The Diversity and Biochemical Composition of Zooplankton as a Potential Indicator of Dietary Requirements for Pikeperch Larvae (Sander lucioperca)
Abstract
1. Introduction
2. Materials and Methods
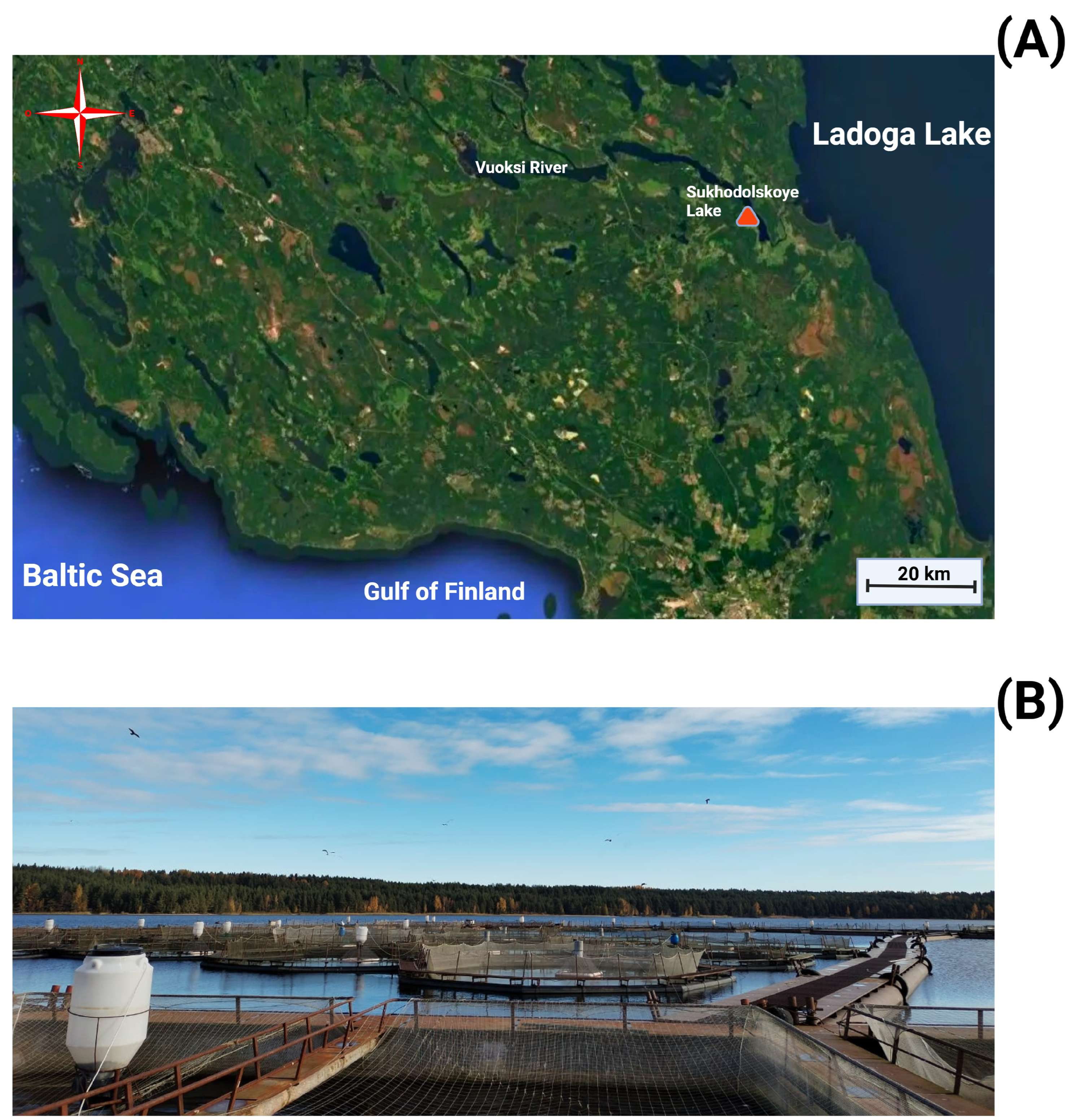
2.1. Pikeperch Larval Rearing Description
2.2. Zooplankton Sampling and Taxon Determination
2.3. Biochemical Analysis of Zooplankton Species
2.4. Hydrochemical Parameters of the Water in the Experiment and Their Monitoring
- The content of phosphorus phosphates (PO4–P) was analyzed using the Murphy–Riley method [47].
- Free CO2 was calculated using the titrimetric method with a 0.02 N Na2CO3 solution.
- Alkalinity was determined using potentiometric titration with 0.02 N H2SO4.
- Nitrite-N (NO2-N) and nitrate nitrogen (NO3-N) contents were measured using spectrophotometry with Bendschneider and Robinson solutions.
- Total nitrogen was analyzed using spectrophotometry with Nessler’s reagent.
- Oxygen dissolved in water was measured by OxyGuard Polaris C (OxyGuard International A/S, Farum, Denmark).
- pH level was measured using an MP-125 digital pH meter (Mettler Toledo, Columbus, OH, USA).
2.5. Statistical Analysis of the Data
3. Results
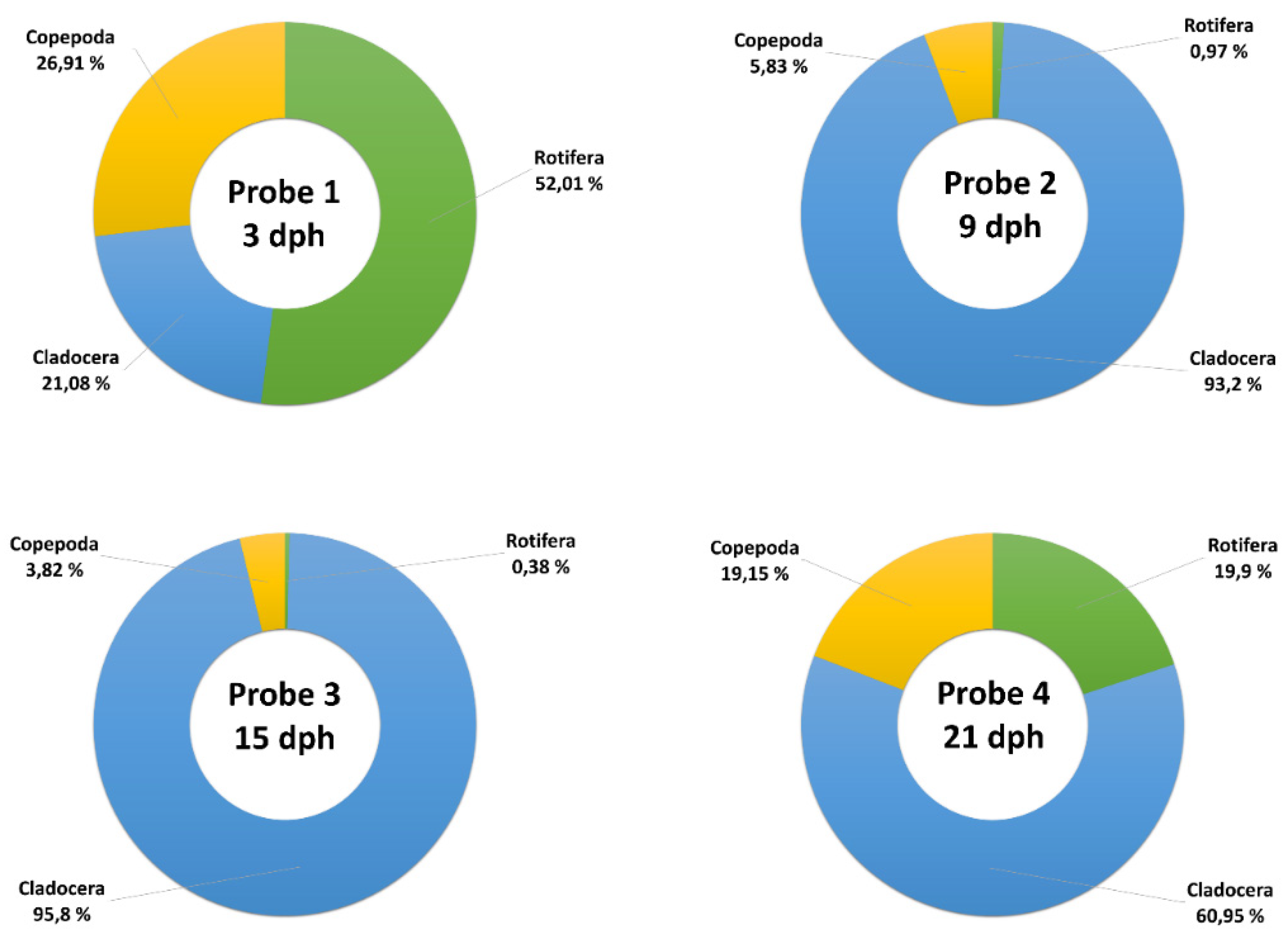
3.1. Taxonomic Diversity of Zooplankton from Sukhodolskoye Lake
3.2. Biochemical Composition of Zooplankton from Sukhodolskoye Lake
3.3. Fatty Acid Composition of the Lipids in Zooplankton from Sukhodolskoye Lake
3.4. Amino Acid Composition of Zooplankton from Sukhodolskoye Lake
| Parameter | Probe 1 | Probe 2 | Probe 3 | Probe 4 | Dietary Requirements for the Larvae of European Perch [50] |
|---|---|---|---|---|---|
| Fish larval developmental stage, days post-hatching (dph) | 3 | 9 | 15 | 21 | - |
| Water temperature, °C | 15.9 | 18.5 | 21.5 | 21.7 | - |
| Essential amino acids (EAAs), % | |||||
| Arginine | 1.9 ± 0.06 a | 5.1 ± 0.06 b | 5.4 ± 0.05 c | 3.7 ± 0.03 d | 4.0 |
| Lysine | 6.9 ± 0.07 a | 6.5 ± 0.06 b | 6.2 ± 0.06 c | 3.5 ± 0.03 d | 5.2 |
| Phenylalanine | 3.0 ± 0.05 a | 2.9 ± 0.04 a | 3.5 ± 0.05 b | 3.3 ± 0.06 c | 2.8 |
| Methionine | 1.1 ± 0.06 a,d | 0.7 ± 0.04 b | 0.9 ± 0.09 c,d | 1.0 ± 0.04 d | 1.9 |
| Threonine | 4.5 ± 0.09 a | 5.1 ± 0.11 b | 5.0 ± 0.06 b | 5.9 ± 0.08 c | 3.3 |
| Histidine | 5.4 ± 0.07 a | 6.2 ± 0.06 b | 7.6 ± 0.05 c | 6.4 ± 0.04 b | 2.0 |
| Valin | 5.7 ± 0.11 a | 6.3 ± 0.06 b | 7.3 ± 0.07 c | 8.9 ± 0.09 d | 3.8 |
| Isoleucine | 4.1 ± 0.06 a | 4.0 ± 0.04 a | 5.1 ± 0.05 b | 6.2 ± 0.07 c | 3.3 |
| Leucine | 4.8 ± 0.04 a | 4.9 ± 0.06 a | 5.6 ± 0.07 b | 5.7 ± 0.06 b | 5.0 |
| ΣEAA | 37.4 ± 0.72 | 41.7 ± 0.96 | 45.6 ± 0.88 | 44.6 ± 1.01 | 31.3 |
| Nonessential amino acids (NEAAs), % | |||||
| Alanine | 25.1 ± 0.14 a | 22.4 ± 0.17 b | 19.3 ± 0.22 c | 18.9 ± 0.18 c | n/d |
| Aspartic acid | 13.2 ± 0.09 a | 12.8 ± 0.10 b | 9.0 ± 0.09 c | 8.7 ± 0.07 d | n/d |
| Glutamic acid | 8.9 ± 0.05 a | 8.7 ± 0.06 b | 9.8 ± 0.08 c | 9.8 ± 0.07 c | n/d |
| Glycine | 4.6 ± 0.03 a | 4.2 ± 0.05 b | 3.4 ± 0.04 c | 3.9 ± 0.03 d | n/d |
| Oxyproline | 0.5 ± 0.01 a | 0.5 ± 0.02 a | 0.8 ± 0.02 b | 0.9 ± 0.02 c | n/d |
| Proline | 2.9 ± 0.02 a | 2.5 ± 0.03 b | 5.4 ± 0.05 c | 5.7 ± 0.05 d | n/d |
| Serin | 6.0 ± 0.04 a | 5.3 ± 0.07 b | 6.1 ± 0.04 c | 6.1 ± 0.06 c | n/d |
| Tyrosine | 1.4 ± 0.02 a | 1.9 ± 0.02 b | 0.6 ± 0.01 c | 1.4 ± 0.02 a | n/d |
| ΣNEAA | 62.6 ± 0.64 | 59.2 ± 0.81 | 52.5 ± 0.75 | 55.4 ± 0.70 | n/d |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | Alpha-linolenic acid |
| APHA | American Public Health Association |
| ARA | Arachidonic acid |
| DHA | Docosahexaenoic acid |
| DPH | Days post-hatching |
| EAA | Essential amino acid |
| EPA | Eicosapentaenoic acid |
| FA | Fatty acid |
| FID | Flame ionization detector |
| LA | Linolenic acid |
| MPC | Maximum permissible concentration |
| MUFA | Monounsaturated fatty acid |
| NFE | Nitrogen-free extract |
| NO2-N | Nitrite-N |
| NO3-N | Nitrate nitrogen |
| NEAA | Nonessential essential amino acid |
| PO4–P | Phosphorus phosphate |
| SFA | Saturated fatty acid |
| UFA | Unsaturated fatty acid |
References
- Alexi, N.; Byrne, D.V.; Nanou, E.; Grigorakis, K. Investigation of sensory profiles and hedonic drivers of emerging aquaculture fish species. J. Sci. Food Agric. 2018, 98, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Colchen, T.; Gisbert, E.; Krauss, D.; Ledoré, Y.; Pasquet, A.; Fontaine, P. Improving pikeperch larviculture by combining environmental, feeding and populational factors. Aquac. Rep. 2020, 17, 100337. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Lund, I.; Thorarinsdottir, R.; Drengstig, A.; Arvonen, K.; Pedersen, P.B. Farming different species in RAS in Nordic countries: Current status and future perspectives. Aquac. Eng. 2013, 53, 2–13. [Google Scholar] [CrossRef]
- Colchen, T.; Ledoré, Y.; Fontaine, P.; Teletchea, F.; Pasquet, A. Larval pikeperch Sander lucioperca cannibals are more efficient predators on zebrafish Danio rerio than non-cannibals. Aquaculture 2023, 575, 739756. [Google Scholar] [CrossRef]
- Kestemont, P.; Dabrowski, K.; Summerfelt, R.C. Biology and Culture of Percid Fishes: Principles and Practices; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Klein Breteler, J. Intensive culture of pike-perch fry with live food. Intensive culture of pikeperch fry with live food. In Aquaculture—A Biotechnology in Progress; De Pauw, N., et al., Eds.; European Aquaculture Society: Bredene, Belgium, 1989; Volume 1, pp. 203–207. [Google Scholar]
- Steffens, W.; Geldhauser, F.; Gerstner, P.; Hilge, V. German experiences in the propagation and rearing of fingerling pikeperch (Stizostedion lucioperca). Ann. Zool. Fenn. 1996, 33, 627–634. [Google Scholar]
- Mani-Ponset, L.; Diaz, J.-P.; Schlumberger, O.; Connes, R. Development of yolk complex, liver and anterior intestine in pike-perch larvae, Stizostedion lucioperca (Percidae), according to the first diet during rearing. Aquat. Living Resour. 1994, 7, 191–202. [Google Scholar] [CrossRef]
- Nyina-Wamwiza, L.; Xu, X.L.; Blanchard, G.; Kestemont, P. Effect of dietary protein, lipid and carbohydrate ratio on growth, feed efficiency and body composition of pikeperch Sander lucioperca fingerlings. Aquac. Res. 2005, 36, 486–492. [Google Scholar] [CrossRef]
- Ostaszewska, T. Developmental changes of digestive system structures in pike-perch (Sander lucioperca L.). Electron. J. Ichthyol. 2005, 2, 65–78. [Google Scholar]
- Dabrowski, K. The role of proteolytic enzymes in fish digestion. Cultiv. Fish Fry Live Food 1979, 4, 107–126. [Google Scholar]
- Lauff, M.; Hofer, R. Proteolytic enzymes in fish development and the importance of dietary enzymes. Aquaculture 1984, 37, 335–346. [Google Scholar] [CrossRef]
- Srichanun, M.; Tantikitti, C.; Vatanakul, V. Digestive enzyme activity during ontogenetic development and effect of live feed in green catfish larvae (Mystus nemurus Cuv. & Val.). Songklanakarin J. Sci. Technol. 2012, 34, 247–254. [Google Scholar]
- El Kertaoui, N.; Lund, I.; Assogba, H.; Domínguez, D.; Izquierdo, M.S.; Baekelandt, S.; Cornet, V.; Mandiki, S.N.M.; Montero, D.; Kestemont, P. Key nutritional factors and interactions during larval development of pikeperch (Sander lucioperca). Sci. Rep. 2019, 9, 7074. [Google Scholar] [CrossRef] [PubMed]
- Kestemont, P.; Henrotte, E. Nutritional Requirements and Feeding of Broodstock and Early Life Stages of Eurasian Perch and Pikeperch. In Biology and Culture of Percid Fishes: Principles and Practices; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 539–564. [Google Scholar]
- Barrows, F.T.; Lellis, W.A.; Nickum, J.G. Intensive Culture of Larval Walleyes with Dry or Formulated Feed: Note on Swim Bladder Inflation. Progress. Fish-Cult. 1988, 50, 160–166. [Google Scholar] [CrossRef]
- Antalfi, A. Propagation and rearing of perch in pond culture. EIFAC Tech. Pap. 1979, 35, 120–125. [Google Scholar]
- Beyerle, G.B. Summary of Attempts to Raise Walleye Fry and Fingerlings on Artificial Diets, with Suggestions on Needed Research and Procedures to be Used in Future Tests. Progress. Fish-Cult. 1975, 37, 103–105. [Google Scholar] [CrossRef]
- Ruuhijärvi, J.; Virtanen, E.; Salminen, M.; Muyunda, M. The growth and survival of pike-perch, Stizostedion lucioperca L., larvae fed on formulated feeds. In Proceedings of the International Symposium on Fish and Crustacean Larviculture, Gent, Belgium, 27–30 August 1991; pp. 154–156. [Google Scholar]
- Schlumberger, O.; Proteau, J. Production de juveniles de sandre (Stizostedion lucioperca). Aqua Rev. 1991, 36, 25–28. [Google Scholar]
- Bischoff, A.A.; Kubitz, M.; Wranik, C.M.; Pfefferkorn, H.; Augustin, C.B.; Hagen, W.; Palm, H.W. Fatty acid utilization of pikeperch (Sander lucioperca (Linnaeus, 1758)) larvae under starvation conditions during early development. Bull. Fish Biol. 2017, 17, 59–73. [Google Scholar]
- Kestemont, P.; Xueliang, X.; Hamza, N.; Maboudou, J.; Imorou Toko, I. Effect of weaning age and diet on pikeperch larviculture. Aquaculture 2007, 264, 197–204. [Google Scholar] [CrossRef]
- Kowalska, A.; Zakęś, Z.; Demska-Zakęś, K. The impact of feeding on the results of rearing larval pikeperch, Sander lucioperca (L.), with regard to the development of the digestive tract. Electron. J. Pol. Agric. Univ. Fish. 2006, 9. Available online: http://www.ejpau.media.pl/volume9/issue2/abs-5.html (accessed on 23 January 2025).
- Ljubobratović, U.; Fazekas, G.; Koljukaj, A.; Ristović, T.; Vass, V.; Ardó, L.; Stanisavljević, N.; Vukotić, G.; Pešić, M.; Milinčić, D.; et al. Pike-perch larvae growth in response to administration of lactobacilli-enriched inert feed during first feeding. Aquaculture 2021, 542, 736901. [Google Scholar] [CrossRef]
- Ljubobratović, U.; Kucska, B.; Feledi, T.; Poleksić, V.; Marković, Z.; Lenhardt, M.; Peteri, A.; Kumar, S.; Rónyai, A. Effect of weaning strategies on growth and survival of pikeperch, Sander lucioperca, larvae. Turk. J. Fish. Aquat. Sci. 2015, 15, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Rønfeldt, J.; Nielsen, J. Filling of Gas Bladder, Growth and the Survival in Pikeperch Larvae (Sander lucioperca) in Intensive Aquaculture. Master’s Thesis, University of Copenhagen, Copenhagen, Denmark, 2010. [Google Scholar]
- Steenfeldt, S.; Vestergaard, M.; Overton, J.; Paulsen, H.; Larsen, V.; Henriksen, N. Development of intensive rearing of pikeperch in Denmark. Den. Anon. Hirtshals. 2010, 303–323. Available online: https://orbit.dtu.dk/files/6581395/228-2010_Videreudvikling-af-intensivt-opdraet-af-sandart-i-Danmark.pdf (accessed on 23 January 2025). (In Danish).
- Szkudlarek, M.; Zakęś, Z. Effect of stocking density on survival and growth performance of pikeperch, Sander lucioperca (L.), larvae under controlled conditions. Aquac. Int. 2007, 15, 67–81. [Google Scholar] [CrossRef]
- Czesny, S.; Kolkovski, S.; Yackey, C.; Dabrowski, K. The effects of (n-3) HUFA enriched Artemia nauplii on growth, survival, and quality of walleye Stizostedion vitreum fry. Aquaculture 1998, 178, 103–115. [Google Scholar] [CrossRef]
- Watanabe, T.; Kitajima, C.; Fujita, S. Nutritional values of live organisms used in Japan for mass propagation of fish: A review. Aquaculture 1983, 34, 115–143. [Google Scholar] [CrossRef]
- Hamza, N.; Kestemont, P.; Khemis, I.B.; Mhetli, M.; Cahu, C. Effect of different sources and levels of dietary phospholipids on performances and fatty acid composition of pikeperch (Sander lucioperca) larvae. Aquac. Nutr. 2012, 18, 249–257. [Google Scholar] [CrossRef]
- Lund, I.; Skov, P.V.; Hansen, B.W. Dietary supplementation of essential fatty acids in larval pikeperch (Sander lucioperca); short and long term effects on stress tolerance and metabolic physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 162, 340–348. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Boruta, A. The effect of diet on the fatty acid composition and liver histology of pikeperch (Sander lucioperca (L.)) larvae. Fish. Aquat. Life 2006, 14, 53–66. [Google Scholar]
- Izquierdo, M.; Koven, W. Lipids. In Larval Fish Nutrition; John Wiley & Sons.: Hoboken, NJ, USA, 2011; pp. 47–81. [Google Scholar]
- Schlumpberger, W. Untersuchungen zur Entwicklung eines industrie-mäβigen Verfahrens für die Produktion von vorgestreckten Zandern (Stizustedion lucioperca [L.]). Ph.D. Thesis, Humboldt-Univ., Berlin, Germany, 1979; p. 232. [Google Scholar]
- Peterka, J.; Matína, J.; Lipka, J. The diet and growth of larval and juvenile pikeperch (Stizostedion lucioperca (L.)): A comparative study of fishponds and a reservoir. Aquac. Int. 2003, 11, 337–348. [Google Scholar] [CrossRef]
- Specziár, A. First Year Ontogenetic Diet Patterns in Two Coexisting Sander Species, S. lucioperca and S. volgensis in Lake Balaton. Hydrobiologia 2005, 549, 115–130. [Google Scholar] [CrossRef]
- Lyutikov, A.A.; Korolev, A.E.; Shumilina, A.K.; Lukina, Y.N.; Vylka, M.M.; Prishchepa, A.S. Comparative Characteristics of the Physiological State of Pikeperch (Sander Lucioperca) from Various Habitat Conditions: Lake (Natural Habitat), Ponds, and Fish Farm Cages. Contemp. Probl. Ecol. 2024, 17, 208–218. [Google Scholar] [CrossRef]
- Woynarovich, E. Aufzucht der Zanderlarven bis zum Raubfischalter. Z. Fur Fisch. 1960, 9, 73–83. [Google Scholar]
- Wang, N.; Eckmann, R. Effects of temperature and food density on egg development, larval survival and growth of perch (Perca fluviatilis L.). Aquaculture 1994, 122, 323–333. [Google Scholar] [CrossRef]
- Alekseev, V.R.; Tsalolikhin, S.A. (Eds.) Taxonomic Key of Zooplankton and Freshwater Zoobenthos of European Russia; KMK Scientific Press: Moscow, Russia, 2010; Volume 1, p. 495. [Google Scholar]
- Eggers, L.F.; Schwudke, D. Liquid Extraction: Folch. In Encyclopedia of Lipidomics; Wenk, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–6. [Google Scholar]
- Sáez-Plaza, P.; José, N.M.; Sławomir, W.; Tadeusz, M.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1998; Volume 1. [Google Scholar]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- Cho, Y.-H.; Nielsen, S.S. Phosphorus Determination by Murphy-Riley Method. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 153–156. [Google Scholar]
- Fiogbé, E.D.; Kestemont, P.; Mélard, C.; Micha, J.C. The effects of dietary crude protein on growth of the Eurasian perch Perca fluviatilis. Aquaculture 1996, 144, 239–249. [Google Scholar] [CrossRef]
- Zakes, Z.; Demska-Zakes, K. Intensive rearing of juvenile Stizostedion lucioperca (Percidae) fed natural and artificial diets. Ital. J. Zool. 1998, 65, 507–509. [Google Scholar] [CrossRef][Green Version]
- Geay, F.; Kestemont, P. Feeding and Nutrition of Percid Fishes During Ongrowing Stages. In Biology and Culture of Percid Fishes: Principles and Practices; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 587–622. [Google Scholar]
- Tay, S.H.; Rajbanshi, V.K.; Ho, W.H.; Chew, J.; Yap, E.A. Culture of cladoceran Moina micrura kurz using agroindustrial wastes. In Fish Nutrition Research in Asia, Proceedings of the Fourth Asian Fish Nutrition Workshop, Manila, Philippines, 3–8 September 1990; Asian Fisheries Society: Manila, Philippines, 1991; p. 1205. [Google Scholar]
- Yurkowski, M.; Tabachek, J.L. Proximate and amino acid composition of some natural fish foods. In Proceedings of the World Symposium on Finfish Nutrition and Fish Feed Technology, Hamburg, Germany, 20–23 June 1978; Heenemann Verlagsgesellschaft: Hamburg, Germany, 1979; pp. 435–448. [Google Scholar]
- Arts, M.T.; Robarts, R.D.; Evans, M.S. Energy Reserve Lipids of Zooplanktonic Crustaceans from an Oligotrophic Saline Lake In Relation to Food Resources and Temperature. Can. J. Fish. Aquat. Sci. 1993, 50, 2404–2420. [Google Scholar] [CrossRef]
- Nanton, D.A.; Castell, J.D. The effects of temperature and dietary fatty acids on the fatty acid composition of harpacticoid copepods, for use as a live food for marine fish larvae. Aquaculture 1999, 175, 167–181. [Google Scholar] [CrossRef]
- Farkas, T.; Herodek, S. The effect of environmental temperature on the fatty acid composition of crustacean plankton. J. Lipid Res. 1964, 5, 369–373. [Google Scholar] [CrossRef]
- Datsomor, A.K.; Zic, N.; Li, K.; Olsen, R.E.; Jin, Y.; Vik, J.O.; Edvardsen, R.B.; Grammes, F.; Wargelius, A.; Winge, P. CRISPR/Cas9-mediated ablation of elovl2 in Atlantic salmon (Salmo salar L.) inhibits elongation of polyunsaturated fatty acids and induces Srebp-1 and target genes. Sci. Rep. 2019, 9, 7533. [Google Scholar] [CrossRef] [PubMed]
- Navas, J.; Doste, S.; Carrillo, M.; Thrush, M.; Jara, J.; Bromage, N. Total lipid in the broodstock diet did not affect fatty acid composition and quality of eggs of sea bass (Dicentrarchus labrax L.). Sci. Mar. (Barc.) 2001, 65. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Palm, H.W. Entwicklung Eines Zooplankton-Reaktors Zur Unterstützung Der Fischlarvenaufzucht Relevanter Zielfischarten in Mecklenburg Vorpommern (MV); Universität Rostock: Rostock, Germany, 2015; p. 67. [Google Scholar]
- Henrotte, É.; Overton, J.L.; Kestemont, P. Effects of dietary n-3 and n-6 fatty acids levels on egg and larval quality of Eurasian perch. Cybium 2008, 32, 271–272. [Google Scholar]
- Claus, C.; Benijts, F.; Vandeputte, G.; Gardner, W. The biochemical composition of the larvae of two strains of Artemia salina (L.) reared on two different algal foods. J. Exp. Mar. Biol. Ecol. 1979, 36, 171–183. [Google Scholar] [CrossRef]
- Bell, J.G.; Ghioni, C.; Sargent, J.R. Fatty acid compositions of 10 freshwater invertebrates which are natural food organisms of Atlantic salmon parr (Salmo salar): A comparison with commercial diets. Aquaculture 1994, 128, 301–313. [Google Scholar] [CrossRef]
- Bigogno, C.; Khozin-Goldberg, I.; Adlerstein, D.; Cohen, Z. Biosynthesis of arachidonic acid in the oleaginous microalga Parietochloris incisa (Chlorophyceae): Radiolabeling studies. Lipids 2002, 37, 209–216. [Google Scholar] [CrossRef]
- Bell, J.G.; Sargent, J.R. Arachidonic acid in aquaculture feeds: Current status and future opportunities. Aquaculture 2003, 218, 491–499. [Google Scholar] [CrossRef]
- Ganga, R.; Tort, L.; Acerete, L.; Montero, D.; Izquierdo, M.S. Modulation of ACTH-induced cortisol release by polyunsaturated fatty acids in interrenal cells from gilthead seabream, Sparus aurata. J. Endocrinol. 2006, 190, 39–45. [Google Scholar] [CrossRef]
- Abi-Ayad, S.M.E.A.; Boutiba, Z.; Mélard, C.; Kestemont, P. Dynamics of Total Body Fatty Acids During Early Ontogeny of Pikeperch (Sander lucioperca) Larvae. Fish Physiol. Biochem. 2004, 30, 129–136. [Google Scholar] [CrossRef]
- Small, B.C.; Soares JR, J.H. Quantitative dietary lysine requirement of juvenile striped bass Morone saxatilis. Aquac. Nutr. 2000, 6, 207–212. [Google Scholar] [CrossRef]
- Alam, M.S.; Teshima, S.-i.; Koshio, S.; Ishikawa, M. Arginine requirement of juvenile Japanese flounder Paralichthys olivaceus estimated by growth and biochemical parameters. Aquaculture 2002, 205, 127–140. [Google Scholar] [CrossRef]
- Costas, B.; Conceição, L.E.; Dias, J.; Novoa, B.; Figueras, A.; Afonso, A. Dietary arginine and repeated handling increase disease resistance and modulate innate immune mechanisms of Senegalese sole (Solea senegalensis Kaup, 1858). Fish Shellfish Immunol. 2011, 31, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Mitra, G.; Mukhopadhyay, P.K.; Ayyappan, S. Biochemical composition of zooplankton community grown in freshwater earthen ponds: Nutritional implication in nursery rearing of fish larvae and early juveniles. Aquaculture 2007, 272, 346–360. [Google Scholar] [CrossRef]
- Srivastava, A.; Hamre, K.; Stoss, J.; Chakrabarti, R.; Tonheim, S.K. Protein content and amino acid composition of the live feed rotifer (Brachionus plicatilis): With emphasis on the water soluble fraction. Aquaculture 2006, 254, 534–543. [Google Scholar] [CrossRef]
- Di Buono, M.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Dietary cysteine reduces the methionine requirement in men1234. Am. J. Clin. Nutr. 2001, 74, 761–766. [Google Scholar] [CrossRef]
- Lehninger, A.L. Biochemistry: The Molecular Basis of Cell Structure and Function; Worth Publishers New York: New York, NY, USA, 1975; Volume 2. [Google Scholar]

| Parameter of Water | M ± se | Permissible Range (PR) |
|---|---|---|
| Oxygen (O2) concentration, mg/L | 9.3 ± 0.3 | ≥6.0 |
| Oxygen saturation, % | 92.6 ± 3.9 | - |
| Temperature, °C | 19.6 ± 2.7 | 15 < PR < 26 |
| pH | 6.8 ± 0.1 | 6.5 < PR < 8.5 |
| CO2 concentration, mg/L | 3.2 ± 0.8 | <20 |
| Ammonium nitrogen (N), mg/L | 0.24 ± 0.1 | <0.40 |
| Nitrites (N), mg/L | <0.010 | <0.020 |
| Nitrates (NO3), mg/L | 0.89 ± 0.16 | <40.0 |
| Phosphates (P), mg/L | <0.010 | <0.15 |
| Parameter | Probe 1 | Probe 2 | Probe 3 | Probe 4 |
|---|---|---|---|---|
| Fish larval developmental stage, days post-hatching (dph) | 3 | 9 | 15 | 21 |
| Water temperature, °C | 15.9 | 18.5 | 21.5 | 21.7 |
| Biochemical parameters, % | ||||
| Dry matter | 10.64 ± 1.64 a | 10.82 ± 1.48 a | 9.43 ± 1.23 b | 9.03 ± 1.12 b |
| Protein | 68.00 ± 2.11 | 68.10 ± 1.98 | 70.50 ± 2.30 | 68.90 ± 1.64 |
| Lipids | 24.88 ± 3.64 a | 24.71 ± 3.05 a | 22.52 ± 2.90 b | 22.27 ± 2.76 b |
| Nitrogen-free extract (NFE) | 3.84 ± 1.74 | 3.23 ± 1.61 | 4.09 ± 1.34 | 3.67 ± 1.29 |
| Ash | 3.28 ± 0.60 a | 3.96 ± 0.52 b | 2.89 ± 0.66 a | 5.16 ± 1.36 c |
| Parameter | Probe 1 | Probe 2 | Probe 3 | Probe 4 |
|---|---|---|---|---|
| Fish larval developmental stage, days post-hatching (dph) | 3 | 9 | 15 | 21 |
| Water temperature, °C | 15.9 | 18.5 | 21.5 | 21.7 |
| Fatty acids (FAs), % | ||||
| 12:0 | - | 0.41 ± 0.08 a | 0.46 ± 0.12 a | 1.27 ± 0.35 b |
| 14:0 | 2.95 ± 0.53 a | 5.68 ± 1.08 b | 6.91 ± 0.92 b | 7.94 ± 2.59 c |
| 15:0 | 1.39 ± 0.44 a | 0.85 ± 0.08 b | 1.18 ± 0.16 b | 1.86 ± 0.42 a |
| 16:0 | 19.64 ± 1.85 a | 19.25 ± 2.14 a | 23.75 ± 2.22 b | 32.68 ± 3.30 c |
| 18:0 | 2.93 ± 0.18 a | 5.23 ± 1.11 b | 5.65 ± 1.14 b | 6.41 ± 0.72 b |
| 22:0 | 0.32 ± 0.13 a | 0.61 ± 0.12 b | 0.38 ± 0.08 a | - |
| 23:0 | 0.33 ± 0.09 a | 1.25 ± 0.35 b | 0.32 ± 0.11 a | 0.50 ± 0.06 c |
| 16:1 n-7 | 0.30 ± 0.04 a | 0.51 ± 0.11 b | 0.43 ± 0.09 b | 0.48 ± 0.09 b |
| 16:1 n-9 | 4.16 ± 1.11 a | 4.29 ± 1.54 a | 6.22 ± 1.13 b | 4.70 ± 1.11 a |
| 17:1 n-9 | 1.72 ± 0.08 a | 1.66 ± 0.19 a | 1.43 ± 0.11 b | 1.34 ± 0.20 b |
| 18:1 n-7 | 5.98 ± 0.92 a | 9.34 ± 2.59 b,c | 5.56 ± 1.08 a | 7.27 ± 0.92 c |
| 18:1 n-9 | 5.50 ± 1.08 a,b | 4.91 ± 1.28 b | 5.86 ± 1.20 a | 6.19 ± 0.96 a |
| 20:1 n-7 | 0.07 ± 0.01 a | - | 0.10 ± 0.01 a | 1.25 ± 0.05 b |
| 22:1 n-9 | 5.87 ± 1.06 a | 12.33 ± 0.61 b | 1.98 ± 1.66 b | 4.46 ± 0.92 a |
| 18:2 n-6 Linolenic acid (LA) | 6.59 ± 1.11 a | 5.18 ± 0.88 a,b | 4.81 ± 0.64 b | 3.05 ± 0.30 c |
| 20:2 n-6 | 0.11 ± 0.01 a | 0.17 ± 0.02 a | - | - |
| 20:4 n-6 Arachidonic acid (ARA) | 21.14 ± 2.89 a | 4.31 ± 0.70 b | 3.31 ± 0.53 c | 1.48 ± 0.93 d |
| 22:2 n-6 | 0.14 ± 0.01 a | 1.55 ± 0.42 b | 1.22 ± 0.38 b | - |
| 18:3 n-3 Alpha-linolenic acid (ALA) | 5.61 ± 0.72 a | 7.55 ± 1.14 b | 8.53 ± 0.92 b | 6.08 ± 0.84 a |
| 18:4 n-3 | 2.87 ± 0.53 a,c | 4.54 ± 1.08 b | 3.13 ± 1.02 a,b,c | 2.07 ± 0.71 c |
| 20:5 n-3 Eicosapentaenoic acid (EPA) | 4.44 ± 0.72 a | 1.64 ± 0.55 b | 3.12 ± 0.53 a | 2.12 ± 0.14 c |
| 22:5 n-3 | 0.39 ± 0.04 a | 0.37 ± 0.03 a | - | - |
| 22:6 n-3 Docosahexaenoic acid (DHA) | 0.70 ± 0.13 a | 2.82 ± 0.20 b | 1.82 ± 0.22 c | 1.64 ± 0.16 c |
| n/d | 6.23 ± 0.56 a | 5.76 ± 0.44 a | 4.64 ± 0.35 b | 7.20 ± 0.58 c |
| Σ SFAs | 27.71 ± 2.84 a | 33.28 ± 3.12 b | 38.65 ± 4.42 b | 50.66 ± 4.88 c |
| Σ MUFAs | 23.69 ± 7.29 a | 33.04 ± 3.09 b | 30.58 ± 3.13 b | 25.69 ± 7.04 a |
| Σ UFAs | 42.37 ± 3.60 a | 27.63 ± 2.07 b | 25.94 ± 1.98 b | 16.44 ± 1.73 c |
| n-3 | 14.01 ± 1.24 a | 16.92 ± 1.36 b | 16.60 ± 1.05 b | 11.91 ± 0.68 c |
| n-6 | 28.36 ± 1.73 a | 10.71 ± 0.89 b | 9.34 ± 0.80 b | 4.53 ± 0.40 c |
| EPA + DHA | 5.14 ± 0.85 a | 4.46 ± 0.75 a | 4.94 ± 0.31 a | 3.76 ± 0.30 b |
| n-3/n-6 | 0.5 | 1.6 | 1.8 | 2.6 |
| ALA/LA | 0.9 | 1.5 | 1.8 | 2.0 |
| DHA/EPA | 0.2 | 1.7 | 0.6 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyutikov, A.; Korolev, A.; Trifonov, A.; Zubareva, A.; Nedoluzhko, A. The Diversity and Biochemical Composition of Zooplankton as a Potential Indicator of Dietary Requirements for Pikeperch Larvae (Sander lucioperca). Hydrobiology 2025, 4, 13. https://doi.org/10.3390/hydrobiology4020013
Lyutikov A, Korolev A, Trifonov A, Zubareva A, Nedoluzhko A. The Diversity and Biochemical Composition of Zooplankton as a Potential Indicator of Dietary Requirements for Pikeperch Larvae (Sander lucioperca). Hydrobiology. 2025; 4(2):13. https://doi.org/10.3390/hydrobiology4020013
Chicago/Turabian StyleLyutikov, Anatoliy, Alexander Korolev, Artem Trifonov, Anastasia Zubareva, and Artem Nedoluzhko. 2025. "The Diversity and Biochemical Composition of Zooplankton as a Potential Indicator of Dietary Requirements for Pikeperch Larvae (Sander lucioperca)" Hydrobiology 4, no. 2: 13. https://doi.org/10.3390/hydrobiology4020013
APA StyleLyutikov, A., Korolev, A., Trifonov, A., Zubareva, A., & Nedoluzhko, A. (2025). The Diversity and Biochemical Composition of Zooplankton as a Potential Indicator of Dietary Requirements for Pikeperch Larvae (Sander lucioperca). Hydrobiology, 4(2), 13. https://doi.org/10.3390/hydrobiology4020013







