Abstract
Natural deep eutectic solvents (NADESs) composed of citric acid and glucose or glycerol and glucose, both with and without the addition of β-cyclodextrin (BCD), were developed and characterized through physicochemical analysis. Parameters such as density, pH, water activity, refractive index, electrical conductivity, and polarity were evaluated. Additionally, their thermal and rheological properties were assessed. The presence of BCD did not significantly affect the polarities of the NADESs but decreased the aw and increased the pH and the apparent viscosity. The designed NADESs were used to extract polyphenolic compounds from Moringa oleifera leaves, providing a sustainable alternative to conventional organic solvents. The results showed that NADESs achieved high extraction yields, demonstrating their effectiveness and potential applications in food and pharmaceutical industries. This study highlights the versatility of NADESs and their relevance in green chemistry and sustainable extraction technologies.
1. Introduction
Efforts are being made to valorize agroindustrial waste by recovering compounds of technological or functional interest [1]. Conventional extraction techniques entail long times and require large quantities of energy and toxic solvents [2]. It is essential to develop innovative extraction strategies that utilize environmentally friendly and efficient solvents and technologies [1,2]. Plant and agroindustrial waste extracts contain bioactive compounds that are valuable due to their antioxidant and antimicrobial properties [1,3]. Moringa oleifera leaves are considered an undervalued source of numerous bioactive compounds such as polyphenols and carotenoids [4], useful for developing functional food ingredients or additives with an extended shelf life and also beneficial for health. Promoting their application could enhance resource utilization at both local and regional levels, providing economic and environmental benefits. M. oleifera extracts have been reported to exhibit antioxidant activity both in vitro and in vivo [5,6].
Natural deep eutectic solvents (NADESs) have emerged as a versatile and sustainable alternative to traditional organic solvents. NADESs have gained interest in both research and industry due to their low or no toxicity, versatility, and adjustable physicochemical properties for specific requirements [7]. They are obtained by combining a hydrogen bond donor and acceptor, commonly found in biological systems, which makes them potentially useful for use in foods [8]. NADESs have a much lower melting point than any of their individual components, mainly due to the generation of intermolecular hydrogen bonds [7,8]. They present notable physicochemical properties, including a remarkable ability to dissolve both polar and non-polar molecules, low vapor pressure that enables their operation at high temperatures, and miscibility with water that allows changes in the polarity of the medium [8]. As a drawback, they have high viscosity, which hinders the extraction process, but this can be addressed by heating or adding water [9,10]. It was reported that extracts obtained using NADESs based on choline chloride, polyalcohol, sugar, and organic acids had good extraction yields of natural compounds such as polyphenols, comparable to those obtained with conventional solvents or even higher [10]. NADESs formulated with citric acid and glucose have been used to increase the solubility of quercetin and they have also demonstrated high efficiency for extracting flavonoids and terpenoids [11]. A glycerol-based NADES has been extensively used for polyphenol extraction from several vegetal sources, including M. oleifera [12].
Cyclodextrins (CDs) are cyclic oligosaccharides produced by the enzymatic degradation of starch, containing six (α-CD), seven (β-CD), or eight (γ-CD) glucose residues linked by α-1,4 glycosidic bonds. They have a truncated cone spatial conformation with a hydrophilic outer surface and a hydrophobic cavity, which gives them the ability to form inclusion complexes with different compounds through non-covalent interactions [13,14]. Molecular inclusion changes the physicochemical properties of the encapsulated compound, increasing, for example, its stability against oxidation or degradation, its bioavailability, and its solubility in water. CDs are considered food additives (E − 457, E − 458, and E − 459) and “Generally Recognized As Safe” (GRAS) so they can be incorporated into foods and medicines [14]. Given their complementary individual properties, combining NADESs and CDs can potentially result in highly interesting materials [15]. Two different approaches have been developed for incorporating CDs into deep eutectic solvents [16]. The first involves introducing CDs into an already prepared DES, and the second method integrates CDs as a fundamental component of the DES, leading to the formation of a novel system known as a supramolecular deep eutectic solvent (SUPRADES) [16,17]. The term “supramolecular” refers to non-covalent interactions between molecules, playing a crucial role in both the drug–CD complex and the formation of a DES [17]. The objective of the present work was to design and characterize NADESs formulated with cyclodextrins to extract antioxidant compounds from M. oleifera leaves.
2. Materials and Methods
2.1. Materials and Chemical Reagents
Dried M. oleifera leaves were purchased in a Buenos Aires (Argentine) local market (Amazonas Laboratory, Asunción, Paraguay). β-Cyclodextrin (containing eight water molecules/a molecule of BCD, Mr. 1135) was purchased from Roquette (Pharma and Nutraceuticals, Lestrem, France). Folin–Ciocalteu reagent; 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), gallic acid, and Nile Red colorant were obtained from Sigma-Aldrich (St. Louis, MO, USA). Citric acid, anhydrous glucose, glycerol, and sodium carbonate trihydrate were purchased from Biopack (Buenos Aires, Argentine). All chemicals were analytical grade.
2.2. NADES Preparation
NADESs were formulated from non-toxic compounds, commonly used in the food industry and considered GRAS and biodegradable. Two natural eutectic solvents of different compositions were formulated. The components were mixed in a certain molar ratio and stirred at 60 °C until a homogeneous system was obtained [18].
- NADESs with citric acid (Cit), anhydrous glucose (Glu), and bidistilled water (W) were mixed in a molar ratio of 1:1:6.5 (Cit:Glu:W), according to Bergua et al. (2021) [11]. This system was also prepared with a 15 mM β-cyclodextrin (BCD) aqueous solution (Cit:Glu:BCD). Water or BCD solution was added to reach a final water content of 30% w/v. The systems were stored at room temperature.
- NADESs with glycerol (Gly) and anhydrous glucose were mixed in a molar ratio of 4:1, according to Jesús et al. (2021) [18]. Subsequently, bidistilled water (Gly:Glu:W) or 15 mM BCD aqueous solution (Gly:Glu:BCD) was added until reaching a final 30% w/v of water content. The systems were stored at room temperature.
2.3. NADES Properties Evaluated
2.3.1. Physicochemical Properties
The density (ρ) of NADESs was determined by pycnometry, measuring the mass of a known volume of a solvent at 25 °C and calculating the quotient between these two values. A digital refractometer (Reichert AR200, Depew, NY, USA) was used to determine the refractive index (nD), with temperature correction at 20 °C. The pH of the solvents was determined using a pH meter (Ohaus ST10, Greifensee, Switzerland), and the electrical conductivity (σ) was measured using a portable conductivity meter (ADWA AD203, Szeged, Hungary). The water activity (aw) was determined using an AquaLab Series 3 dew point hygrometer (Decagon Devices, Washington, USA).
Polarity measurements were carried out using Nile Red as a solvatochromic probe. Solvatochromic absorbance probes provide a measure of polarity through the change in the UV-vis absorbance spectrum when the molecule interacts with the solvent of interest [19]. A stock solution was prepared by dissolving 1 g/L of Nile Red in ethanol and stored at 4 °C. A volume of 50 μL of Nile Red stock solution was added to the sample and mixed. The sample was then placed in a 1 cm2 quartz cuvette and the UV spectra were immediately acquired, at room temperature, in duplicate [20]. A NADES sample blank was acquired before each determination. For each sample, the wavelength where the absorbance was maximum ()) was determined and the related parameter was calculated. represents the molar transition energy of the dye at room temperature and normal pressure using the following equation:
with in kcal.mol−1 and in nm.
Solvents with higher polarity produce a bathochromic effect of the of Nile Red (shift to higher wavelength values), which produces lower values. In contrast, a hypsochromic shift occurs in nonpolar solvents [19]. By measuring the changes in of Nile Red relative to the value it exhibits in a reference solvent, it is possible to calculate the relative polarity of the solvent of interest [20]. In addition, the polarity of ultrapure water, 15 mM BCD solution, and ethanol/water 80:20% v/v was determined by the same method.
2.3.2. Thermal and Rheological Properties
Differential scanning calorimetry (DSC) was used to study the thermal properties of NADES using a calorimeter (Mettler-Toledo AG Model 822, Urtenen-Schönbühl, Switzerland). The equipment was calibrated with indium and zinc. Samples (9.00 to 13.80 ± 0.01 mg) were weighed, placed in a 40 µL aluminum pans, and hermetically sealed. The capsules were immersed in liquid nitrogen before measuring to achieve rapid freezing. An empty pan was used as a reference. Scans were performed in an inert atmosphere of nitrogen gas with a flow rate of 200 mL/min between −120 and 60 °C at 10 °C/min. Thermograms were evaluated using STARe Thermal Analysis System v. 1.0 software. 6.1 (Mettler Toledo, Switzerland) and plotted using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA).
A strain sweep was performed at a constant temperature (25 °C) from 0.1 to 100%. A strain percentage value was selected within the linear viscoelasticity range in a rheometer (Anton Paar Rheometer MCR 102e, Ostfildern, Germany). A frequency sweep was then performed from 0.01 to 50 Hz, keeping the strain percentage constant (0.5%). Subsequently, a temperature sweep was performed from 10 to 70 °C and the apparent viscosity value (ηapp, mPa.s) was recorded. All determinations were performed in duplicate.
2.3.3. Use of NADES in Antioxidant Compound Extraction from M. oleifera Leaves
Ground-dried leaves of M. oleifera and the corresponding NADES extraction solvent were mixed at an extraction ratio of 1:50 w/v. Samples were then subjected to sonication for 5 min. The mixture was stirred at 1000 rpm using a magnetic stirrer at 25 and 50 °C for 1 h. The samples were then centrifuged (15 min, 5000 rpm, 25 °C). The total polyphenol content (TPC) and antioxidant capacity (AO) evaluated by a DPPH assay of the obtained extracts were determined. The TPC of the extracts was determined using the Folin–Ciocalteu (FC) method with modifications [21]. An aliquot (50 μL) of the extracted sample, 800 μL of distilled water, 125 μL of sodium carbonate solution (20% w/v) in NaOH 0.1 M, and 125 μL of FC reagent (1 M) were mixed. After 30 min at room temperature, the absorbance of the solutions was measured at 765 nm. A DPPH assay reaction mixture was prepared by adding 50 µL of each extracted sample to 950 µL of an ethanolic solution of a DPPH radical with an initial absorbance equal to 1 at 517 nm [22]. The absorbance at 517 nm was determined after 30 min of reaction in the dark. The percentage of the inhibition of the radical DPPH was calculated according to Equation (2):
where is the absorbance of each sample after 30 min of reaction, and is the absorbance of the DPPH control solution. The results were expressed in mg of gallic acid equivalent per gram of dry leaf (mg GAE/g d.m.) considering the calibration curve (R2 = 0.99). The extraction efficiency of NADESs and the effect of temperature (25 or 50 °C) on the extraction efficiency of NADESs was evaluated and compared concerning water, 15 mM BCD solution, and ethanol/water 80:20 v/v.
3. Results and Discussion
3.1. NADES Characterization
The knowledge of the physicochemical properties of the NADES is essential for designing solvents with specific characteristics for their potential application. Table 1 shows the results obtained for the studied formulations.

Table 1.
Physicochemical properties, glass transition temperature (Tg), and apparent viscosity (ηapp) at 25 °C of NADES.
As expected, the density values (ρ) of the NADESs were higher than that of pure water. The density of Cit:Glu systems was approximately 10% higher than Gly:Glu. No significant differences in the density of the systems were observed due to the incorporation of BCD. Density provides structural insights into a liquid as it is inversely proportional to the molar volume, which reflects the molecular packing [11]. Most NADESs exhibit densities higher than that of water, typically ranging from 1.10 to 1.40 g/mL [23]. The density value is influenced by the degree of hydrogen bonding between the components of the NADES and their molar ratio. A higher degree of hydrogen bonding reduces the size of holes within the liquid, leading to an increase in density [11,17]. In Cit:Glu systems, citric acid and glucose are found in an equal molar ratio (1:1) together with 9 moles of water. Citric acid has three carboxyl groups and one hydroxyl group that act as hydrogen bond donors and a total of seven oxygen groups that can act as hydrogen bond acceptors. The glucose molecule has five hydroxyl groups that act as hydrogen bond donors and a total of six oxygen groups that act as hydrogen bond acceptors, which gives glucose molecules the ability to interact more efficiently with each other and with water molecules. In contrast, glycerol has only three hydroxyls that can act as both hydrogen bond donors and/or acceptors. Although the molar ratio in Gly:Glu systems is 4:1, they are found in proportion with 13 moles of water. Therefore, there is a lower efficiency of interaction between the components since they depend on the accommodation of the molecules in space and their interaction with the water present in the system, which could explain the lower density of these solvents [24]. On the other hand, the refractive indices (nD) of the developed NADESs ranged between 1.420 and 1.456, with no significant differences between the different systems, although the nD of the systems with glycerol was slightly lower than those of the systems with citric acid. The refractive index also indicates the degree of molecular packing since the vacuum facilitates the propagation of light; therefore, the less compact the fluid, the lower the nD values will be [11]. Pure glycerol has a refractive index of 1.47, and the glycerol-based NADES presented nD lower than this value, so it is inferred that the interaction with glucose, water, and BCD generates less compaction of the molecules.
The aw of the NADES Cit:Glu was 0.761 for the system with water and 0.746 for the system with BCD. In the NADES based on glycerol and glucose, an aw value of 0.672 was obtained for the system with water and 0.661 for the system with BCD. Therefore, it was observed that in both systems, there was a significant difference due to the presence of cyclodextrin. The aw of the systems with BCD is lower than that of the corresponding ones without cyclodextrin. This difference could be attributed to an increased interaction of water through hydrogen bonds with the hydroxyl groups exposed on the surface of BCD [25]. On the other hand, it is observed that the aw of the Gly:Glu systems is significantly lower than that of the Cit:Glu systems. This difference could indicate a greater interaction of water in the systems with glycerol due to the formation of hydrogen bonds that compromise water molecules in the structure of the NADES. In the case of the citric acid and glucose-based systems, the interaction between these two components could avoid or limit the interactions with the water molecules, leaving them as free water. The pH of the NADES with citric acid was less than 1 (0.68 with water and 0.77 with BCD), which is explained by the high acid concentration. In the NADES formed with glycerol and glucose, the resulting pH was 3.14 in the system with water and 3.51 in the system containing BCD. In both cases, a significant difference is observed due to the presence of cyclodextrin, which results in higher pH values. This may indicate again that BCD could be interacting with water and/or with the other components of the NADES as a hydrogen bond acceptor, making the deprotonation of the acids or hydrogen donors disfavored [25]. Regarding electrical conductivity (σ), glycerol-based NADESs exhibited zero conductivity, which may be because none of the constituent compounds can form ions. In contrast, in the NADES with citric acid, considerably high conductivity values were obtained, associated with the movement of citrate ions. It can also be observed that conductivity decreases in the presence of BCD, which could be associated with greater intermolecular interaction and less deprotonation of the acid, as mentioned above.
Regarding thermal properties, for all the studied NADESs, a jump in the heat flow was observed in the thermogram, corresponding to the glass transition temperature (Tg), at which the structure changed from a glassy state (amorphous solid) to a supercooled liquid (or “rubbery”) state. The presence of a Tg allows NADESs to be classified as glass-forming materials [20]. The glass transition temperatures were around −74 °C for the Cit:Glu NADES and −102 °C for the Gly:Glu systems (Table 1). In these systems, the Tg was similar to that reported for pure glycerol (−103 °C). No significant differences were observed in the Tg values due to the incorporation of BCD. Tg values lower than −50 °C were also reported for other NADESs composed of choline chloride, sugars, organic acids and polyalcohol [7]. Melting peaks were not observed in the temperature range studied for any of the systems, indicating that these NADESs are in a stable liquid state at room temperature and within a wide temperature range [7]. The absence of endothermic transitions has also been reported by other authors in the characterization of NADESs [17].
The apparent viscosity at 25 °C of the Cit:Glu systems was significantly higher than the viscosity of the NADES Gly:Glu. This difference is probably related to the fact that the original constituents employed to prepare the Cit:Glu systems are in a solid state. In contrast, glycerol used to prepare the Gly:Glu systems is liquid, resulting in a more fluid mixture at room temperature. On the other hand, higher viscosity is associated with stronger intermolecular interactions [24]. Benvenutti et al. (2019) [26] reported that eutectic solvents containing lactic acid (with one carboxyl group) have a lower viscosity than those formed by citric acid (with three carboxyl groups) due to the increase in intermolecular forces. Solvents formed by polyalcohols have a lower viscosity compared to those formed by acid or alcohol, which provides weaker molecular interactions. The viscosity of both systems decreased with increasing temperature, which is essential to take into consideration when evaluating the application of these solvents for extraction processes.
Another physicochemical property evaluated in the obtained NADESs was their polarity, a key factor in the solvent’s ability to solubilize and interact with different compounds [17,19], thereby directly affecting the extraction yields. In general, NADESs are hydrophilic due to their high electronegativity and ability to form hydrogen bonds interactions [26]. Figure 1 shows the polarity values obtained for the NADESs developed.
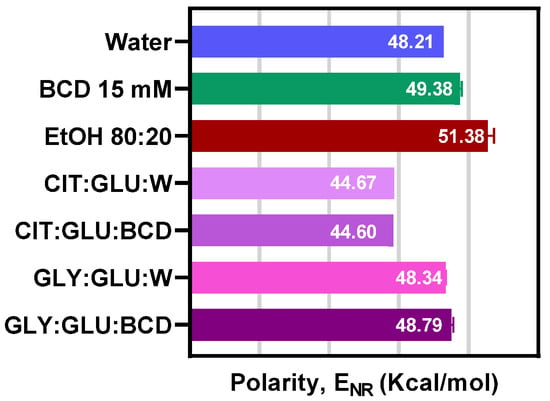
Figure 1.
The polarity of the developed NADESs based on citric acid and glucose (Cit:Glu) or glycerol and glucose (Gly:Glu) with water (W) or β-cyclodextrin (BCD) compared with the polarity of different solvents such as water, ethanol/water 80:20 and 15 mM BCD solution.
The polarity of Cit:Glu was found to be around 44.6 kcal/mol, with no significant differences between systems with and without BCD. Previous work indicates that NADESs that have organic acids as hydrogen bond donors have a higher polarity and that the polarity of sugar-based NADESs is similar to that of water (48.21 kcal/mol) [7]. Craveiro et al. (2016) [20] reported a polarity of 47.81 kcal/mol for NADESs formulated combining glucose with citric acid and a water content of 5%. The NADES Cit:Glu formulated in this work has a water content of 30%. An increase in the water content produces a shift of to higher values, which indicates an increase in polarity. On the other hand, the NADES Gly:Glu presented lower polarity (48.87 kcal/mol) than the citric acid systems. In general, NADESs based on sugars and polyalcohols have a polarity similar to that of methanol (51.89 kcal/mol) [7]. This difference could arise from the fact that smaller molecules with a higher number of hydroxyl and carboxyl substitutes generate intermolecular interactions of greater intensity [7].
3.2. Application of NADES for Extraction of Antioxidant Compounds from M. oleifera
Figure 2 shows the total polyphenolic content (a) and antioxidant activity (AO) by DPPH (b) determined for the extracts obtained using the NADESs as solvents. The extraction yields of polyphenols and antioxidant activity did not present significant differences between NADESs at a temperature of 25 °C and were lower than the yield achieved in water, BCD aqueous solution and with ethanol 80:20. However, the polyphenol extraction yield using NADES at 50 °C was similar to that obtained with water. Particularly, the antioxidant activity measured by DPPH was higher in the Gly:Glu systems than in the aqueous extract. Furthermore, in all NADES systems, a higher polyphenol extraction and AO was observed with increasing temperature. These results suggest a certain improvement in the stability of the extracted compounds. Polyphenols are typically sensitive to temperatures above 50 °C, so the use of a NADES in extraction processes at these temperatures could prevent their degradation [27,28].
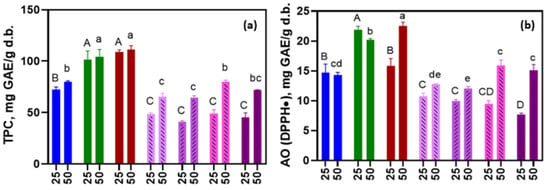
Figure 2.
Total polyphenolic content (a) and antioxidant activity by DPPH assay (b) expressed in mg of gallic acid equivalent (GAE) per gram of dry basis of dried M. oleifera leaves at 25 and 50 °C, using the following as extraction solvent:  water,
water,  15 mM BCD solution,
15 mM BCD solution,  ethanol/water 80:20,
ethanol/water 80:20,  citric acid/glucose/water,
citric acid/glucose/water,  citric acid/glucose/BCD,
citric acid/glucose/BCD,  glycerol/glucose/water, and
glycerol/glucose/water, and  glycerol/glucose/BCD. Different uppercase and lowercase letters represent significant differences between samples at 25 °C and at 50 °C, respectively, with p < 0.05 using one-way ANOVA.
glycerol/glucose/BCD. Different uppercase and lowercase letters represent significant differences between samples at 25 °C and at 50 °C, respectively, with p < 0.05 using one-way ANOVA.
 water,
water,  15 mM BCD solution,
15 mM BCD solution,  ethanol/water 80:20,
ethanol/water 80:20,  citric acid/glucose/water,
citric acid/glucose/water,  citric acid/glucose/BCD,
citric acid/glucose/BCD,  glycerol/glucose/water, and
glycerol/glucose/water, and  glycerol/glucose/BCD. Different uppercase and lowercase letters represent significant differences between samples at 25 °C and at 50 °C, respectively, with p < 0.05 using one-way ANOVA.
glycerol/glucose/BCD. Different uppercase and lowercase letters represent significant differences between samples at 25 °C and at 50 °C, respectively, with p < 0.05 using one-way ANOVA.
Also, increasing the temperature decreases the viscosity of the NADESs, which allows for the greater mobility of the solids and solvent, enhancing the diffusion capacity [8]. The TPC and antioxidant activity of the extracts obtained with NADESs based on glycerol were higher than those of the systems based on citric acid. This may be a consequence of the lower apparent viscosity and polarity of the former. Conversely, NADESs formulated with citric acid (characterized by higher polarity, viscosity, and acidity) were less efficient in polyphenol extraction. This behavior is not expected since polyphenols are predominantly polar compounds. These results underscore the importance of solvent design and characterization for their application in the food industry. It is essential not only to analyze the extraction yields but also the solvent design tailored to specific extraction requirements and the subsequent stabilization of antioxidant compounds. The extraction yield with BCD aqueous solution was higher that than with NADESs; this confirms that CDs are not only a host molecule but also interact in NADES H- bonds [16]. This idea agrees with the concept of SUPRADESs, where CDs play an important role in NADES stability and properties. In Table 1, we observed that the presence of BCD affects NADES physicochemical properties.
4. Conclusions
The present results demonstrate the variations in the properties of NADESs when modifying their composition, either in terms of hydrogen donors and acceptors or the presence of water or cyclodextrins. The presence of BCD did not affect significantly the polarities of the NADES but decreased the aw and increased the pH and the apparent viscosity. These is probably due to the interaction between CD and NADES components and aligns with the idea of SUPRADES, a novel type of supramolecular eutectic solvent. The analysis of thermal properties (Tg), polarity, and viscosity provide information on the intensity of interactions between the components of the NADES formulated. The viscosity and polarity of citric acid-based NADESs were significantly higher than glycerol NADESs. The obtained data also highlight the importance of characterizing these systems to enable their efficient application. The yield and specificity of the extraction can be improved by selecting NADESs with suitable properties. It is also important to consider that the decrease in the viscosity of NADESs with increasing temperature facilitates the diffusion of the compounds to be extracted. This factor is important when considering the application of NADES and improving the efficiency of extractions using these solvents. Furthermore, it was observed that NADESs have the advantage of being natural and non-toxic and could be designed with particular characteristics to favor the extraction of specific compounds and also to stabilize them during extraction and storage.
Author Contributions
Conceptualization, M.F.M. and C.I.d.S.F.; methodology, Melina I. Lionello; software, C.I.d.S.F.; validation, M.E.L. and C.I.d.S.F.; formal analysis, M.E.L., M.F.M. and C.I.d.S.F.; investigation, M.E.L., M.F.M. and C.I.d.S.F.; resources, M.F.M.; data curation, M.E.L. and C.I.d.S.F.; writing—original draft preparation, M.F.M. and C.I.d.S.F.; writing—review and editing, M.F.M. and C.I.d.S.F.; visualization, C.I.d.S.F.; supervision, M.F.M. and C.I.d.S.F.; project administration, M.F.M. and C.I.d.S.F.; funding acquisition, M.F.M. and C.I.d.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agencia Nacional de Promoción Científica (PICT 2020 Nº 01909), Universidad de Buenos Aires (UBACYT 20020220300231BA, UBACYT 20020220400331BA) and CONICET (PIP N° 11220210100702CO).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green non-conventional techniques for the extraction of polyphenols from agricultural food by-products: A review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef] [PubMed]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.; Sasireka, A.; Chandrasekaran, M.; Chung, I. Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Biosci. 2018, 26, 23–29. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pagnossa, J.P.; Blasi, F.; Cossignani, L.; Hilsdorf Piccoli, R.; Zengin, G.; Montesano, D.; Cocconcelli, P.S.; Lucini, L. Phenolic profiling and in vitro bioactivity of Moringa oleifera leaves as affected by different extraction solvents. Food Res. Int. 2020, 127, 108712. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2019, 60, 2564–2592. [Google Scholar] [CrossRef]
- Bergua, F.; Delso, I.; Muñoz-Embid, J.; Lafuente, C.; Artal, M. Structure and properties of two glucose-based deep eutectic systems. Food Chem. 2021, 336, 127717. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Lalas, S. Glycerol and Glycerol-Based Deep Eutectic Mixtures as Emerging Green Solvents for Polyphenol Extraction: The Evidence So Far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Buera, P.; Mazzobre, F. Novel trends in cyclodextrins encapsulation. Applications in food science. Curr. Opin. Food Sci. 2017, 16, 106–113. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Dugoni, G.; Di Pietro, M.E.; Ferro, M.; Castiglione, F.; Ruellan, S.; Moufawad, T.; Moura, L.; Costa Gomes, M.F.; Fourmentin, S.; Mele, A. Effect of Water on Deep Eutectic Solvent/β-Cyclodextrin Systems. ACS Sustain. Chem. Eng. 2019, 7, 7277–7285. [Google Scholar] [CrossRef]
- Balenzano, G.; Racaniello, G.F.; Arduino, I.; Lopedota, A.A.; Lopalco, A.; Laquintana, V.; Denora, N. Cyclodextrin-based supramolecular deep eutectic solvent (CycloDES): A vehicle for the delivery of poorly soluble drugs. Int. J. Pharm. 2023, 647, 123553. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Jesus, A.R.; Meneses, L.; Duarte, A.R.C.; Paiva, A. Natural deep eutectic systems, an emerging class of cryoprotectant agents. Cryobiology 2021, 101, 95–104. [Google Scholar] [CrossRef]
- Farooq, M.Q.; Abbasi, N.M.; Anderson, J.L. Deep eutectic solvents in separations: Methods of preparation, polarity, and applications in extractions and capillary electrochromatography. J. Chromatogr. A 2020, 1633, 461613. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.; Dionísio, M.; Barreiros, S.; Reis, R.; Duarte, A.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chaillou, L.; Nazareno, M. New Method to Determine Antioxidant Activity of Polyphenols. J. Agric. Food Chem. 2006, 54, 8397–8402. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef] [PubMed]
- Joules, A.; Burrows, T.I.; Dosa, P.; Hubel, A. Characterization of eutectic mixtures of sugars and sugar-alcohols for cryopreservation. J. Mol. Liq. 2023, 371, 120937. [Google Scholar] [CrossRef]
- Makoś-Chełstowska, P.; Słupek, E.; Fourmentin, S.; Gębicki, J. Supramolecular deep eutectic solvents in extraction processes: A review. Environ. Chem. Lett. 2024, 23, 41–65. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Maraulo, G.E.; Santos Ferreira, C.; Mazzobre, M.F. β-cyclodextrin enhanced ultrasound-assisted extraction as a green method to recover olive pomace bioactive compounds. J. Food Process. Preserv. 2021, 45, e15194. [Google Scholar] [CrossRef]
- Ratnasooriya, C.C.; Rupasinghe, H.P.V. Extraction of phenolic compounds from grapes and their pomace using β-cyclodextrin. Food Chem. 2012, 134, 625–631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).