Obtaining a Functional Food from Andean Grains through Lactic Acid Fermentation †
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Sensory Analysis of the Fermented Products
2.4. Characterization of the Final Product
2.5. Statistical Analysis
3. Results and Discussion
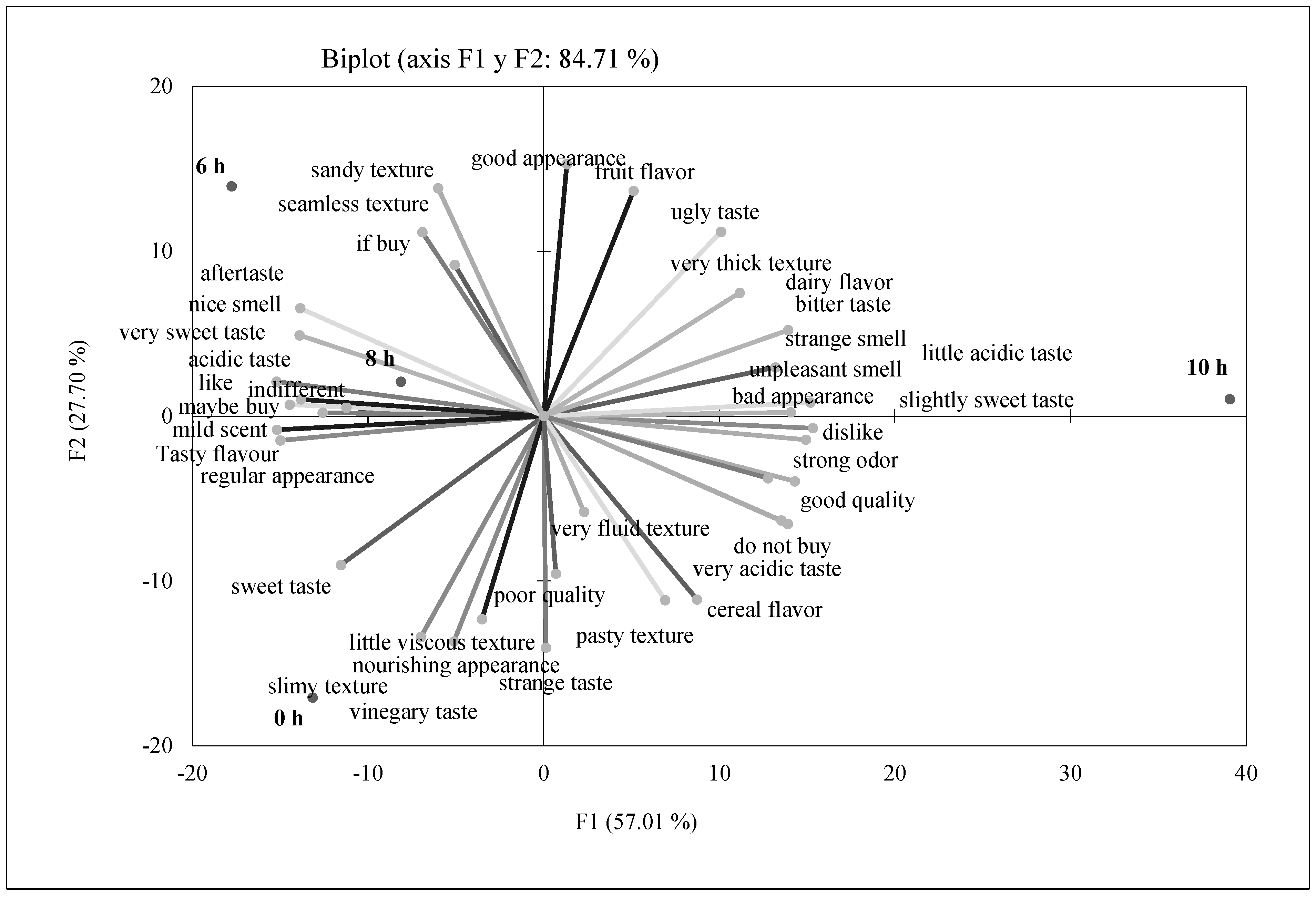
3.1. Sensory Analysis of Fermented Purees
3.2. Formulation of the Food and Its Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corbo, M.R.; Bevilaqua, A.; Petruzzelli, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Bekatoron, A.; Kanellaki, M.; Koutinas, A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Limitone, A.; Minervini, F.; Carnevali, P.; Corsetti, A.; Gaenzle, M.; Ciati, R.; Gobbetti, M. Glucan and fructan production by sourdough Weissella cibaria and Lactobacillus plantarum. J. Agric. Food Chem. 2006, 54, 9873–9881. [Google Scholar] [CrossRef]
- Pramudya, R.C.; Seo, H.-S. Using Check-All-That-Apply (CATA) method for determining product temperature dependent sensory-attribute variations: A case study of cooked rice. Food Res. Int. 2018, 105, 724–732. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis International, 16th ed.; EEUU: Gaithsburgf, MA, USA; Washintong, DC, USA, 1995. [Google Scholar]
- ICMSF. Microorganismos de los alimentos. In Técnicas de Análisis Microbiológicos, 2nd ed.; Editorial Acribia: Zaragoza, Spain, 1983; Volume 1, pp. 113–115. [Google Scholar]
- Segundo, C.; Román, L.; Gómez, M.; Martínez, M. Mechanically fractionated flour isolated from green bananas (M. cavendishii var. nanica) as a tool to increase the dietary fiber and phytochemical bioactivity of layer and sponge cakes. Food Chem. 2016, 219, 240–248. [Google Scholar] [CrossRef]
- Zannini, E.; Jeske, S.; Lynch, K.M.; Arendt, E.K. Development of novel quinoa based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef]
- Argentine Food Code. Chapter XVII, Article 1389. Available online: https://www.argentina.gob.ar/sites/default/files/anmat_caa_capitulo_xvii_dieteticosactualiz_2021-07.pdf (accessed on 1 January 2022).
- Palombini, S.V.; Claus, T.; Maruyama, S.A.; Gohara, A.K.; Souza, A.H.P.; Souza, N.E.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Evaluation of nutritional compounds in new amaranth and quinoa cultivars. Food Sci. Technol. 2013, 33, 339–344. [Google Scholar] [CrossRef]
- Carrizo, S.L.; Montes de Oca, C.E.; Hébert, M.E.; Saavedra, L.; Vignolo, G.; LeBlanc, J.G.; Rollán, G. Lactic Acid Bacteria from Andean Grain Amaranth: A Source of Vitamins and Functional Value Enzymes. J. Mol. Microbiol. Biotechnol. 2017, 27, 289–298. [Google Scholar] [CrossRef]
| Fermentation Time (h) | Acceptability | Purchase Interest | ||||
|---|---|---|---|---|---|---|
| Like (%) | Dislike (%) | Indifferent (%) | Buy (%) | Do Not Buy (%) | Maybe Buy (%) | |
| 0 | 62 | 19 | 19 | 29 | 43 | 29 |
| 6 | 67 | 14 | 19 | 43 | 29 | 29 |
| 8 | 52 | 19 | 29 | 24 | 38 | 38 |
| 10 | 38 | 52 | 10 | 29 | 57 | 14 |
| Humidity | Proteins | Lipids | Ash | Carbohydrates | |
|---|---|---|---|---|---|
| Fermented puree | 78.21 ± 0.10 | 1.74 ± 0.17 | 0.39 ± 0.04 | 0.06 ± 0.03 | 19.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas Alcon, C.E.; Jiménez, M.D.; Lobo, M.O.; Sammán, N.C. Obtaining a Functional Food from Andean Grains through Lactic Acid Fermentation. Biol. Life Sci. Forum 2022, 17, 11. https://doi.org/10.3390/blsf2022017011
Salinas Alcon CE, Jiménez MD, Lobo MO, Sammán NC. Obtaining a Functional Food from Andean Grains through Lactic Acid Fermentation. Biology and Life Sciences Forum. 2022; 17(1):11. https://doi.org/10.3390/blsf2022017011
Chicago/Turabian StyleSalinas Alcon, Cintya Elizabeth, María Dolores Jiménez, Manuel Oscar Lobo, and Norma Cristina Sammán. 2022. "Obtaining a Functional Food from Andean Grains through Lactic Acid Fermentation" Biology and Life Sciences Forum 17, no. 1: 11. https://doi.org/10.3390/blsf2022017011
APA StyleSalinas Alcon, C. E., Jiménez, M. D., Lobo, M. O., & Sammán, N. C. (2022). Obtaining a Functional Food from Andean Grains through Lactic Acid Fermentation. Biology and Life Sciences Forum, 17(1), 11. https://doi.org/10.3390/blsf2022017011






