Ecological Status Should Be Considered When Evaluating and Mitigating the Effects of River Connectivity Losses in European Rivers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Sources
2.2.1. River Network
2.2.2. Ecological Status
2.2.3. Barrier Data
2.3. Connectivity Calculations
- (1)
- S_NB, a scenario considering only natural barriers and segment river length;
- (2)
- W_NB, a scenario considering only natural barriers and segment river length, weighted by its probability of achieving GES;
- (3)
- S_AB, a scenario considering all the barriers that are present (natural and artificial barriers and reservoirs) and segment river length;
- (4)
- W_AB, a scenario considering all the barriers that are present and segment river length, weighted by its probability of achieving GES.
- (5)
- W_AB_IQ, a scenario considering all the barriers that are present and segment river length, weighted by the probability of achieving GES in a scenario of ecological quality improvement;
- (6)
- W_AB_IC, a scenario considering all the barriers that are present with higher values of permeability (natural barriers—0.5; artificial barriers—0.75; and reservoirs—0.9) and segment river length, weighted by its probability of achieving GES;
- (7)
- W_AB_IQP scenario considering all the barriers that are present with higher values of permeability (natural barriers—0.5; artificial barriers—0.75; and reservoirs—0.9) and segment river length, weighted by the probability of achieving GES in a scenario of ecological quality improvement.
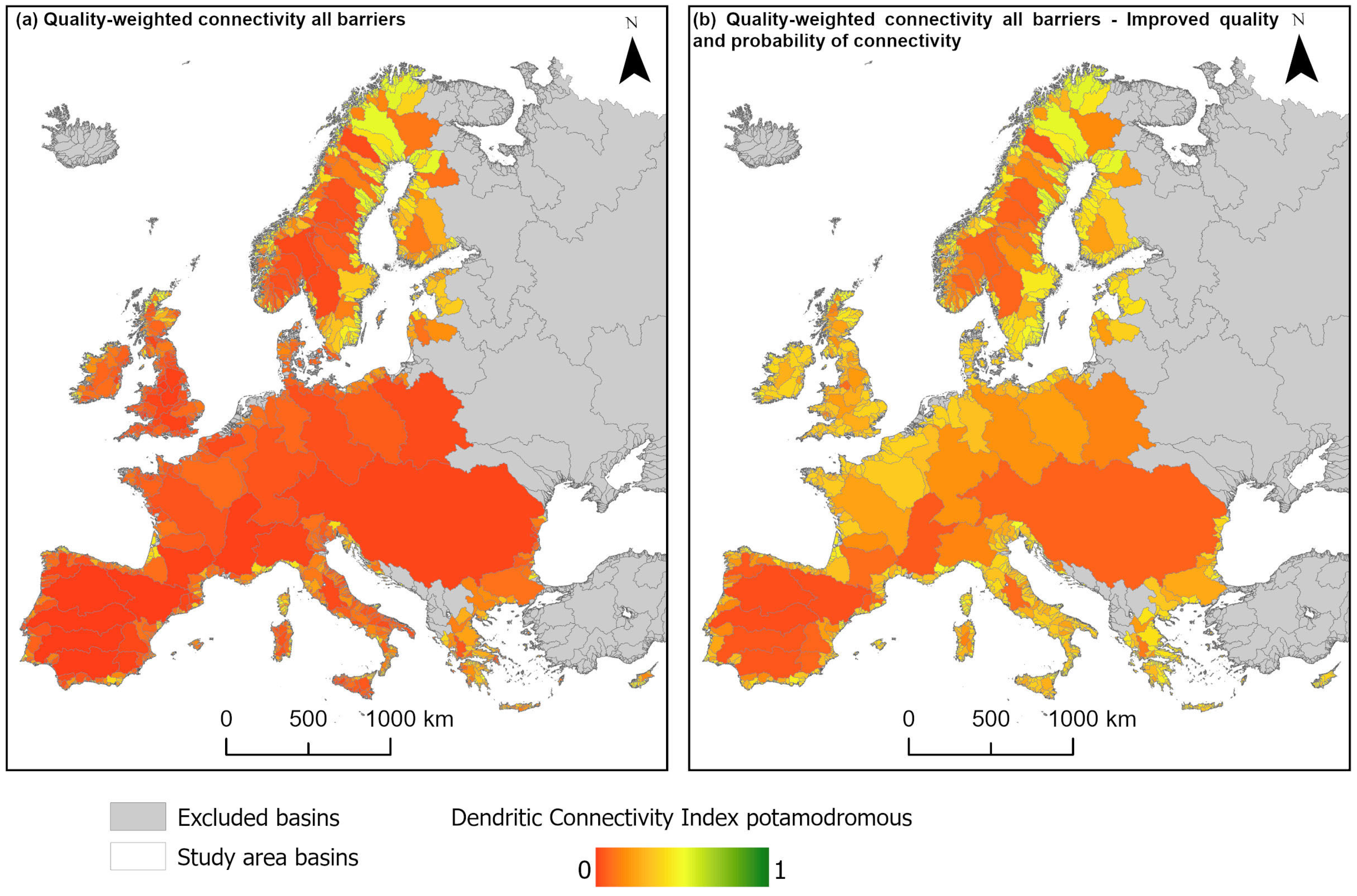
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Sayer, C.A.; Fernando, E.; Jimenez, R.R.; Macfarlane, N.B.W.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Contreras-MacBeath, T.; Cox, N.A.; Harrison, I.; et al. One-quarter of freshwater fauna threatened with extinction. Nature 2025, 638, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.; Mackay, E.B.; Cardoso, A.C.; Baattrup-Pedersen, A.; Birk, S.; Blackstock, K.L.; Borics, G.; Borja, A.; Feld, C.K.; Ferreira, M.T.; et al. Protecting and restoring Europe’s waters: An analysis of the future development needs of the water framework directive. Sci. Total Environ. 2019, 658, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. State of Nature in the EU: Results from Reporting under the Nature Directives 2013–2018; Publications Office of the European Union: Luxembourg, 2020.
- European Environment Agency. European Waters: Assessment of Status and Pressures 2018; Publications Office of the European Union: Luxembourg, 2018.
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Pereira, H.M.; Leadley, P.W.; Proença, V.; Alkemade, R.; Scharlemann, J.P.W.; Fernandez-Manjarrés, J.F.; Araújo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.L.; et al. Scenarios for global biodiversity in the 21st century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef]
- Rands, M.R.W.; Adams, W.M.; Bennun, L.; Butchart, S.H.M.; Clements, A.; Coomes, D.; Entwistle, A.; Hodge, I.; Kapos, V.; Scharlemann, J.P.W.; et al. Biodiversity conservation: Challenges beyond 2010. Science 2010, 329, 1298–1303. [Google Scholar] [CrossRef]
- Su, G.; Logez, M.; Xu, J.; Tao, S.; Villéger, S.; Brosse, S. Human impacts on global freshwater fish biodiversity. Science 2021, 371, 835–838. [Google Scholar] [CrossRef]
- Poff, N.L.; Zimmerman, J.K.H. Ecological responses to altered flow regimes: A literature review to inform the science and management of environmental flows. Freshw. Biol. 2010, 55, 194–205. [Google Scholar] [CrossRef]
- Suen, J.-P. Determining the ecological flow regime for existing reservoir operation. Water Resour. Manag. 2011, 25, 817–835. [Google Scholar] [CrossRef]
- Díaz, G.; Górski, K.; Heino, J.; Arriagada, P.; Link, O.; Habit, E. The longest fragment drives fish beta diversity in fragmented river networks: Implications for river management and conservation. Sci. Total Environ. 2021, 766, 144323. [Google Scholar] [CrossRef]
- Ward, J.V. The four-dimensional nature of lotic ecosystems. J. N. Am. Benthol. Soc. 1989, 8, 2–8. [Google Scholar] [CrossRef]
- Ward, J.V.; Tockner, K.; Schiemer, F. Biodiversity of floodplain river ecosystems: Ecotones and connectivity. Regul. Rivers. Mgmt. 1999, 15, 125–139. [Google Scholar] [CrossRef]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Water Policy; European Union: Brussels, Belgium, 2000. [Google Scholar]
- Hall, C.J.; Jordaan, A.; Frisk, M.G. The historic influence of dams on diadromous fish habitat with a focus on river herring and hydrologic longitudinal connectivity. Landscape. Ecol. 2011, 26, 95–107. [Google Scholar] [CrossRef]
- Liermann, C.R.; Nilsson, C.; Robertson, J.; Ng, R.Y. Implications of dam obstruction for global freshwater fish diversity. Biosci. 2012, 62, 539–548. [Google Scholar] [CrossRef]
- Lucas, M.; Baras, E. Migration of Freshwater Fishes; Blackwell Science: Oxford, UK, 2001; ISBN 978-0-632-05754-2. [Google Scholar]
- Barbarossa, V.; Schmitt, R.J.P.; Huijbregts, M.A.J.; Zarfl, C.; King, H.; Schipper, A.M. Impacts of current and future large dams on the geographic range connectivity of freshwater fish worldwide. Proc. Natl. Acad. Sci. USA 2020, 117, 3648–3655. [Google Scholar] [CrossRef]
- Humphries, P.; Winemiller, K.O. Historical impacts on river fauna, shifting baselines, and challenges for restoration. BioScience 2009, 59, 673–684. [Google Scholar] [CrossRef]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef]
- Northcote, T.G. Migratory Behaviour of Fish and Its Significance to Movement Trough Riverine Fish Passage Facilities. In Fish Migration and Fish Bypasses; Fishing News Books: Oxford, UK, 1998; pp. 3–18. [Google Scholar]
- Mader, H.; Maier, C. A method for prioritizing the reestablishment of river continuity in Austrian rivers. Hydrobiologia 2008, 609, 277–288. [Google Scholar] [CrossRef]
- Fetting, C. The European Green Deal; ESDN: Vienna, Austria, 2020. [Google Scholar]
- European Commission Directorate-General for Environment. EU Biodiversity Strategy for 2030—Bringing Nature Back into Our Lives; European Commission: Brussels, Belgium, 2021.
- Schwarz, U. The Potential of Barrier Removal to Reconnect Europe’s Rivers; WWF: Gland, Switzerland, 2021. [Google Scholar]
- European Commission Directorate-General for Environment. Nature Restoration Law—For People, Climate, and Planet, Proposal for a Regulation on Nature Restoration; European Commission: Brussels, Belgium, 2022.
- European Environment Agency. Europe’s State of Water 2024: The Need for Improved Water Resilience; Publications Office of the European Union: Luxembourg, 2024.
- Petsas, P.; Almpanidou, V.; Mazaris, A.D. Landscape connectivity analysis: New metrics that account for patch quality, neighbors’ attributes and robust connections. Landsc. Ecol. 2021, 36, 3153–3168. [Google Scholar] [CrossRef]
- Saura, S.; Pascual-Hortal, L. A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landsc. Urban Plan. 2007, 83, 91–103. [Google Scholar] [CrossRef]
- European Commission. Overall Approach to the Classification of Ecological Status and Ecological Potential: Guidance Document; No. 13; European Commission: Brussels, Belgium, 2003.
- Vigiak, O.; Udias, A.; Pistocchi, A.; Zanni, M.; Aloe, A.; Grizzetti, B. Probability maps of anthropogenic impacts affecting ecological status in European rivers. Ecol. Indic. 2021, 126, 107684. [Google Scholar] [CrossRef] [PubMed]
- De Jager, A.; Vogt, J. Rivers and Catchments of Europe—Catchment Characterisation Model (CCM); European Commission: Brussels, Belgium, 2007.
- European Waterfalls 2020. Available online: https://www.europeanwaterfalls.com/,2020 (accessed on 16 September 2021).
- Swan, B.; Gross, D. World Waterfall Database. 2012. Available online: https://www.worldwaterfalldatabase.com/ (accessed on 16 September 2021).
- Google Earth. Google Earth Pro; Google: Menlo Park, CA, USA, 2022. [Google Scholar]
- Duarte, G.; Peponi, A.; Leite, T.; Faro, A.; Moreno, D.; Anjinho, P.; Segurado, P.; Borgwardt, F.; Baattrup-Pedersen, A.; Hering, D.; et al. MERLIN Deliverable D3.1: Screening Maps: Europe-Wide Maps of the Needs and Potentials to Restore Floodplains, Rivers, and Wetlands with a Range of Restoration Measures; EU H2020 Research and Innovation Project MERLIN Deliverable; Merlin Entertainments: Poole, UK, 2023; p. 348. [Google Scholar]
- AMBER Consortium. The AMBER Barrier Atlas—A Pan-European Database of Artificial Instream Barriers; European Environment Agency: Copenhagen, Denmark, 2020.
- Wang, J.; Walter, B.A.; Yao, F.; Song, C.; Ding, M.; Maroof, A.S.; Zhu, J.; Fan, C.; McAlister, J.M.; Sikder, S.; et al. GeoDAR: Georeferenced global dams and reservoirs dataset for bridging attributes and geolocations. Earth Syst. Sci. Data 2022, 14, 1869–1899. [Google Scholar] [CrossRef]
- Mulligan, M.; van Soesbergen, A.; Sáenz, L. GOODD, a global dataset of more than 38,000 georeferenced dams. Sci. Data 2020, 7, 31. [Google Scholar] [CrossRef]
- Cote, D.; Kehler, D.G.; Bourne, C.; Wiersma, Y.F. A new measure of longitudinal connectivity for stream networks. Landsc. Ecol. 2009, 24, 101–113. [Google Scholar] [CrossRef]
- Bourne, C.M.; Kehler, D.G.; Wiersma, Y.F.; Cote, D. Barriers to fish passage and barriers to fish passage assessments: The impact of assessment methods and assumptions on barrier identification and quantification of watershed connectivity. Aquat. Ecol. 2011, 45, 389–403. [Google Scholar] [CrossRef]
- Grill, G.; Ouellet Dallaire, C.; Fluet Chouinard, E.; Sindorf, N.; Lehner, B. Development of new indicators to evaluate river fragmentation and flow regulation at large scales: A case study for the Mekong River basin. Ecol. Indic. 2014, 45, 148–159. [Google Scholar] [CrossRef]
- Duarte, G.; Segurado, P.; Oliveira, T.; Haidvogl, G.; Pont, D.; Ferreira, M.T.; Branco, P. The river network toolkit—Rivtool. Ecography 2019, 42, 549–557. [Google Scholar] [CrossRef]
- Diebel, M.W.; Fedora, M.; Cogswell, S.; O’Hanley, J.R. Effects of road crossings on habitat connectivity for stream-resident fish. River Res. Appl. 2015, 31, 1251–1261. [Google Scholar] [CrossRef]
- Maitland, B.M.; Poesch, M.; Anderson, A.E. Prioritising culvert removals to restore habitat for at-risk salmonids in the boreal forest. Fish. Manag. Ecol. 2016, 23, 489–502. [Google Scholar] [CrossRef]
- Shaw, E.A.; Lange, E.; Shucksmith, J.D.; Lerner, D.N. Importance of partial barriers and temporal variation in flow when modelling connectivity in fragmented river systems. Ecol. Eng. 2016, 91, 515–528. [Google Scholar] [CrossRef]
- Pringle, C. What is hydrologic connectivity and why is it ecologically important? Hydrol. Process. 2003, 17, 2685–2689. [Google Scholar] [CrossRef]
- Schmutz, S.; Sendzimir, J. (Eds.) Riverine Ecosystem Management: Science for Governing Towards a Sustainable Future; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-73249-7. [Google Scholar]
- Branco, P.; Amaral, S.D.; Ferreira, M.T.; Santos, J.M. Do small barriers affect the movement of freshwater fish by increasing residency? Sci. Total Environ. 2016, 581–582, 486–494. [Google Scholar] [CrossRef]
- Hayes, D.S.; Branco, P.; Santos, J.M.; Ferreira, T. Oxygen depletion affects kinematics and shoaling cohesion of cyprinid fish. Water 2019, 11, 642. [Google Scholar] [CrossRef]
- Le Pichon, C.; Lestel, L.; Courson, E.; Merg, M.-L.; Tales, E.; Belliard, J. Historical changes in the ecological connectivity of the Seine River for fish: A focus on physical and chemical barriers since the mid-19th century. Water 2020, 12, 1352. [Google Scholar] [CrossRef]
- Brettschneider, D.J.; Spring, T.; Blumer, M.; Welge, L.; Dombrowski, A.; Schulte-Oehlmann, U.; Sundermann, A.; Oetken, M.; Oehlmann, J. Much effort, little success: Causes for the low ecological efficacy of restoration measures in German surface waters. Environ. Sci. Eur. 2023, 35, 31. [Google Scholar] [CrossRef]

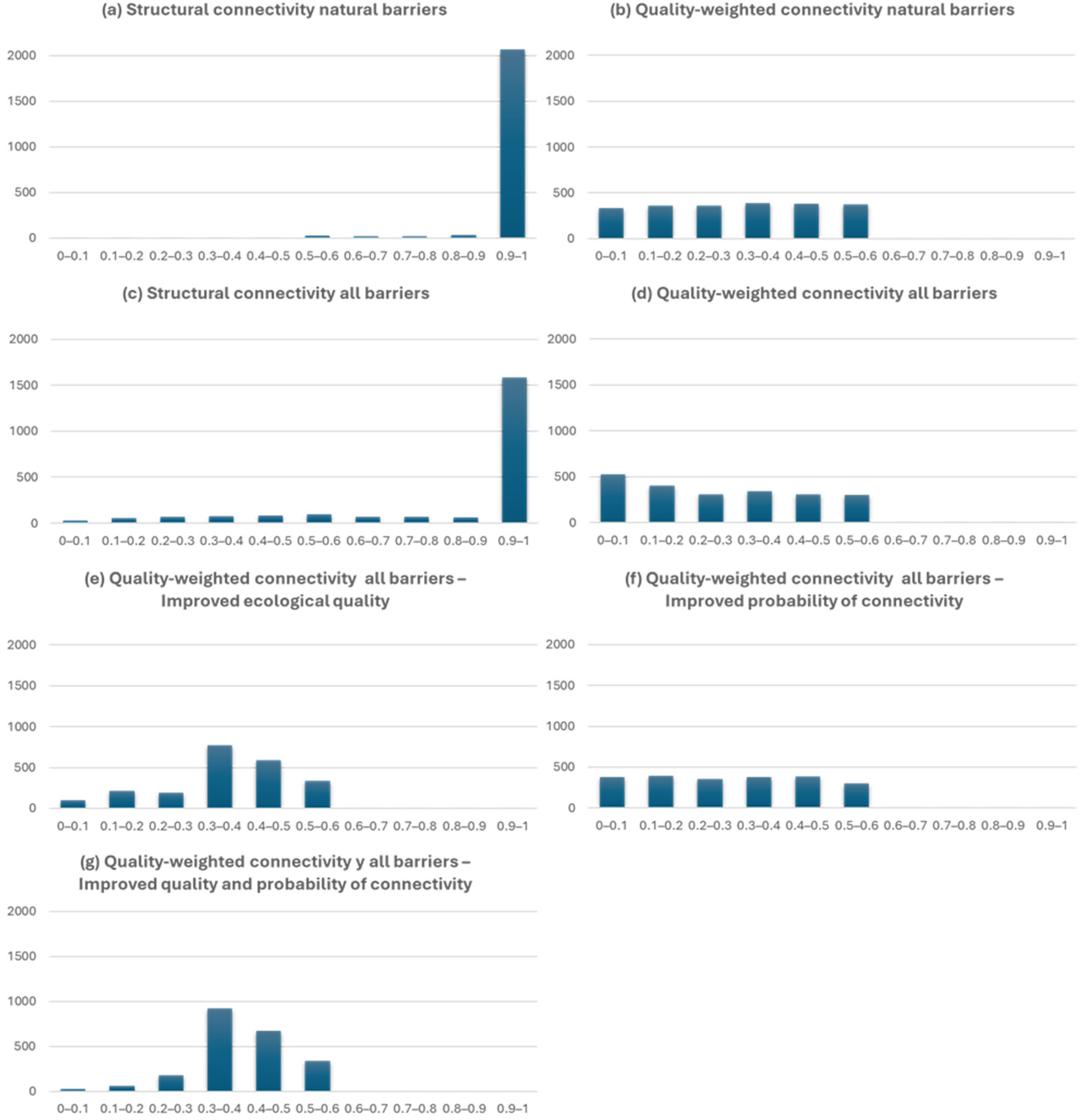
| S_NB | W_NB | S_AB | W_AB | W_AB_IQ | W_AB_IC | W_AB_IQC | |
|---|---|---|---|---|---|---|---|
| Mean | 0.98 | 0.30 | 0.85 | 0.27 | 0.37 | 0.29 | 0.40 |
| Std. Deviation | 0.09 | 0.16 | 0.26 | 0.17 | 0.13 | 0.16 | 0.26 |
| Minimum | 0.25 | 0.00 | 0.02 | 0.00 | 0.01 | 0.00 | 0.02 |
| Maximum | 1.00 | 0.57 | 1.00 | 0.57 | 0.57 | 0.57 | 0.57 |
| Weighted Mean (by area) | 0.93 | 0.23 | 0.54 | 0.09 | 0.15 | 0.13 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovar Faro, A.; Duarte, G.; Leite, T.; Ferreira, M.T.; Branco, P. Ecological Status Should Be Considered When Evaluating and Mitigating the Effects of River Connectivity Losses in European Rivers. Hydrobiology 2025, 4, 18. https://doi.org/10.3390/hydrobiology4030018
Tovar Faro A, Duarte G, Leite T, Ferreira MT, Branco P. Ecological Status Should Be Considered When Evaluating and Mitigating the Effects of River Connectivity Losses in European Rivers. Hydrobiology. 2025; 4(3):18. https://doi.org/10.3390/hydrobiology4030018
Chicago/Turabian StyleTovar Faro, António, Gonçalo Duarte, Tamara Leite, Maria Teresa Ferreira, and Paulo Branco. 2025. "Ecological Status Should Be Considered When Evaluating and Mitigating the Effects of River Connectivity Losses in European Rivers" Hydrobiology 4, no. 3: 18. https://doi.org/10.3390/hydrobiology4030018
APA StyleTovar Faro, A., Duarte, G., Leite, T., Ferreira, M. T., & Branco, P. (2025). Ecological Status Should Be Considered When Evaluating and Mitigating the Effects of River Connectivity Losses in European Rivers. Hydrobiology, 4(3), 18. https://doi.org/10.3390/hydrobiology4030018









