Key Habitat and Predatory Influences on the Community- and Species-Level Population Dynamics of Spring-Breeding Amphibian Larvae Within a Remnant Tupelo-Cypress Wetland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Larval Amphibian and Aquatic Predator Surveys
2.3. Site- and Habitat-Specific Covariates
2.4. Covariate Scaling and Selection
2.5. Modeling
2.6. Occupancy Model
2.7. Abundance Model
2.8. Detection Model
2.9. Priors and Inference
2.10. Modeling Methods
3. Results
3.1. Dipnet Surveys
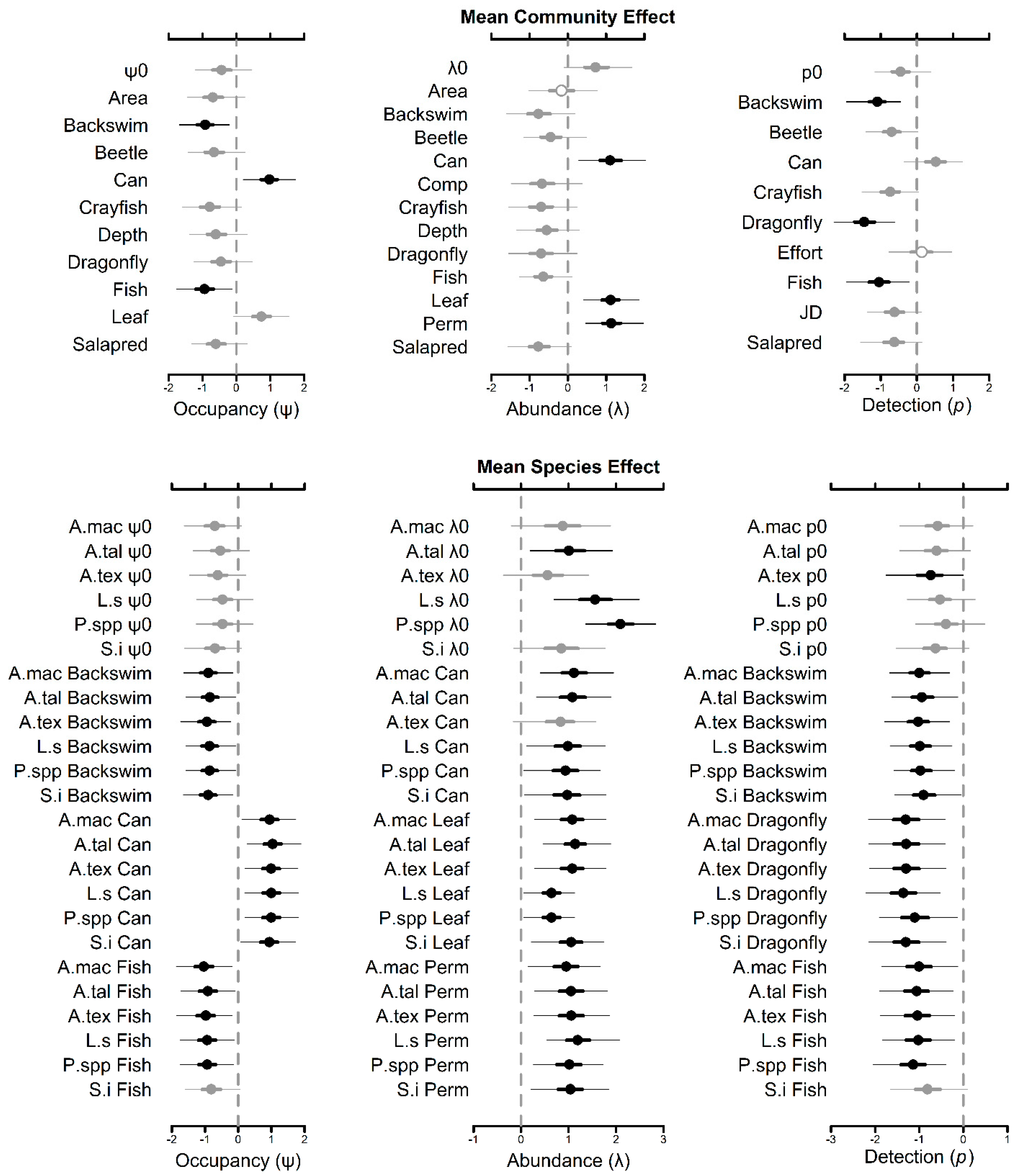
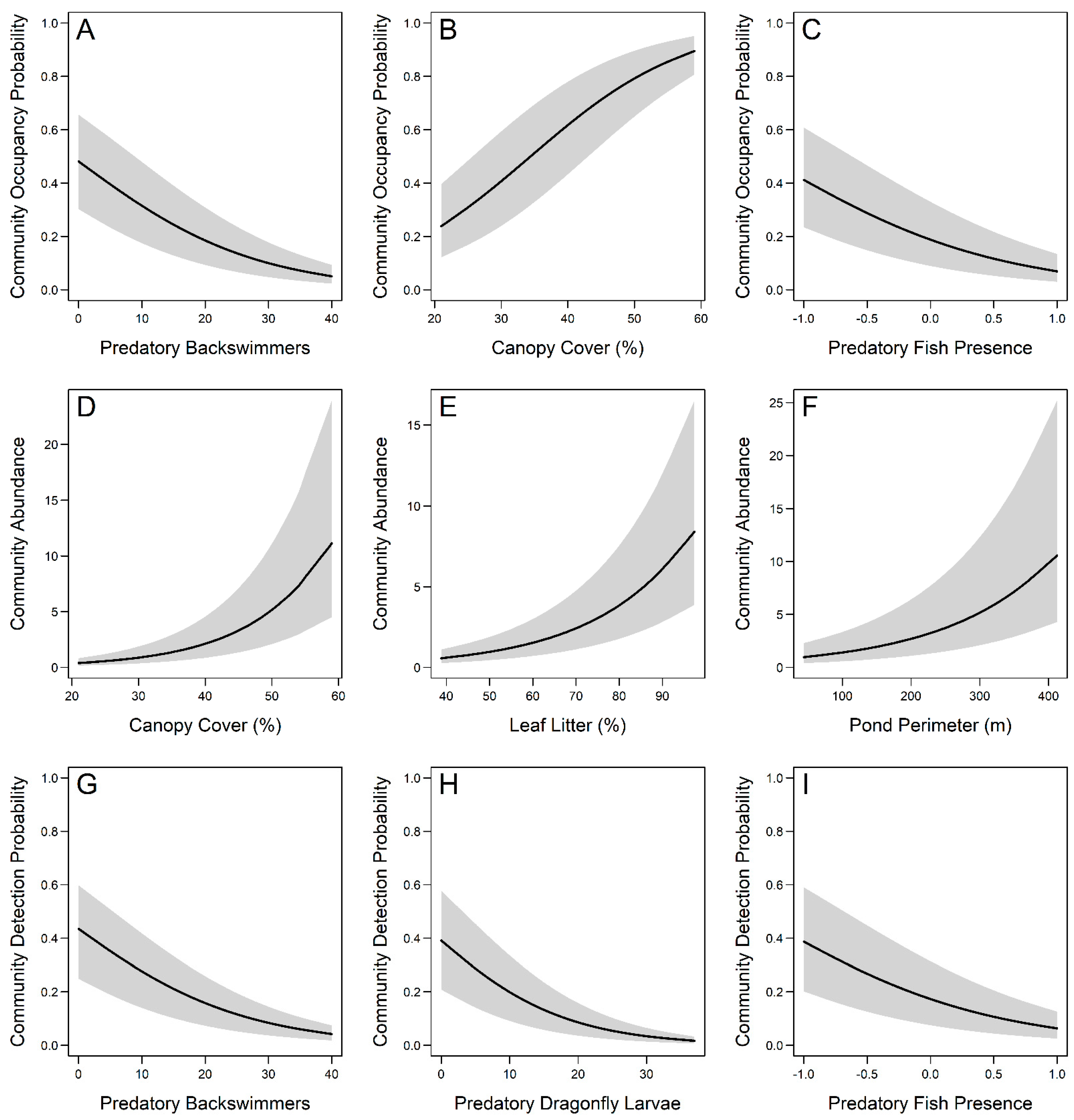
3.2. Community-Level Occupancy Responses
3.3. Community-Level Abundance Responses
3.4. Community-Level Detection Responses
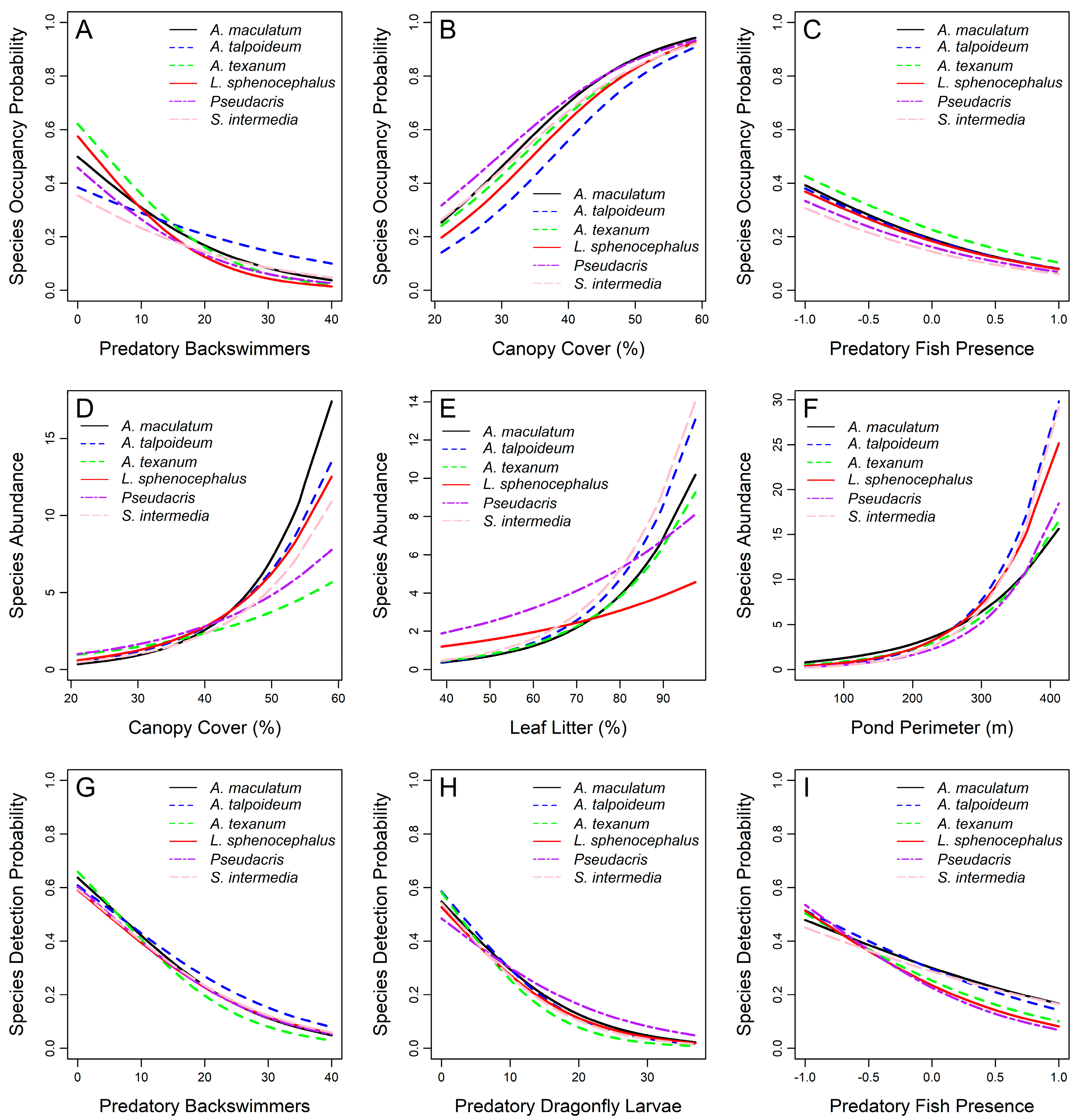
3.5. Species-Level Occupancy Responses
3.6. Species-Level Abundance Responses
3.7. Species-Level Detection Responses
4. Discussion
4.1. Occupancy
4.2. Abundance
4.3. Detection
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiegs, S.D.; Berven, K.A.; Carmack, D.J.; Capps, K.A. Stoichiometric implications of a biphasic life cycle. Oecologia 2016, 180, 853–863. [Google Scholar] [CrossRef]
- Rumrill, C.T.; Scott, D.E.; Lance, S.L. Delayed effects and complex life cycles: How the larval aquatic environment influences terrestrial performance and survival. Environ. Toxicol. Chem. 2018, 37, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.J.; Price, T.D. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am. Nat. 2004, 164, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Nolan, N.; Hayward, M.W.; Klop-Toker, K.; Mahony, M.; Lemckert, F.; Callen, A. Complex organisms must deal with complex threats: How does amphibian conservation deal with biphasic life cycles? Animals 2023, 13, 1634. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. The contribution of population and community biology to conservation science. Annu. Rev. Ecol. Syst. 1988, 19, 473–511. [Google Scholar] [CrossRef]
- Dorazio, R.M.; Royle, J.A.; Soderstrom, B.; Glimskar, A. Estimating species richness and accumulation by modeling species occurrence and detectability. Ecology 2006, 87, 842–854. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Svenning, J.C. The role of biotic interactions in shaping distributions and realized assemblages of species: Implications for species distribution modeling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- Burton, T.M.; Likens, G.E. Energy flow and nutrient cycling in salamander populations in the Hubbard Brook Experimental Forest, New Hampshire. Ecology 1975, 56, 1068–1080. [Google Scholar] [CrossRef]
- Seale, D.B. Influence of amphibian larvae on primary production, nutrient flux, and competition in a pond ecosystem. Ecology 1980, 61, 1531–1550. [Google Scholar] [CrossRef]
- Hocking, D.J.; Babbitt, K.J. Amphibian contributions to ecosystem services. Herpetol. Conserv. Biol. 2014, 9, 1–17. [Google Scholar]
- West, J. Importance of Amphibians: A Synthesis of Their Environmental Functions, Benefits to Humans, and Need for Conservation. Ph.D. Thesis, Bridgewater State University, Bridgewater, MA, USA, 2018. [Google Scholar]
- Houlahan, J.E.; Findlay, C.S.; Schmidt, B.R.; Meyer, A.H.; Kuzmin, S.L. Quantitative evidence for global amphibian population declines. Nature 2000, 404, 752–755. [Google Scholar] [CrossRef]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Stuart, S.N. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.E. Amphibian metamorphosis: Growth rate, predation risk, and the optimal size at transformation. Am. Nat. 1986, 128, 319–341. [Google Scholar] [CrossRef]
- Warne, R.W.; Crespi, E.J.; Brunner, J.L. Escape from the pond: Stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct. Ecol. 2011, 25, 139–146. [Google Scholar] [CrossRef]
- Dastansara, N.; Vaissi, S.; Mosavi, J.; Sharifi, M. Impacts of temperature on growth, development, and survival of larval Bufo (Pseudepidalea) viridis (Amphibia: Anura): Implications of climate change. Zool. Ecol. 2017, 27, 228–234. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. Amphibian immunity–stress, disease, and climate change. Dev. Comp. Immunol. 2017, 66, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Denver, R.J. Environmental stress as a developmental cue: Corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm. Behav. 1997, 31, 169–179. [Google Scholar] [CrossRef]
- Beck, C.W.; Congdon, J.D. Effects of age and size at metamorphosis on performance and metabolic rates of Southern Toad, Bufo terrestris, metamorphs. Funct. Ecol. 2000, 14, 32–38. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Critical elements for biologically based recovery plans of aquatic-breeding amphibians. Conserv. Biol. 2002, 16, 619–629. [Google Scholar] [CrossRef]
- Warne, R.W.; Crespi, E.J. Larval growth rate and sex determine resource allocation and stress responsiveness across life stages in juvenile frogs. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2015, 323, 191–201. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Skelly, D.K. Ecology and conservation of pool-breeding amphibians. In Science and Conservation of Vernal Pools in Northeastern North America; CRC Press: Boca Raton, FL, USA, 2008; pp. 127–148. [Google Scholar]
- Brooks, G.C.; Kindsvater, H.K. Early development drives variation in amphibian vulnerability to global change. Front. Ecol. Evol. 2022, 10, 813414. [Google Scholar] [CrossRef]
- Newman, R.A. Ecological constraints on amphibian metamorphosis: Interactions of temperature and larval density with responses to changing food level. Oecologia 1998, 115, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Warne, R.W.; Kardon, A.; Crespi, E.J. Physiological, behavioral, and maternal factors that contribute to size variation in larval amphibian populations. PLoS ONE 2013, 8, e76364. [Google Scholar] [CrossRef]
- Rohr, J.R.; Elskus, A.A.; Shepherd, B.S.; Crowley, P.H.; McCarthy, T.M.; Niedzwiecki, J.H.; Palmer, B.D. Multiple stressors and salamanders: Effects of an herbicide, food limitation, and hydroperiod. Ecol. Appl. 2004, 14, 1028–1040. [Google Scholar] [CrossRef]
- Denver, R.J.; Glennemeier, K.A.; Boorse, G.C. Endocrinology of complex life cycles: Amphibians. In Hormones, Brain and Behavior; Pfaff, D.W., Arnold, A.P., Etgen, A.M., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 469–XI. [Google Scholar]
- Linder, G.; Palmer, B.D.; Little, E.E.; Rowe, C.L.; Henry, P. Physiological ecology of amphibians and reptiles. In Ecotoxicology of Amphibians and Reptiles; CRC Press: Pensacola, FL, USA, 2010; pp. 105–166. [Google Scholar]
- Crespi, E.J.; Warne, R.W. Environmental conditions experienced during the tadpole stage alter post-metamorphic glucocorticoid response to stress in an amphibian. Integr. Comp. Biol. 2013, 53, 989–1001. [Google Scholar] [CrossRef]
- Székely, D.; Cogălniceanu, D.; Székely, P.; Armijos-Ojeda, D.; Espinosa-Mogrovejo, V.; Denoël, M. How to recover from a bad start: Size at metamorphosis affects growth and survival in a tropical amphibian. BMC Ecol. 2020, 20, 24. [Google Scholar] [CrossRef]
- Burraco, P.; Valdés, A.E.; Orizaola, G. Metabolic costs of altered growth trajectories across life transitions in amphibians. J. Anim. Ecol. 2020, 89, 855–866. [Google Scholar] [CrossRef]
- Hamer, A.J.; Barta, B.; Bohus, A.; Gál, B.; Schmera, D. Roads reduce amphibian abundance in ponds across a fragmented landscape. Glob. Ecol. Conserv. 2021, 28, e01663. [Google Scholar] [CrossRef]
- Hamer, A.J.; Schmera, D.; Mahony, M.J. Multi-species occupancy modeling provides novel insights into amphibian metacommunity structure and wetland restoration. Ecol. Appl. 2021, 31, e2293. [Google Scholar] [CrossRef]
- Amburgey, S.M.; Bailey, L.L.; Murphy, M.; Muths, E.; Funk, W.C. The effects of hydropattern and predator communities on amphibian occupancy. Can. J. Zool. 2014, 92, 927–937. [Google Scholar] [CrossRef]
- O’Regan, S.M.; Palen, W.J.; Anderson, S.C. Climate warming mediates negative impacts of rapid pond drying for three amphibian species. Ecology 2014, 95, 845–855. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and indirect effects of climate change on amphibian populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Han, B.A.; Relyea, R.A.; Johnson, P.T.; Buck, J.C.; Gervasi, S.S.; Kats, L.B. The complexity of amphibian population declines: Understanding the role of cofactors in driving amphibian losses. Ann. N. Y. Acad. Sci. 2011, 1223, 108–119. [Google Scholar] [CrossRef]
- Morin, P.J. Predation, competition, and the composition of larval anuran guilds. Ecol. Monogr. 1983, 53, 119–138. [Google Scholar] [CrossRef]
- Cocciardi, J.M.; Ohmer, M.E. Drivers of intraspecific variation in thermal traits and their importance for resilience to global change in amphibians. Integr. Comp. Biol. 2024, 64, 882–899. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.A.; Grant, E.H.C.; Muths, E.; Amburgey, S.M.; Adams, M.J.; Joseph, M.B.; Sigafus, B.H. Quantifying climate sensitivity and climate-driven change in North American amphibian communities. Nat. Commun. 2018, 9, 3926. [Google Scholar] [CrossRef]
- Lavergne, S.; Mouquet, N.; Thuiller, W.; Ronce, O. Biodiversity and climate change: Integrating evolutionary and ecological responses of species and communities. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 321–350. [Google Scholar] [CrossRef]
- Vellend, M.; Lajoie, G.; Bourret, A.; Múrria, C.; Kembel, S.W.; Garant, D. Drawing ecological inferences from coincident patterns of population- and community-level biodiversity. Mol. Ecol. 2014, 23, 2890–2901. [Google Scholar] [CrossRef]
- Guillera-Arroita, G. Modelling of species distributions, range dynamics and communities under imperfect detection: Advances, challenges and opportunities. Ecography 2017, 40, 281–295. [Google Scholar] [CrossRef]
- Skelly, D.K. Distributions of pond-breeding anurans: An overview of mechanisms. Isr. J. Zool. 2001, 47, 313–332. [Google Scholar] [CrossRef]
- Drayer, A.N.; Richter, S.C. Physical wetland characteristics influence amphibian community composition differently in constructed wetlands and natural wetlands. Ecol. Eng. 2016, 93, 166–174. [Google Scholar] [CrossRef]
- Meerhoff, M.; Beklioğlu, M. Shallow lakes and ponds. In Wetzel’s Limnology; Academic Press: Amsterdam, The Netherlands, 2024; pp. 859–892. [Google Scholar]
- Ruiz-Gutiérrez, V.; Zipkin, E.F. Detection biases yield misleading patterns of species persistence and colonization in fragmented landscapes. Ecosphere 2011, 2, 1–14. [Google Scholar] [CrossRef]
- Dorazio, R.M.; Royle, J.A. Estimating size and composition of biological communities by modeling the occurrence of species. J. Am. Stat. Assoc. 2005, 100, 389–398. [Google Scholar] [CrossRef]
- Dorazio, R.M. Accounting for imperfect detection and survey bias in statistical analysis of presence-only data. Glob. Ecol. Biogeogr. 2014, 23, 1472–1484. [Google Scholar] [CrossRef]
- Kéry, M.; Royle, J.A. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS, 1st ed.; Academic Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Zipkin, E.F.; Dewan, A.; Royle, J.A. Impacts of forest fragmentation on species richness: A hierarchical approach to community modelling. J. Appl. Ecol. 2009, 46, 815–822. [Google Scholar] [CrossRef]
- Devarajan, K.; Morelli, T.L.; Tenan, S. Multi-species occupancy models: Review, roadmap, and recommendations. Ecography 2020, 43, 1612–1624. [Google Scholar] [CrossRef]
- Dorazio, R.M.; Kéry, M.; Royle, J.A.; Plattner, M. Models for inference in dynamic metacommunity systems. Ecology 2010, 91, 2466–2475. [Google Scholar] [CrossRef]
- Mihaljevic, J.R.; Joseph, M.B.; Johnson, P.T. Using multispecies occupancy models to improve the characterization and understanding of metacommunity structure. Ecology 2015, 96, 1783–1792. [Google Scholar] [CrossRef]
- Gomez, J.P.; Robinson, S.K.; Blackburn, J.K.; Ponciano, J.M. An efficient extension of N-mixture models for multi-species abundance estimation. Methods Ecol. Evol. 2018, 9, 340–353. [Google Scholar] [CrossRef]
- Link, W.A. Modeling pattern in collections of parameters. J. Wildl. Manag. 1999, 63, 1017–1027. [Google Scholar] [CrossRef]
- Zipkin, E.F.; Royle, J.A.; Dawson, D.K.; Bates, S. Multi-species occurrence models to evaluate the effects of conservation and management actions. Biol. Conserv. 2010, 143, 479–484. [Google Scholar] [CrossRef]
- Thorson, J.T.; Barnett, L.A. Comparing estimates of abundance trends and distribution shifts using single-and multispecies models of fishes and biogenic habitat. ICES J. Mar. Sci. 2017, 74, 1311–1321. [Google Scholar] [CrossRef]
- Ramsar Convention. The Ramsar Convention on Wetlands. Available online: http://www.ramsar.org (accessed on 15 April 2024).
- Demissie, M.; Soong, T.W.; Keefer, L.L.; Allgire, R.L.; Makowski, P.B. Cache River Basin: Hydrology, Hydraulics, and Sediment Transport; ISWS: Champaign, IL, USA, 1990; Volume 1. [Google Scholar]
- Hutton, J.M.; Macedo, A.D.; Warne, R.W. Factors influencing the occupancy and detection of nonbreeding Hyla chrysoscelis within artificial polyvinyl chloride refugia. Herpetologica 2024, 80, 221–233. [Google Scholar] [CrossRef]
- Hutton, J.M.; Macedo, A.D.; Brown, J.L.; Warne, R.W. A novel indicator to assess PIT tag retention in an aquatic amphibian: Impact of body size. Anim. Biotelemetry 2024, 12, 37. [Google Scholar] [CrossRef]
- Phillips, C.A.; Crawford, J.A.; Kuhns, A.R. Field Guide to Amphibians and Reptiles of Illinois; University of Illinois Press: Champaign, IL, USA, 2022. [Google Scholar]
- Denton, R.D.; Richter, S.C. A quantitative comparison of two common amphibian sampling techniques for wetlands. Herpetol. Rev. 2012, 43, 44–47. [Google Scholar]
- Knape, J.; Korner-Nievergelt, F. Estimates from non-replicated population surveys rely on critical assumptions. Methods Ecol. Evol. 2015, 6, 298–306. [Google Scholar] [CrossRef]
- Altig, R.; McDiarmid, R.W. Handbook of Larval Amphibians of the United States and Canada; Cornell University Press: Ithaca, NY, USA, 2017. [Google Scholar]
- Merritt, R.W.; Cummins, K.W. (Eds.) An Introduction to the Aquatic Insects of North America; Kendall Hunt: Dubuque, IA, USA, 1996. [Google Scholar]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; Johns Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Altig, R.; McDiarmid, R.W. (Eds.) Tadpoles: The Biology of Anuran Larvae; University of Chicago Press: Chicago, IL, USA, 1999. [Google Scholar]
- Lannoo, M.J. (Ed.) Amphibian Declines: The Conservation Status of United States Species; University of California Press: Berkeley, CA, USA, 2005. [Google Scholar]
- Anderson, J.T.; Zilli, F.L.; Montalto, L.; Marchese, M.R.; McKinney, M.; Park, Y.L. Sampling and processing aquatic and terrestrial invertebrates in wetlands. In Wetland Techniques; Anderson, J.T., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 143–195. [Google Scholar]
- Peterman, W.E.; Anderson, T.L.; Drake, D.L.; Ousterhout, B.H.; Semlitsch, R.D. Maximizing pond biodiversity across the landscape: A case study of larval ambystomatid salamanders. Anim. Conserv. 2014, 17, 275–285. [Google Scholar] [CrossRef]
- Simpkins, C.A.; Shuker, J.D.; Lollback, G.W.; Castley, J.G.; Hero, J.M. Environmental variables associated with the distribution and occupancy of habitat specialist tadpoles in naturally acidic, oligotrophic waterbodies. Aust. Ecol. 2014, 39, 95–105. [Google Scholar] [CrossRef]
- Werner, E.E.; Skelly, D.K.; Relyea, R.A.; Yurewicz, K.L. Amphibian species richness across environmental gradients. Oikos 2007, 116, 1697–1712. [Google Scholar] [CrossRef]
- Anderson, T.L.; Heemeyer, J.L.; Peterman, W.E.; Everson, M.J.; Ousterhout, B.H.; Drake, D.L.; Semlitsch, R.D. Automated analysis of temperature variance to determine inundation state of wetlands. Wetl. Ecol. Manag. 2015, 23, 1039–1047. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Royle, J.A.; Langtimm, C.A. Estimating site occupancy rates when detection probabilities are less than one. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Gelman, A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 2008, 27, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Wit, E.; Heuvel, E.V.D.; Romeijn, J.W. ‘All models are wrong…’: An introduction to model uncertainty. Stat. Neerl. 2012, 66, 217–236. [Google Scholar] [CrossRef]
- Bürkner, P.C.; Scholz, M.; Radev, S.T. Some models are useful, but how do we know which ones? Towards a unified Bayesian model taxonomy. Statist. Surv. 2023, 17, 216–310. [Google Scholar] [CrossRef]
- Newling, J.; Bassett, B.; Hlozek, R.; Kunz, M.; Smith, M.; Varughese, M. Parameter estimation with Bayesian estimation applied to multiple species in the presence of biases and correlations. Mon. Not. R. Astron. Soc. 2012, 421, 913–925. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef]
- Herwig, B.R.; Schroeder, L.W.; Zimmer, K.D.; Hanson, M.A.; Staples, D.F.; Wright, R.G.; Younk, J.A. Fish influences on amphibian presence and abundance in prairie and parkland landscapes of Minnesota, USA. J. Herpetol. 2013, 47, 489–497. [Google Scholar] [CrossRef]
- Hamer, A.J.; Horányi, J. Improving inference within freshwater community studies: Accounting for variable detection rates of amphibians and fish. Ecol. Evol. 2024, 14, e70383. [Google Scholar] [CrossRef]
- Kéry, M.; Schaub, M. Estimation of occupancy and species distributions from detection/nondetection data in metapopulation designs using site-occupancy models. In Bayesian Population Analysis Using WinBUGS: A Hierarchical Perspective; Academic Press: Cambridge, MA, USA, 2012; pp. 413–461. [Google Scholar]
- MacKenzie, D.I. Modeling the probability of resource use: The effect of, and dealing with, detecting a species imperfectly. J. Wildl. Manag. 2006, 70, 367–374. [Google Scholar] [CrossRef]
- Kéry, M.; Royle, J.A. Hierarchical Bayes estimation of species richness and occupancy in spatially replicated surveys. J. Appl. Ecol. 2008, 45, 589–598. [Google Scholar] [CrossRef]
- Latif, Q.S.; Valente, J.J.; Johnston, A.; Davis, K.L.; Fogarty, F.A.; Green, A.W.; Zhao, Q. Designing count-based studies in a world of hierarchical models. J. Wildl. Manag. 2024, 88, e22622. [Google Scholar] [CrossRef]
- Royle, J.A. N-mixture models for estimating population size from spatially replicated counts. Biometrics 2004, 60, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Royle, J.A.; Nichols, J.D.; Kéry, M. Modelling occurrence and abundance of species when detection is imperfect. Oikos 2005, 110, 353–359. [Google Scholar] [CrossRef]
- Kéry, M.; Dorazio, R.M.; Soldaat, L.; Van Strien, A.; Zuiderwijk, A.; Royle, J.A. Trend estimation in populations with imperfect detection. J. Appl. Ecol. 2009, 46, 1163–1172. [Google Scholar] [CrossRef]
- Knape, J.; Arlt, D.; Barraquand, F.; Berg, Å.; Chevalier, M.; Pärt, T.; Żmihorski, M. Sensitivity of binomial N-mixture models to overdispersion: The importance of assessing model fit. Methods Ecol. Evol. 2018, 9, 2102–2114. [Google Scholar] [CrossRef]
- Link, W.A.; Schofield, M.R.; Barker, R.J.; Sauer, J.R. On the robustness of N-mixture models. Ecology 2018, 99, 1547–1551. [Google Scholar] [CrossRef]
- Williams, P.J.; Robb, J.R.; Karns, D.R. Occupancy dynamics of breeding Crawfish Frogs in southeastern Indiana. Wildl. Soc. Bull. 2012, 36, 350–357. [Google Scholar] [CrossRef]
- Hartel, T.; Nemes, S.; Cogălniceanu, D.; Öllerer, K.; Schweiger, O.; Moga, C.I.; Demeter, L. The effect of fish and aquatic habitat complexity on amphibians. Hydrobiologia 2007, 583, 173–182. [Google Scholar] [CrossRef]
- Lewis, T. Environmental Influences on the Population Ecology of Sixteen Anuran Amphibians in a Seasonally Flooded Neotropical Forest. Ph.D. Thesis, Farnborough College of Technology, Farnborough, UK, 2009. [Google Scholar]
- Hamer, A.J.; Parris, K.M. Predation modifies larval amphibian communities in urban wetlands. Wetlands 2013, 33, 641–652. [Google Scholar] [CrossRef]
- Jeffries, M.J.; Epele, L.B.; Studinski, J.M.; Vad, C.F. Invertebrates in temporary wetland ponds of the temperate biomes. In Invertebrates in Freshwater Wetlands: An International Perspective on Their Ecology; Springer: Dordrecht, The Netherlands, 2016; pp. 105–139. [Google Scholar]
- Pacifici, K.; Zipkin, E.F.; Collazo, J.A.; Irizarry, J.I.; DeWan, A. Guidelines for a priori grouping of species in hierarchical community models. Ecol. Evol. 2014, 4, 877–888. [Google Scholar] [CrossRef]
- Beasley, E.M. Ecologically informed priors improve Bayesian model estimates of species richness and occupancy for undetected species. Ecol. Appl. 2024, 34, e2941. [Google Scholar] [CrossRef]
- Anholt, B.R.; Skelly, D.K.; Werner, E.E. Factors modifying antipredator behavior in larval toads. Herpetologica 1996, 52, 301–313. [Google Scholar]
- Nicieza, A.G. Interacting effects of predation risk and food availability on larval anuran behaviour and development. Oecologia 2000, 123, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Oakley, J.E.; O’Hagan, A. Probabilistic sensitivity analysis of complex models: A Bayesian approach. J. R. Stat. Soc. B 2004, 66, 751–769. [Google Scholar] [CrossRef]
- Conn, P.B.; Johnson, D.S.; Williams, P.J.; Melin, S.R.; Hooten, M.B. A guide to Bayesian model checking for ecologists. Ecol. Monogr. 2018, 88, 526–542. [Google Scholar] [CrossRef]
- Schaub, M.; Kéry, M. Integrated Population Models: Theory and Ecological Applications with R and JAGS; Academic Press: London, UK, 2021. [Google Scholar]
- Cumming, G.; Finch, S. Inference by eye: Confidence intervals and how to read pictures of data. Am. Psychol. 2005, 60, 170. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.T.; Ferreira, M.A.; Kays, R.W.; Forrester, T.D.; Kalies, E.L.; McShea, W.J.; Millspaugh, J.J. A multispecies occupancy model for two or more interacting species. Methods Ecol. Evol. 2016, 7, 1164–1173. [Google Scholar] [CrossRef]
- Plummer, M. JAGS Version 3.4.0 User Manual. Available online: https://sourceforge.net/projects/mcmc-jags/files/Manuals/ (accessed on 29 January 2025).
- Su, Y.S.; Yajima, M. R2jags: Using R to Run ‘JAGS’. R Package Version 0.5-7. Available online: https://cran.r-project.org/web/packages/R2jags/ (accessed on 29 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 457–472. [Google Scholar] [CrossRef]
- Brooks, S.P.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar] [CrossRef]
- Olson, G.S.; Anthony, R.G.; Forsman, E.D.; Ackers, S.H.; Loschl, P.J.; Reid, J.A.; Ripple, W.J. Modeling of site occupancy dynamics for northern spotted owls, with emphasis on the effects of barred owls. J. Wildl. Manag. 2005, 69, 918–932. [Google Scholar] [CrossRef]
- Roloff, G.J.; Grazia, T.E.; Millenbah, K.F.; Kroll, A.J. Factors associated with amphibian detection and occupancy in southern Michigan forests. J. Herpetol. 2011, 45, 15–22. [Google Scholar] [CrossRef]
- Gelman, A.; Meng, X.L.; Stern, H. Posterior predictive assessment of model fitness via realized discrepancies. Stat. Sin. 1996, 6, 733–760. [Google Scholar]
- Stolen, E.D.; Oddy, D.M.; Gann, S.L.; Holloway-Adkins, K.G.; Legare, S.A.; Weiss, S.K.; Breininger, D.R. Accounting for heterogeneity in false-positive detection rate in southeastern beach mouse habitat occupancy models. Ecosphere 2019, 10, e02893. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; Van Der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. B 2002, 64, 583–639. [Google Scholar] [CrossRef]
- Hooten, M.B.; Hobbs, N.T. A guide to Bayesian model selection for ecologists. Ecol. Monogr. 2015, 85, 3–28. [Google Scholar] [CrossRef]
- Stretz, P.; Anderson, T.L.; Burkhart, J.J. Macroinvertebrate foraging on larval Ambystoma maculatum across ontogeny. Copeia 2019, 107, 244–249. [Google Scholar] [CrossRef]
- Pilliod, D.S.; Hossack, B.R.; Bahls, P.F.; Bull, E.L.; Corn, P.S.; Hokit, G.; Wyrick, A. Non-native salmonids affect amphibian occupancy at multiple spatial scales. Divers. Distrib. 2010, 16, 959–974. [Google Scholar] [CrossRef]
- Gehlbach, F.R.; Kennedy, S.E. Population ecology of a highly productive aquatic salamander Siren intermedia. Southwest. Nat. 1978, 23, 423–430. [Google Scholar] [CrossRef]
- Raymond, L.R. Seasonal activity of Siren intermedia in northwestern Louisiana (Amphibia: Sirenidae). Southwest. Nat. 1991, 36, 144–147. [Google Scholar] [CrossRef]
- Jackson, M.E.; Semlitsch, R.D. Paedomorphosis in the salamander Ambystoma talpoideum: Effects of a fish predator. Ecology 1993, 74, 342–350. [Google Scholar] [CrossRef]
- Frese, P.W.; Mathis, A.; Wilkinson, R. Population characteristics, growth, and spatial activity of Siren intermedia in an intensively managed wetland. Southwest. Nat. 2003, 48, 534–542. [Google Scholar] [CrossRef]
- Sawyer, J.A.; Trauth, S.E. Seasonal activity, population characteristics, and age estimation in the aquatic salamander Siren intermedia nettingi (Goin). J. Ark. Acad. Sci. 2011, 65, 117–125. [Google Scholar] [CrossRef]
- Thornton, B. Population characteristics of the lesser siren (Siren intermedia) in central Illinois. Ill. State Acad. Sci. Trans. 2023, 116, 7–11. [Google Scholar]
- Semlitsch, R.D. Reproductive strategy of a facultatively paedomorphic salamander Ambystoma talpoideum. Oecologia 1985, 65, 305–313. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Harris, R.N.; Wilbur, H.M. Paedomorphosis in Ambystoma talpoideum: Maintenance of population variation and alternative life-history pathways. Evolution 1990, 44, 1604–1613. [Google Scholar] [PubMed]
- Baker, J.; Beebee, T.; Buckley, J.; Gent, T.; Orchard, D. Amphibian Habitat Management Handbook; Amphibian and Reptile Conservation: Bournemouth, UK, 2011; p. 39. [Google Scholar]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batáry, P.; Westphal, C. Landscape moderation of biodiversity patterns and processes—Eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef]
- Baldwin, R.F.; Calhoun, A.J.K.; deMaynadier, P.G. The significance of hydroperiod and stand maturity for pool-breeding amphibians in forested landscapes. Can. J. Zool. 2006, 84, 1604–1615. [Google Scholar] [CrossRef]
- Earl, J.E.; Luhring, T.M.; Williams, B.K.; Semlitsch, R.D. Biomass export of salamanders and anurans from ponds is affected differentially by changes in canopy cover. Freshw. Biol. 2011, 56, 2473–2482. [Google Scholar] [CrossRef]
- Peterman, W.E.; Rittenhouse, T.A.; Earl, J.E.; Semlitsch, R.D. Demographic network and multi-season occupancy modeling of Rana sylvatica reveal spatial and temporal patterns of population connectivity and persistence. Landsc. Ecol. 2013, 28, 1601–1613. [Google Scholar] [CrossRef]
- Burrow, A.K.; Maerz, J.C. Experimental confirmation of effects of leaf litter type and light on tadpole performance for two priority amphibians. Ecosphere 2021, 12, e03729. [Google Scholar] [CrossRef]
- Stephens, J.P.; Berven, K.A.; Tiegs, S.D. Anthropogenic changes to leaf litter input affect the fitness of a larval amphibian. Freshw. Biol. 2013, 58, 1631–1646. [Google Scholar] [CrossRef]
- Curtis, A.E.; Paton, P.W. Assessing detection probabilities of larval amphibians and macroinvertebrates in isolated ponds. Wetlands 2010, 30, 901–914. [Google Scholar] [CrossRef]
- Skelly, D.K.; Richardson, J.L. Larval sampling. In Amphibian Ecology and Conservation: A Handbook of Techniques; Oxford University Press: Oxford, UK, 2010; pp. 55–70. [Google Scholar]
- Twining, J.P.; Fuller, A.K.; Sun, C.C.; Calderón-Acevedo, C.A.; Schlesinger, M.D.; Berger, M.; Frair, J.L. Integrating presence-only and detection/non-detection data to estimate distributions and expected abundance of difficult-to-monitor species on a landscape-scale. J. Appl. Ecol. 2024, 61, 1441–1459. [Google Scholar] [CrossRef]
- Albecker, M.; Vance-Chalcraft, H.D. Mismatched anti-predator behavioral responses in predator-naïve larval anurans. PeerJ 2015, 3, e1472. [Google Scholar] [CrossRef]
- Brown, T.A.; Fraker, M.E.; Ludsin, S.A. Space use of predatory larval dragonflies and tadpole prey in response to chemical cues of predation. Am. Midl. Nat. 2019, 181, 53–62. [Google Scholar] [CrossRef]
- Flores, C.A.; Crossland, M.R.; Arreortúa, M.; González-Bernal, E. Responses of Showy Leopard Frog Larvae (Lithobates spectabilis) to the Presence of Introduced Rainbow Trout in Mexico. Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4254. [Google Scholar] [CrossRef]
- Relyea, R.A. Fine-tuned phenotypes: Tadpole plasticity under 16 combinations of predators and competitors. Ecology 2004, 85, 172–179. [Google Scholar] [CrossRef]
- Wilson, R.S.; Kraft, P.G.; Van Damme, R. Predator-specific changes in the morphology and swimming performance of larval Rana lessonae. Funct. Ecol. 2005, 19, 238–244. [Google Scholar] [CrossRef]
- Schmidt, B.R.; Băncilă, R.I.; Hartel, T.; Grossenbacher, K.; Schaub, M. Shifts in amphibian population dynamics in response to a change in the predator community. Ecosphere 2021, 12, e03528. [Google Scholar] [CrossRef]
- Bennett, S.H.; Waldron, J.L.; Welch, S.M. Light bait improves capture success of aquatic funnel-trap sampling for larval amphibians. Southeast. Nat. 2012, 11, 49–58. [Google Scholar] [CrossRef]
- Petitot, M.; Manceau, N.; Geniez, P.; Besnard, A. Optimizing occupancy surveys by maximizing detection probability: Application to amphibian monitoring in the Mediterranean region. Ecol. Evol. 2014, 4, 3538–3549. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Zuiderwijk, A. Sampling efficiency, bias and shyness in funnel trapping aquatic newts. Amphibia-Reptilia 2020, 41, 413–420. [Google Scholar] [CrossRef]
- Baumgardt, J.A.; Morrison, M.L.; Brennan, L.A.; Thornley, M.; Campbell, T.A. Variation in herpetofauna detection probabilities: Implications for study design. Environ. Monit. Assess. 2021, 193, 658. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, J.D.; Gimenez, O. Detecting and estimating density dependence in wildlife populations. J. Wildl. Manag. 2013, 77, 12–23. [Google Scholar] [CrossRef]
- Sih, A.; Crowley, P.; McPeek, M.; Petranka, J.; Strohmeier, K. Predation, competition, and prey communities: A review of field experiments. Annu. Rev. Ecol. Syst. 1985, 16, 269–311. [Google Scholar] [CrossRef]
- Amir, Z.; Sovie, A.; Luskin, M.S. Inferring predator–prey interactions from camera traps: A Bayesian co-abundance modeling approach. Ecol. Evol. 2022, 12, e9627. [Google Scholar] [CrossRef]
- Crane, A.L.; Bryshun, R.; McCormack, M.; Achtymichuk, G.H.; Rivera-Hernández, I.A.; Ferrari, M.C. Evidence for the Predator Attraction Hypothesis in an amphibian predator–prey system. J. Evol. Bio. 2023, 36, 57–66. [Google Scholar] [CrossRef]
- Wizen, G.; Gasith, A. An unprecedented role reversal: Ground beetle larvae (Coleoptera: Carabidae) lure amphibians and prey upon them. PLoS ONE 2011, 6, e25161. [Google Scholar] [CrossRef]
- Legendre, P.; Fortin, M.J. Spatial pattern and ecological analysis. Vegetatio 1989, 80, 107–138. [Google Scholar] [CrossRef]
- Guzy, J.C.; Halloran, K.M.; Homyack, J.A.; Thornton-Frost, J.E.; Willson, J.D. Differential responses of amphibian and reptile assemblages to size of riparian buffers within managed forests. Ecol. Appl. 2019, 29, e01995. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutton, J.M.; Warne, R.W. Key Habitat and Predatory Influences on the Community- and Species-Level Population Dynamics of Spring-Breeding Amphibian Larvae Within a Remnant Tupelo-Cypress Wetland. Hydrobiology 2025, 4, 15. https://doi.org/10.3390/hydrobiology4020015
Hutton JM, Warne RW. Key Habitat and Predatory Influences on the Community- and Species-Level Population Dynamics of Spring-Breeding Amphibian Larvae Within a Remnant Tupelo-Cypress Wetland. Hydrobiology. 2025; 4(2):15. https://doi.org/10.3390/hydrobiology4020015
Chicago/Turabian StyleHutton, Jacob M., and Robin W. Warne. 2025. "Key Habitat and Predatory Influences on the Community- and Species-Level Population Dynamics of Spring-Breeding Amphibian Larvae Within a Remnant Tupelo-Cypress Wetland" Hydrobiology 4, no. 2: 15. https://doi.org/10.3390/hydrobiology4020015
APA StyleHutton, J. M., & Warne, R. W. (2025). Key Habitat and Predatory Influences on the Community- and Species-Level Population Dynamics of Spring-Breeding Amphibian Larvae Within a Remnant Tupelo-Cypress Wetland. Hydrobiology, 4(2), 15. https://doi.org/10.3390/hydrobiology4020015





