Effect of Sage (Salvia officinalis L.) Extract on the Survival of Staphylococcus aureus in Portuguese Alheira Sausage during Maturation †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Procedure
2.2. Antioxidant and Antimicrobial Activity of the Sage Extract
2.3. Inoculation of S. aureus in Alheiras
2.4. Microbiological and Physicochemical Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant and Antimicrobial Activities of the Sage Extract
3.2. Maturation Process of Lab-Scale Produced Alheiras
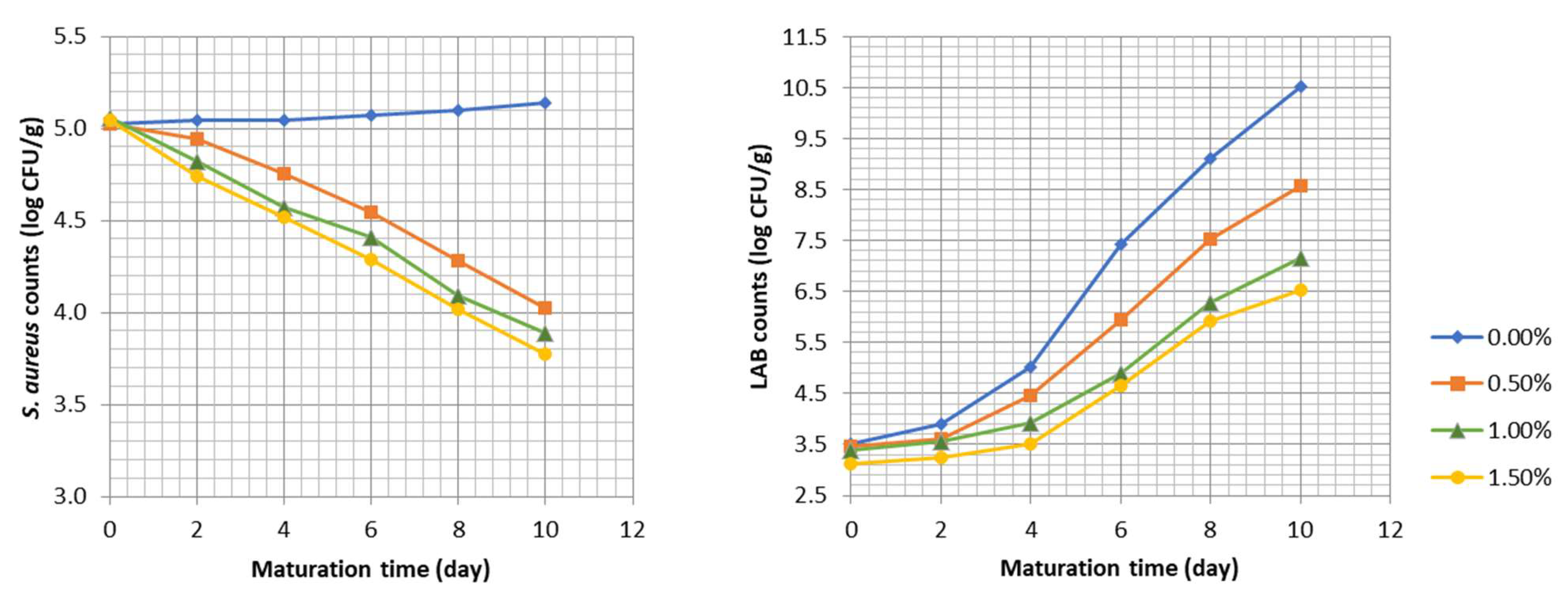
3.3. Effect of the Sage Extract on S. aureus and Lactic Acid Bacteria in Alheiras
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Božin, B.; Mimica-Dukić, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Fernandes, S.; Zefanias, O.; Rodrigues, G.; Faria, A.S.; Fernandes, Â.; Barros, L.; Cadavez, V.; Gonzales-Barron, U. Microbiological and physicochemical assessment of artisanally produced “Alheira” fermented sausages in Northern Portugal. Proceedings 2021, 70, 16. [Google Scholar] [CrossRef]
- Sojic, B.; Pavlic, B.; Zekovic, Z.; Tomovic, V.; Ikonic, P.; Kocic-Tanackov, S.; Dzinic, N. The effect of essential oil and extract from sage (Salvia officinalis L.) herbal dust (food industry by-product) on the oxidative and microbial stability of fresh pork sausages. LWT—Food Sci. Tech. 2018, 89, 749–755. [Google Scholar] [CrossRef]
- De Sá, I.S.; Peron, A.P.; Leimann, F.V.; Bressan, G.N.; Krum, B.N.; Fachinetto, R.; Pinela, J.; Calhelha, R.C.; Barreiro, M.F.; Ferreira, I.C.; et al. In vitro and in vivo evaluation of enzymatic and antioxidant activity, cytotoxicity and genotoxicity of curcumin-loaded solid dispersions. Food Chem. Toxicol. 2019, 125, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, J.; Chen, J.; Tai, A. A method for evaluation of antioxidant activity based on inhibition of free radical-induced erythrocyte hemolysis. Methods Mol. Biol. 2010, 594, 287–296. [Google Scholar] [CrossRef] [PubMed]
- ISO 152147:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 6888-1:1999/Amd 1: 2003. (1999); Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Technique Using Baird-Parker Agar Medium. International Organization for Standardization: Geneva, Switzerland, 2003.
- Bianchin, M.; Pereira, D.; Almeida, J.D.F.; Moura, C.D.; Pinheiro, R.S.; Heldt, L.F.S.; Haminiuk, C.W.I.; Carpes, S.T. Antioxidant properties of lyophilized rosemary and sage extracts and its effect to prevent lipid oxidation in poultry pátê. Molecules 2020, 25, 5160. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.; Ramirez, R.; Ventanas, S.; Cava, R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pate. LWT Food Sci. Technol. 2007, 40, 58–65. [Google Scholar] [CrossRef]
- Borgi, H. Prevalence and Molecular Characterisation of Salmonella spp. Isolated from Alheira, a Traditional Portuguese Meat Product. Master’s Thesis, Instituto Politécnico de Bragança, Bragança, Portugal, 2020. Available online: https://bibliotecadigital.ipb.pt/handle/10198/22748 (accessed on 21 June 2021).
| Activity | Mean ± SD |
|---|---|
| TBARS a (IC50, µg/mL) | 206 ± 5 |
| OxHLIAb (IC50, µg/mL) | |
| Δt 60 min | 23.9 ± 0.9 |
| Δt 120 min | 56.0 ± 2.0 |
| MIC—E. coli (mg/mL) | 1.250 ± 0.00 |
| MIC—S. aureus (mg/mL) | 0.625 ± 0.00 |
| Maturation Day | pH * | aw * | Weight Loss (%) * |
|---|---|---|---|
| 0 | 6.03 (0.019) a | 0.9931 (0.0032) a | - |
| 2 | 5.98 (0.026) a | 0.9870 (0.0037) b | 12.09 (1.23) a |
| 4 | 5.79 (0.026) b | 0.9769 (0.0009) c | 18.16 (0.86) b |
| 6 | 5.55 (0.022) b | 0.9751 (0.0082) c | 21.28 (0.68) c |
| 8 | 5.49 (0.034) b | 0.9734 (0.0010) c | 22.12 (1.25) c |
| Bacterium | Test | Mean Difference (log CFU/g) | F/t Value | Pr(>F)/Pr(>|t|) |
|---|---|---|---|---|
| ANOVA | ||||
| S. aureus | Day | - | 16.79 | <0.001 |
| Concentration | - | 23.50 | <0.001 | |
| Comparison of means | ||||
| 0.5%–0.0% | −0.476 | −5.407 | <0.001 | |
| 1.0%–0.0% | −0.598 | −6.786 | <0.001 | |
| 1.5%–0.0% | −0.672 | −7.626 | <0.001 | |
| 1.0%–0.5% | −0.122 | −1.379 | 0.520 | |
| 1.5%–0.5% | −0.196 | −2.219 | 0.136 | |
| 1.5%–1.0% | −0.074 | −0.841 | 0.835 | |
| ANOVA | ||||
| LAB | Day | - | 85.35 | <0.001 |
| Concentration | - | 27.27 | <0.001 | |
| Comparison of means | ||||
| 0.5%–0.0% | −0.983 | −3.910 | 0.002 | |
| 1.0%–0.0% | −1.760 | −7.003 | <0.001 | |
| 1.5%–0.0% | −2.085 | −8.295 | <0.001 | |
| 1.0%–0.5% | −0.777 | −3.093 | 0.018 | |
| 1.5%–0.5% | −1.102 | −4.385 | <0.001 | |
| 1.5%–1.0% | −0.325 | −1.292 | 0.573 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho-Fernandes, S.; Rodrigues, G.; Faria, A.S.; Caleja, C.; Pereira, E.; Pinela, J.; Carocho, M.; Barros, L.; Cadavez, V.; Gonzales-Barron, U. Effect of Sage (Salvia officinalis L.) Extract on the Survival of Staphylococcus aureus in Portuguese Alheira Sausage during Maturation. Biol. Life Sci. Forum 2021, 6, 125. https://doi.org/10.3390/Foods2021-11047
Coelho-Fernandes S, Rodrigues G, Faria AS, Caleja C, Pereira E, Pinela J, Carocho M, Barros L, Cadavez V, Gonzales-Barron U. Effect of Sage (Salvia officinalis L.) Extract on the Survival of Staphylococcus aureus in Portuguese Alheira Sausage during Maturation. Biology and Life Sciences Forum. 2021; 6(1):125. https://doi.org/10.3390/Foods2021-11047
Chicago/Turabian StyleCoelho-Fernandes, Sara, Gisela Rodrigues, Ana Sofia Faria, Cristina Caleja, Eliana Pereira, José Pinela, Márcio Carocho, Lillian Barros, Vasco Cadavez, and Ursula Gonzales-Barron. 2021. "Effect of Sage (Salvia officinalis L.) Extract on the Survival of Staphylococcus aureus in Portuguese Alheira Sausage during Maturation" Biology and Life Sciences Forum 6, no. 1: 125. https://doi.org/10.3390/Foods2021-11047
APA StyleCoelho-Fernandes, S., Rodrigues, G., Faria, A. S., Caleja, C., Pereira, E., Pinela, J., Carocho, M., Barros, L., Cadavez, V., & Gonzales-Barron, U. (2021). Effect of Sage (Salvia officinalis L.) Extract on the Survival of Staphylococcus aureus in Portuguese Alheira Sausage during Maturation. Biology and Life Sciences Forum, 6(1), 125. https://doi.org/10.3390/Foods2021-11047













