Defense Limitations of Single Parents in the Biparental Convict Cichlid Fish: A Field Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site Description
2.2. Identification of Convict Cichlid Families and Parental Treatment Group Set up
2.3. Data Collection
- Intruder event: The encroachment of a conspecific or heterospecific fish within an approximate distance of 0.5 m or less of the target family’s territory boundary. Territory area was visually estimated as the area encompassing the parent(s) and fry when no intruders were present.
- Intruder chase: The number of times a conspecific or heterospecific intruder was chased away from the territory by a parent. If a pair of intruders were chased away simultaneously, it was recorded as a single intruder event.
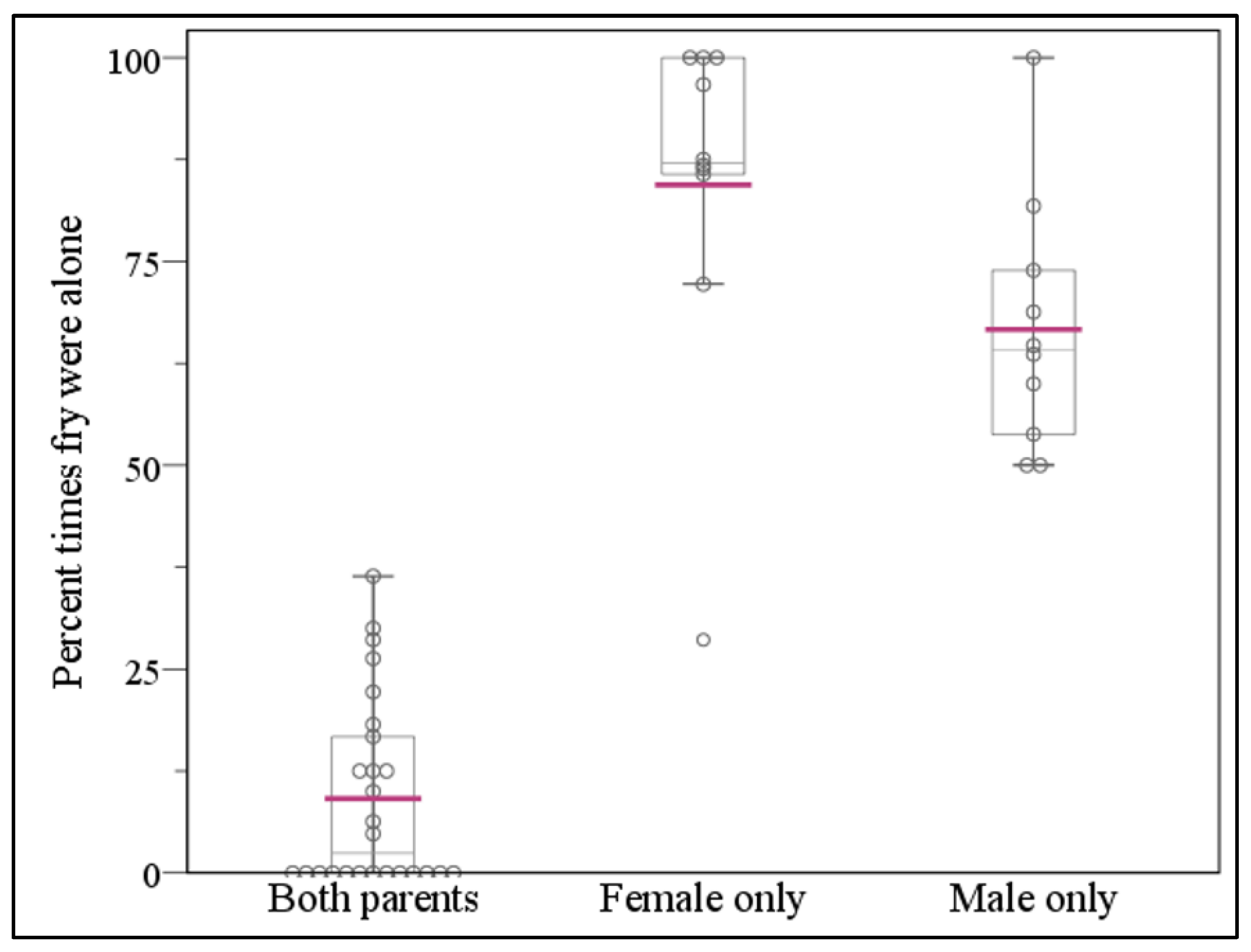
- Times a shoal was left unattended: The percentage of intruder events (intruders within at least 0.5 m of the territory) where a parent was not within at least ~3 body lengths of the offspring shoal.
- Pelvic fin-flicks: The number of pelvic fin-flicks performed by parents during and within 5 s immediately after an intruder event. A fin-flick was characterized as the quick anterior extension of the paired pectoral fins while simultaneously exerting forward thrusts using the anal and caudal fins leading to no net movement during the flicking behavior [23].
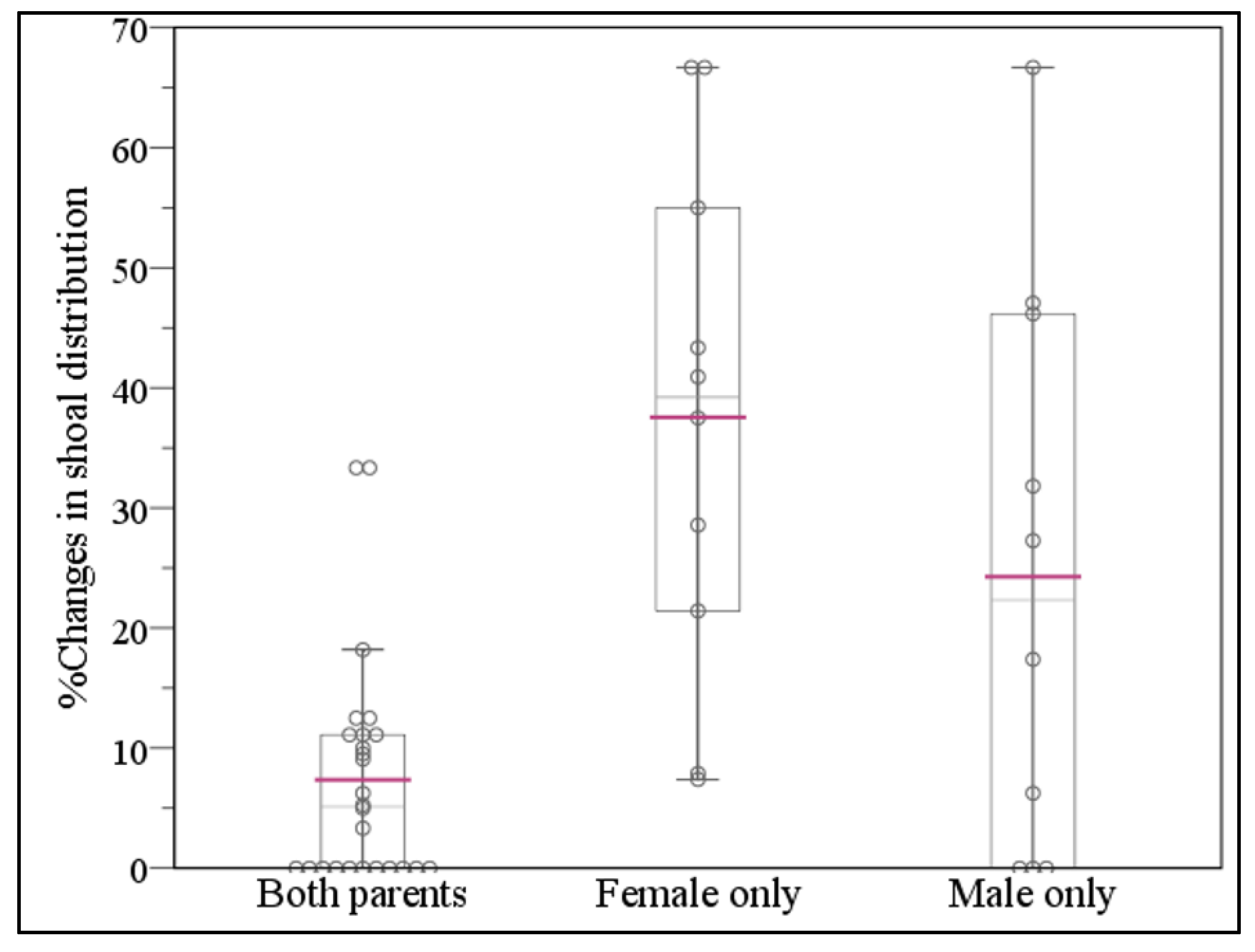
- Changes in shoal distribution: The percent of intruder events where a change in the horizontal or vertical distribution of the offspring shoal was observed compared to just before the intruder event. A change could occur in either direction, but most changes resulted in less cohesive shoals.
2.4. Statistical Analysis
3. Results
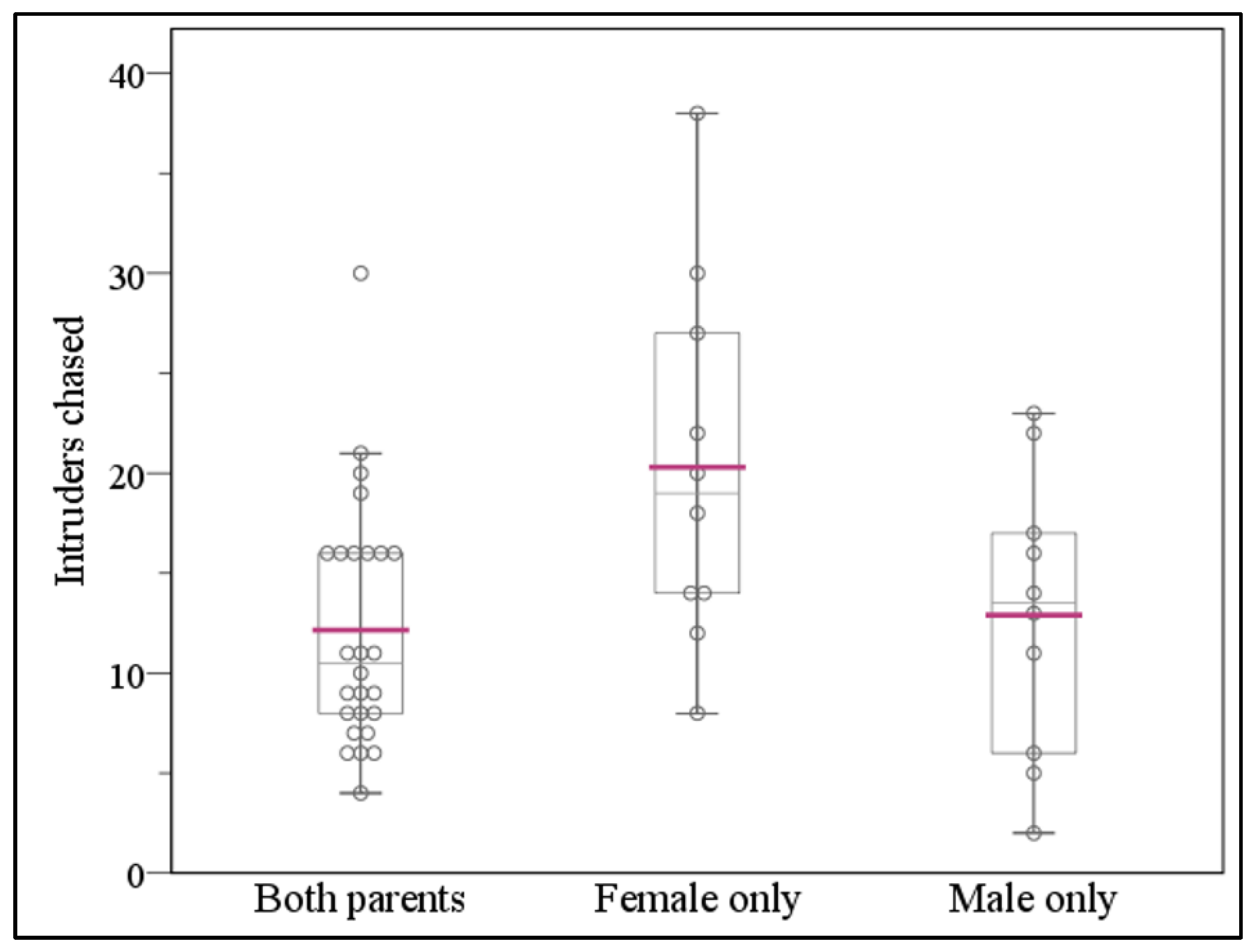
3.1. Single-Females Chase More Intruders than Single-Males or Both Parents Combined
3.2. Offspring Shoals Were Unattended More Often in Single Versus Both-Parent Treatments
3.3. Fin-Flicks Following Intruder Events
3.4. Changes in Offspring Shoal Distribution
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mock, D.W. Parental care in birds. Curr. Biol. 2022, 32, R1132–R1136. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, M.F.; Bell, A.M. Insights into Parental Care from Studies on Non-mammalian Vertebrates. Affect. Sci. 2022, 3, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.; Zaret, T.M. Parental Care Patterns of Fishes. Am. Nat. 1979, 113, 351–361. [Google Scholar] [CrossRef]
- Sargent, R.C.; Gross, M.R. The Behaviour of Teleost Fishes; The Johns Hopkins University Press: Baltimore, MD, USA, 1986; pp. 275–293. [Google Scholar] [CrossRef]
- Boesch, C. Clutton-Brock T. H. 1991. The Evolution of Parental Care. Princeton University Press. 352 pp., P/b: $19.95, H/b $ 49.50. ISBN: 0-691-02516-9. J. Evol. Biol. 1992, 5, 719–721. [Google Scholar] [CrossRef]
- Keenleyside, M.H.A.; Mackereth, R.W. Effects of loss of male parent on brood survival in a biparental cichlid fish. Environ. Biol. Fishes 1992, 34, 207–212. [Google Scholar] [CrossRef]
- Wisenden, B.D. Effect of Predation on Shaping Parental Brood Defense and Larval Ontogeny of Convict Cichlids Leading to Population Divergence. Diversity 2020, 12, 136. [Google Scholar] [CrossRef]
- Mrowka, W. Effect of removal of the mate on the parental care behaviour of the biparental cichlid Aequidens paraguayensis. Anim. Behav. 1982, 30, 295–297. [Google Scholar] [CrossRef]
- Wisenden, B.D. Reproductive behaviour of free-ranging convict cichlids, Cichlasoma nigrofasciatum. Environ. Biol. Fishes 1995, 43, 121–134. [Google Scholar] [CrossRef]
- Alonzo, J.J.; McKaye, K.R.; Berghe, E.P. Parental defense of young by the convict cichlid, Archocentrus nigrofasciatus, in Lake Xiloá, Nicaragua. J. Aquaric. Aquat. Sci. 1999, 9, 208–228. [Google Scholar]
- McKaye, K.R. Competition for Breeding Sites between the Cichlid Fishes of Lake Jiloa, Nicaragua. Ecology 1977, 58, 291–302. [Google Scholar] [CrossRef]
- Snekser, J.L.; Itzkowitz, M. Contrasting Parental Tasks Influence Parental Roles for Paired and Single Biparental Cichlid Fish. Ethology 2014, 120, 483–491. [Google Scholar] [CrossRef]
- Sowersby, W.; Lehtonen, T.K.; Wong, B.B.M. Threat sensitive adjustment of aggression by males and females in a biparental cichlid. Behav. Ecol. 2018, 29, 761–768. [Google Scholar] [CrossRef]
- Lavery, R.J.; Reebs, S.G. Effect of Mate Removal on Current and Subsequent Parental Care in the Convict Cichlid (Pisces: Cichlidae). Ethology 1994, 97, 265–277. [Google Scholar] [CrossRef]
- Gagliardi-Seeley, J.L.; Itzkowitz, M. Male size predicts the ability to defend offspring in the biparental convict cichlid Archocentrus nigrofasciatus. J. Fish. Biol. 2006, 69, 1239–1244. [Google Scholar] [CrossRef]
- Breukelen, N.A.v.; Itzkowitz, M. Mate removal leads to increase in parental defence behaviour in free-ranging convict cichlids. Anim. Behav. 2011, 82, 1023–1026. [Google Scholar] [CrossRef]
- Wisenden, B.D.; Stumbo, A.D.; Self, P.A.; Snekser, J.L.; McEwen, D.C.; Wisenden, P.A.; Keenleyside, M.H.A.; Itzkowitz, M.; Brisch, E. Co-evolution of offspring antipredator competence and parental brood defense in convict cichlids. Hydrobiologia 2015, 748, 259–272. [Google Scholar] [CrossRef]
- Marlin, T.; Snekser, J.L.; Leese, J.M. Juvenile convict cichlids shoaling decisions in relation to shoal size and age. Ethology 2019, 125, 485–491. [Google Scholar] [CrossRef]
- Cole, J.E.; Ward, J.A. The Communicative Function of Pelvic Fin-Flickering in Etroplus maculatus (Pisces, Cichlidae). Behaviour 1969, 35, 179–199. [Google Scholar] [CrossRef]
- Ostrander, G.K.; Ward, J.A. The function of the pelvic fins during courtship and spawning in the orange chromide, Etroplus maculatus. Environ. Biol. Fishes. 1985, 13, 203–210. [Google Scholar] [CrossRef]
- Shennan, M.G.C.; Waas, J.R.; Lavery, R.J. The warning signals of parental convict cichlids are socially facilitated. Anim. Behav. 1994, 47, 974–976. [Google Scholar] [CrossRef]
- Lee-Jenkins, S.S.Y.; Jeswiet, S.B.; Godin, J.-G.J. Spatial separation from family in the mobile young of a biparental fish: Risks and dynamics of returning home. Naturwissenschaften 2014, 101, 11–15. [Google Scholar] [CrossRef]
- Sutrisno, R.; Schotte, P.M.; Schultz, S.K.; Wisenden, B.D. Fin-flicking behaviour as a means of cryptic olfactory sampling under threat of predation. Ecol. Freshw. Fish 2014, 23, 656–658. [Google Scholar] [CrossRef]
- Wisenden, B.D. Factors affecting mate desertion by males in free-ranging convict cichlids (Cichlasoma nigrofasciatum). Behav. Ecol. 1994, 5, 439–447. [Google Scholar] [CrossRef]
- Snekser, J.L.; Santangelo, N.; Nyby, J.; Itzkowitz, M. Sex differences in biparental care as offspring develop: A field study of convict cichlids (Amatitlania siquia). Environ. Biol. Fishes. 2011, 91, 15–25. [Google Scholar] [CrossRef]
- Breukelen, N.A.v.; Santangelo, N. Parental male and female convict cichlids assess and respond to threats differently depending on intruder species. Behav. Process. 2021, 187, 104396. [Google Scholar] [CrossRef]
- Wisenden, B.D.; Snekser, J.L.; Stumbo, A.D.; Leese, J.M. Parental defence of an empty nest after catastrophic brood loss. Anim. Behav. 2008, 76, 2059–2067. [Google Scholar] [CrossRef]
- Itzkowitz, M. Sexual Differences in Offspring Defense in a Monogamous Cichlid Fish. Z. Für Tierpsychol. 1985, 70, 247–255. [Google Scholar] [CrossRef]
- Neil, S.J. Field studies of the behavioral ecology and agonistic behavior of Cichlasoma meeki (Pisces: Cichlidae). Environ. Biol. Fishes 1984, 10, 59–68. [Google Scholar] [CrossRef]
- Santangelo, N. Female breeding experience affects parental care strategies of both parents in a monogamous cichlid fish. Anim. Behav. 2015, 104, 31–37. [Google Scholar] [CrossRef]
- Paciorek, T.; Joseph, L. Behavioral and Endocrine Alterations to Partner Interactions and Offspring Care during Periods of Conflict. Integr. Org. Biol. 2020, 2, obaa002. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Jones, R.; Moscicki, M.; Clotfelter, E.; Earley, R.L. Seeing orange: Breeding convict cichlids exhibit heightened aggression against more colorful intruders. Behav. Ecol. Sociobiol. 2016, 70, 647–657. [Google Scholar] [CrossRef]
- Wisenden, B.D.; Mammenga, E.A.; Storseth, C.N.; Berglund, N.J. Odour tracking by young convict cichlids and a mechanism for alloparental brood amalgamation. Anim. Behav. 2014, 93, 201–206. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shaer, L.; Baumann, B.; Itzkowitz, M. Defense Limitations of Single Parents in the Biparental Convict Cichlid Fish: A Field Study. Hydrobiology 2025, 4, 14. https://doi.org/10.3390/hydrobiology4020014
Al-Shaer L, Baumann B, Itzkowitz M. Defense Limitations of Single Parents in the Biparental Convict Cichlid Fish: A Field Study. Hydrobiology. 2025; 4(2):14. https://doi.org/10.3390/hydrobiology4020014
Chicago/Turabian StyleAl-Shaer, Layla, Brandon Baumann, and Murray Itzkowitz. 2025. "Defense Limitations of Single Parents in the Biparental Convict Cichlid Fish: A Field Study" Hydrobiology 4, no. 2: 14. https://doi.org/10.3390/hydrobiology4020014
APA StyleAl-Shaer, L., Baumann, B., & Itzkowitz, M. (2025). Defense Limitations of Single Parents in the Biparental Convict Cichlid Fish: A Field Study. Hydrobiology, 4(2), 14. https://doi.org/10.3390/hydrobiology4020014





