The Bioactivity of Byproducts from the Blackberry (Rubus fruticosus) Juice Industry †
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Characterization of Byproducts and Fermented Fractions
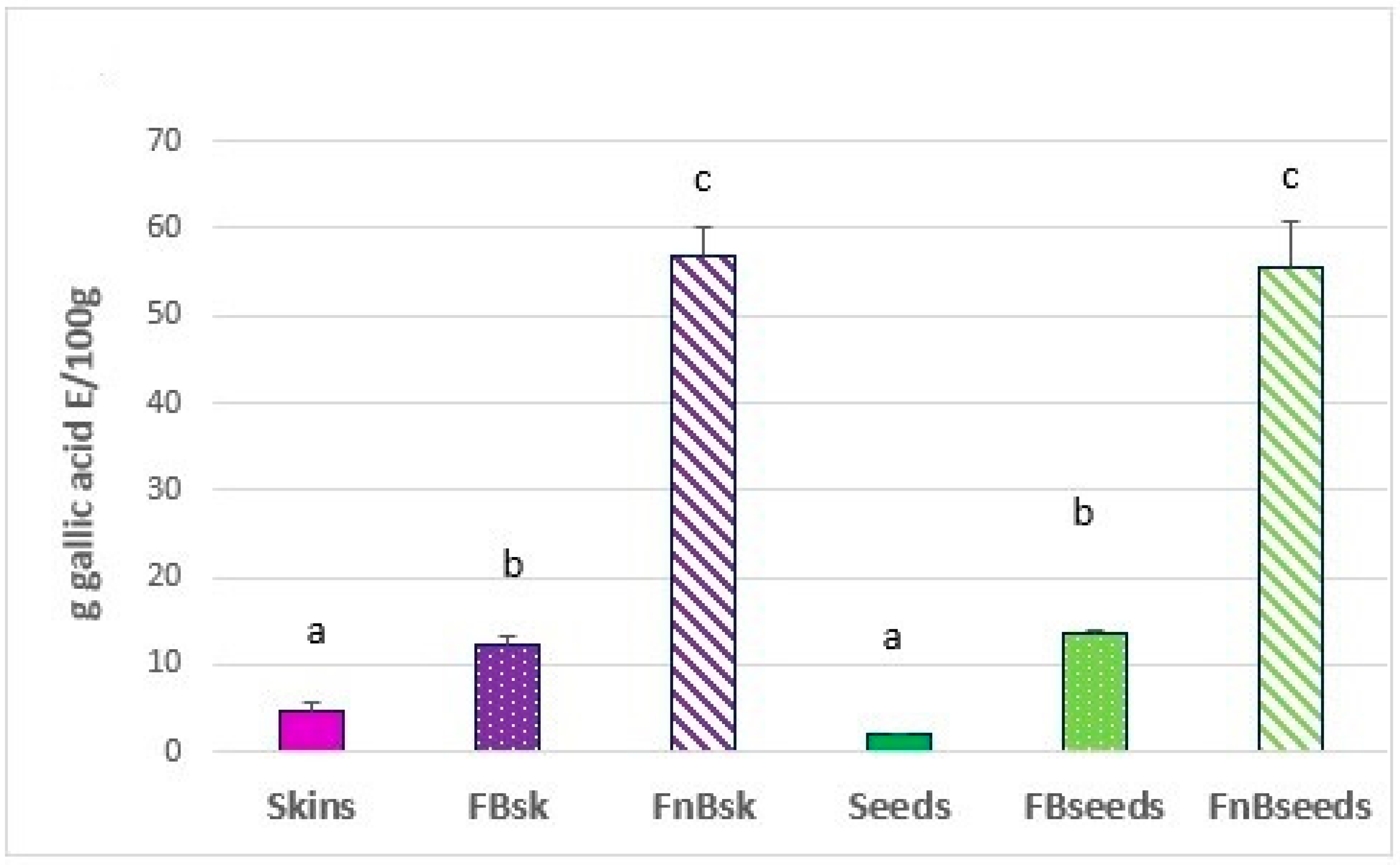
2.2.1. Folin–Ciocalteu Assay (FC)
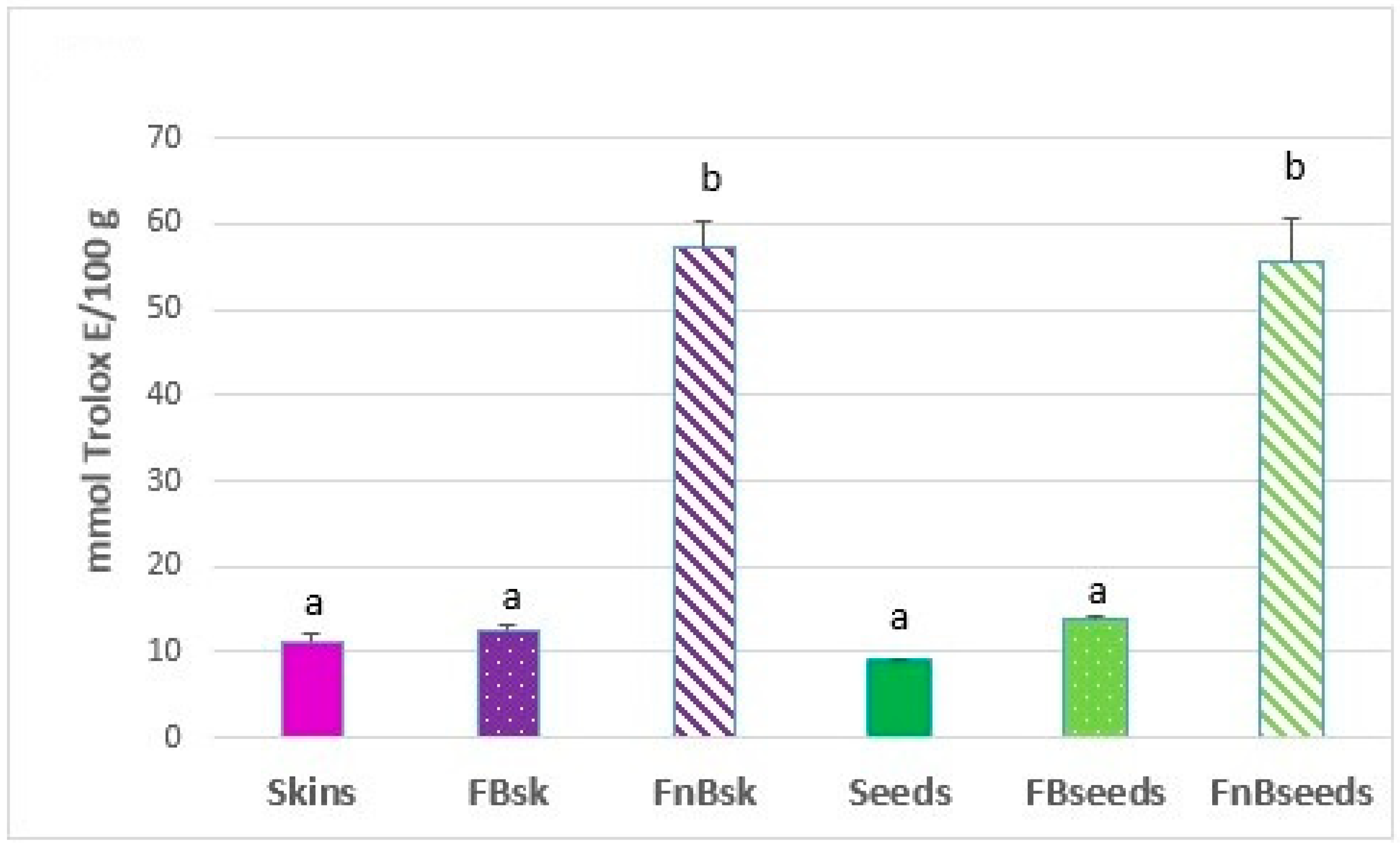
2.2.2. Antioxidant Capacity (ABTS Assay)
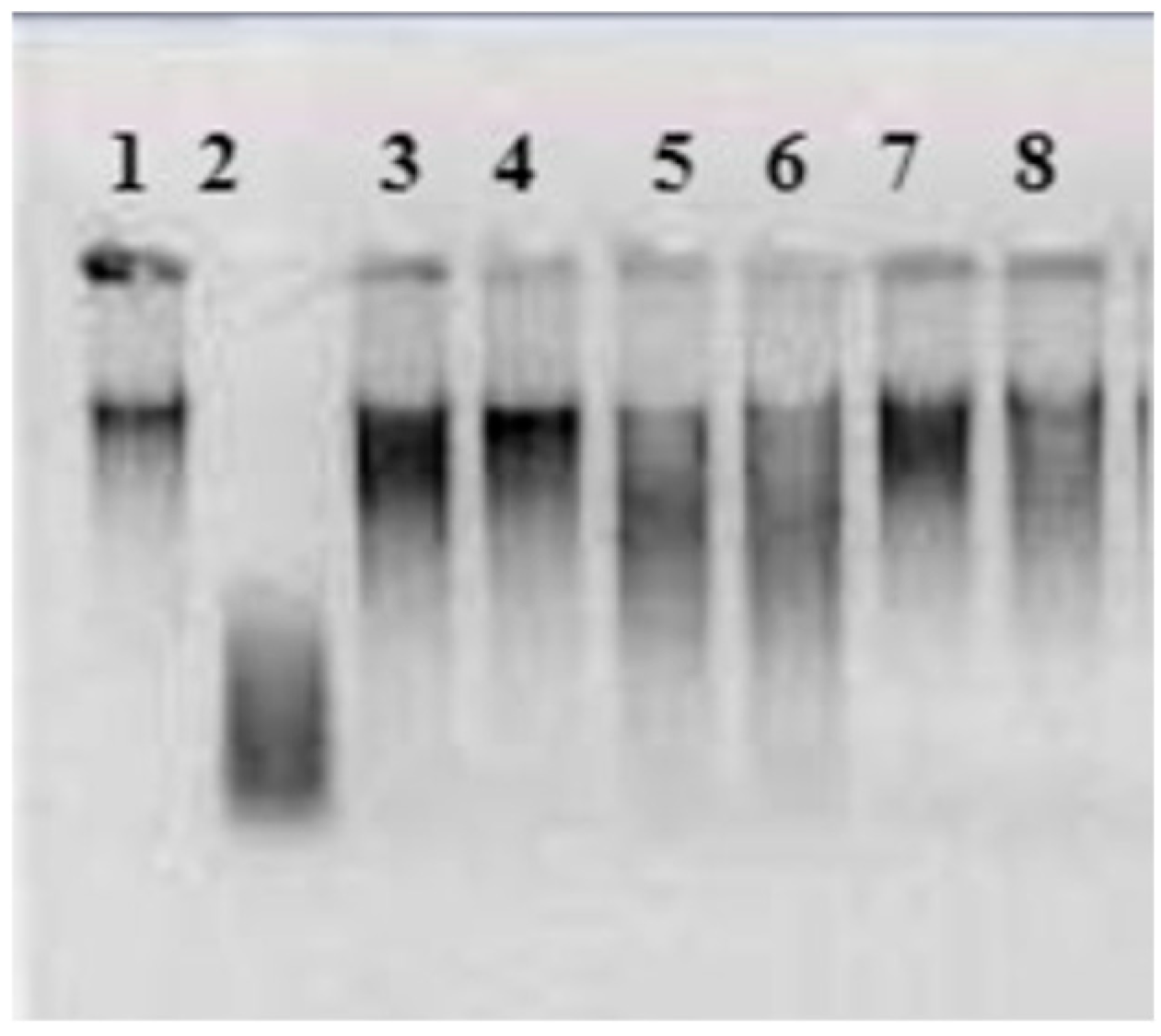
2.3. Genotoxicity
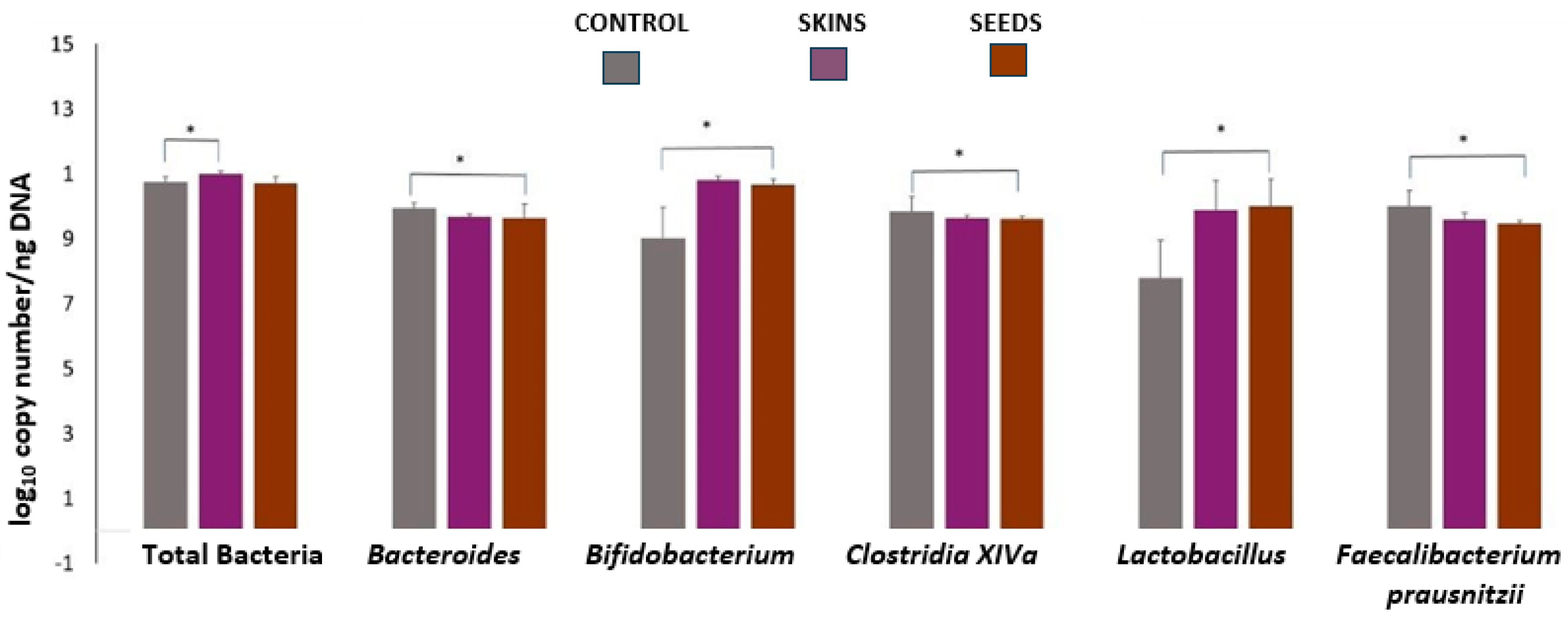
2.4. Microbiota Analysis
- Total Bacteria: F-CGGTGAATACGTTCCCGG and R-TACGGCTACCTTGTTACGACTT;
- Bacteroides: F-GAGAGGAAGGTCCCCCAC and R-CGCACTTGGCTGGTTCAG;
- Bifidobacterium: F-GATTCTGGCTCAGGATGAACGC and R-CTGATAGGACGCGACCCCAT;
- Clostridia XIVa: F-CGGTACCTGACTAAGAAGC and R-AGTTTYATTCTTGCGAACG;
- F. prausnitzii: F-GGAGGAAGAAGGTCTTCGG and R-AATTCCCGCCTACCTCTGCACT.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rutz, J.K.; Voss, G.B.; Zambiazi, R. Influence of the degree of maturation on the bioactive compounds in blackberry (Rubus spp.) cv. Tupy. Food Nutr. Sci. 2012, 3, 1452–1460. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Glišić, I.; Mladenović, J. Fruit quality attributes of blackberry grown under limited environmental conditions. Plant Soil Environ. 2012, 58, 322–327. [Google Scholar] [CrossRef]
- Jazić, M.; Kukrić, Z.; Vulić, J.; Četojević-Simin, D. Polyphenolic composition, antioxidant and antiproliferative effects of wild and cultivated blackberries (Rubus fruticosus L.) pomace. Int. J. Food Sci. Technol. 2019, 54, 194–201. [Google Scholar] [CrossRef]
- Choe, U.; Li, Y.; Yu, L.; Gao, B.; Wang, T.T.Y.; Sun, J.; Chen, P.; Yu, L. Chemical composition of cold-pressed blackberry seed flour extract and its potential health-beneficial properties. Food Sci. Nutr. 2020, 20, 1215–1225. [Google Scholar] [CrossRef]
- Seeram, N.P. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burillo, S.; Rufian-Henares, J.A.; Pastoriza, S. Towards an improved global antioxidant response method (GAR+): Physiological-resembling in vitro digestion-fermentation method. Food Chem. 2018, 239, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolic Compounds with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, P.; Valls, V.; Perez-Broseta, C.; Iradi, A.; Climen, J.V.; Oliva, M.R.; Tormo-Saez, G. The role of 8-hydroxy-2′-deoxyguanosine in rifamycin-induced DNA damage. Free Radic. Biol. Med. 1995, 18, 747–775. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Seraglio, S.K.T.; Della Betta, F.; Nehring, P.; Valese, A.C.; Daguer, H.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Blackberry (Rubus ulmifolius Schott): Chemical composition, phenolic compounds and antioxidant capacity in two edible stages. Food Res. Int. 2019, 122, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Ryan, L. Improving public health?: The role of antioxidant-rich fruit and vegetable beverages. Food Res. Int. 2011, 44, 3135–3148. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J. Dietary fiber: Still alive. Food Chem. 2024, 439, 138076. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Oliva, E.; Mondor, M.; Arcand, Y.; Herández-Alvarez, A.J. In vitro gastrointestinal digestion impact on stability, bioaccessibility and antioxidant activity of polyphenols from wild and commercial blackberries (Rubus spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef] [PubMed]
- Cigarran Guldris, S.; González Parra, E.; Cases Amenós, A. Gut microbiota in chronic kidney disease. Nefrologia 2017, 37, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Agnant, K.; Lamuela-Raventós, R.M.; St-Onge, M.P. Dietary polyphenols and sleep modulation: Current evidence and perspectives. Sleep Med. Rev. 2023, 72, 101844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Chen, C.; Huang, Q.; Fu, X. In vitro digestion of the whole blackberry fruit: Bioaccessibility, bioactivite variation of active and impacts on human gut microbiota. Food Chem. 2022, 370, 131001. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Pérez, M.D.; Gerardi, G.; Cavia-Saiz, M.; Ortega-Heras, M.; Muñiz, P. The Bioactivity of Byproducts from the Blackberry (Rubus fruticosus) Juice Industry. Biol. Life Sci. Forum 2024, 40, 52. https://doi.org/10.3390/blsf2024040052
Rivero-Pérez MD, Gerardi G, Cavia-Saiz M, Ortega-Heras M, Muñiz P. The Bioactivity of Byproducts from the Blackberry (Rubus fruticosus) Juice Industry. Biology and Life Sciences Forum. 2024; 40(1):52. https://doi.org/10.3390/blsf2024040052
Chicago/Turabian StyleRivero-Pérez, Maria Dolores, Gisela Gerardi, Mónica Cavia-Saiz, Miriam Ortega-Heras, and Pilar Muñiz. 2024. "The Bioactivity of Byproducts from the Blackberry (Rubus fruticosus) Juice Industry" Biology and Life Sciences Forum 40, no. 1: 52. https://doi.org/10.3390/blsf2024040052
APA StyleRivero-Pérez, M. D., Gerardi, G., Cavia-Saiz, M., Ortega-Heras, M., & Muñiz, P. (2024). The Bioactivity of Byproducts from the Blackberry (Rubus fruticosus) Juice Industry. Biology and Life Sciences Forum, 40(1), 52. https://doi.org/10.3390/blsf2024040052





