known photoactive compound bergapten was used as a positive control.
Immunoblotting analysis was also carried out in order to assess the apoptotic response. After each treatment, C32 cells were lysed for total protein extraction, and proteins were then resolved on 10% SDS-polyacrylamide gel, transferred to a nitrocellulose membrane and probed with Cyclin D1, p21, p53, PARP and GAPDH antibodies (Santa Cruz Biotechnology). Membranes were then incubated with peroxidase-coupled goat anti-mouse or goat anti-rabbit antibodies, and the antigen–antibody complex was shown using the ECL System (Amersham Pharmacia) [12].
Data were analyzed using GraphPad Prism Software (San Diego, CA, USA). Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey’s or Dunnett’s post hoc test.
3. Results and Discussion
Traditional maceration resulted in a significantly higher yield (14.7%) compared with the other two utilized techniques PCSL and S-CO2 extraction (3.6% and 2.4%, respectively).
The phytochemical composition was assessed with GC–MS. Overall, the four linear furanocoumarins psoralen, xanthotoxin, bergapten and isopimpinellin were detected, together with their precursor marmesin, with the S-CO2 extract showing the highest number of these compounds. The same sample contained the highest number of simple coumarins, with the coumarin derivative osthole being the major one. Moreover, different fatty acids, such as myristic, palmitic, linoleic, behenic and lignoceric acids, and four terpenes were identified in C. ferulacea extracts.
The TM sample showed the highest phenolic content (17.99 ± 0.50 mg/g of plant material). Lower amounts were detected in the other two samples (4.14 ± 0.24 and 2.32 ± 0.09 mg/g for PCSL and S-CO2, respectively). The same trend was observed for total flavonoids, with the TM extract showing the highest amount (0.63 ± 0.06 mg/g).
Consistently with the phenolic content, the sample obtained through traditional maceration showed the best radical scavenging activity (IC50 value = 77.37 ± 1.58 µg/mL) and better results in the β-carotene bleaching test (IC50 = 19.57 ± 0.67 and 27.94 ± 0.48 µg/mL after 30 and 60 min of incubation, respectively).
The photobiological properties of C. ferulacea were investigated on C32 cell cultures irradiated with UVA light for 1 h at a dose of 1.08 J/cm2. All the three C. ferulacea extracts induced a concentration-dependent photocytotoxic activity (Figure 1). IC50 values equal to 27.95 ± 0.67 and 25.90 ± 1.23 µg/mL were obtained for the TM and PCSL samples, respectively. The extract obtained through supercritical CO2 extraction was the most active, with an IC50 value of 4.91 ± 0.15 µg/mL. Interestingly, no extract affected cell viability in the dark.
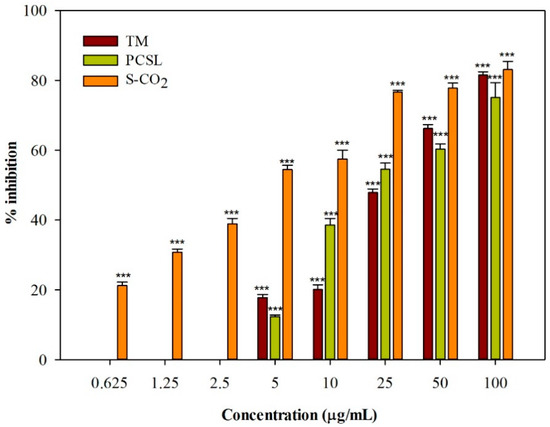
Figure 1.
Photocytotoxic activity of C. ferulacea (L.) Calest. extracts. TM, traditional maceration; PCSL, pressurized cyclic solid–liquid extraction; S-CO2, supercritical CO2 extraction. Data are expressed as the mean ± S.E.M. (n = 4). *** p < 0.001 compared with control (Dunnett’s multiple comparisons test).
The proliferative arrest was evidenced by the down-regulation of Cyclin D1 in C32 UV-treated cells. However, a consistent reduction in the same protein, together with an up-regulation of the cyclin-dependent kinase inhibitor p21, was given by the S-CO2 extraction method in UV-treated melanoma cells. The latter protein, as previously reported, is a modulator of apoptotic responses through different pathways [13]. This is in line with the sustained increase in the proteolytic form of PARP, a marker of DNA damage, observed in the melanoma cells which underwent UVA light treatment.
4. Conclusions
The present study demonstrated that C. ferulacea extracts, mainly S-CO2 sample, contain important photoactive constituents responsible for their photocytotoxic activity. Investigated samples induced promising cytotoxic effects on malignant melanoma cells upon irradiation with UVA light, without affecting cell viability in the dark. Future studies could be useful to further optimize the extraction method and to continue investigating the interesting photobiological properties of this species.
Author Contributions
Phytochemical investigation, M.M. and M.R.P.; biological investigation, M.M., V.A. and F.G.; data curation, M.M. and F.G.; writing—original draft preparation, M.M. and F.G.; writing—review and editing, F.C., M.L.P. and G.S.; supervision, M.L.P. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. M.R. Perri was supported by POR Calabria FESR/FSE 2014–2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Melough, M.M.; Cho, E.; Chun, O.K. Furocoumarins: A review of biochemical activities, dietary sources and intake, and potential health risks. Food Chem. Toxicol. 2018, 113, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Menichini, G.; Provenzano, E.; Conforti, F. Applications of natural compounds in the photodynamic therapy of skin cancer. Curr. Med. Chem. 2014, 21, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F. Phototherapy of cutaneous T-cell lymphomas. Photochem. Photobiol. Sci. 2018, 17, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Tarabadkar, E.S.; Shinohara, M.M. Skin directed therapy in cutaneous T-cell lymphoma. Front. Oncol. 2019, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in anticancer therapy–For and against. Fitoterapia 2020, 142, 104492. [Google Scholar] [CrossRef]
- Menichini, G.; Alfano, C.; Provenzano, E.; Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Cachrys pungens Jan inhibits human melanoma cell proliferation through photo-induced cytotoxic activity. Cell Prolif. 2012, 45, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Perri, M.R.; Amodeo, V.; Giordano, F.; Statti, G.A.; Panno, M.L.; Conforti, F. Assessment of Photo-Induced Cytotoxic Activity of Cachrys sicula and Cachrys libanotis Enriched-Coumarin Extracts against Human Melanoma Cells. Plants 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, V.; Marrelli, M.; Pontieri, V.; Cassano, R.; Trombino, S.; Conforti, F.; Statti, G. Chenopodium album L. and Sisymbrium officinale (L.) Scop.: Phytochemical Content and In Vitro Antioxidant and Anti-Inflammatory Potential. Plants 2019, 8, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrelli, M.; Conforti, F.; Araniti, F.; Casacchia, T.; Statti, G. Seasonal and environmental variability of non-cultivated edible Cichorioideae (Asteraceae). Plant Biosyst. 2018, 152, 759–766. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.A.; Tundis, R.; Menichini, F.; Conforti, F. Fatty acids, coumarins and polyphenolic compounds of Ficus carica L. cv. Dottato: Variation of bioactive compounds and biological activity of aerial parts. Nat. Prod. Res. 2014, 28, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Naimo, G.D.; Nigro, A.; Romeo, F.; Paolì, A.; De Amicis, F.; Vivacqua, A.; Morelli, C.; Mauro, L.; Panno, M.L. Valproic acid addresses neuroendocrine differentiation of LNCaP cells and maintains cell survival. Drug Des. Dev. Ther. 2019, 13, 4265–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).