Assessing Methods to Monitor Aquatic Invertebrates in a Large River: Comparing Rock Baskets and Hess Samplers in the Snake River, Wyoming, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Methods
2.3. Invertebrate Processing and Bioassessment Metrics
2.4. Invertebrate Assemblages
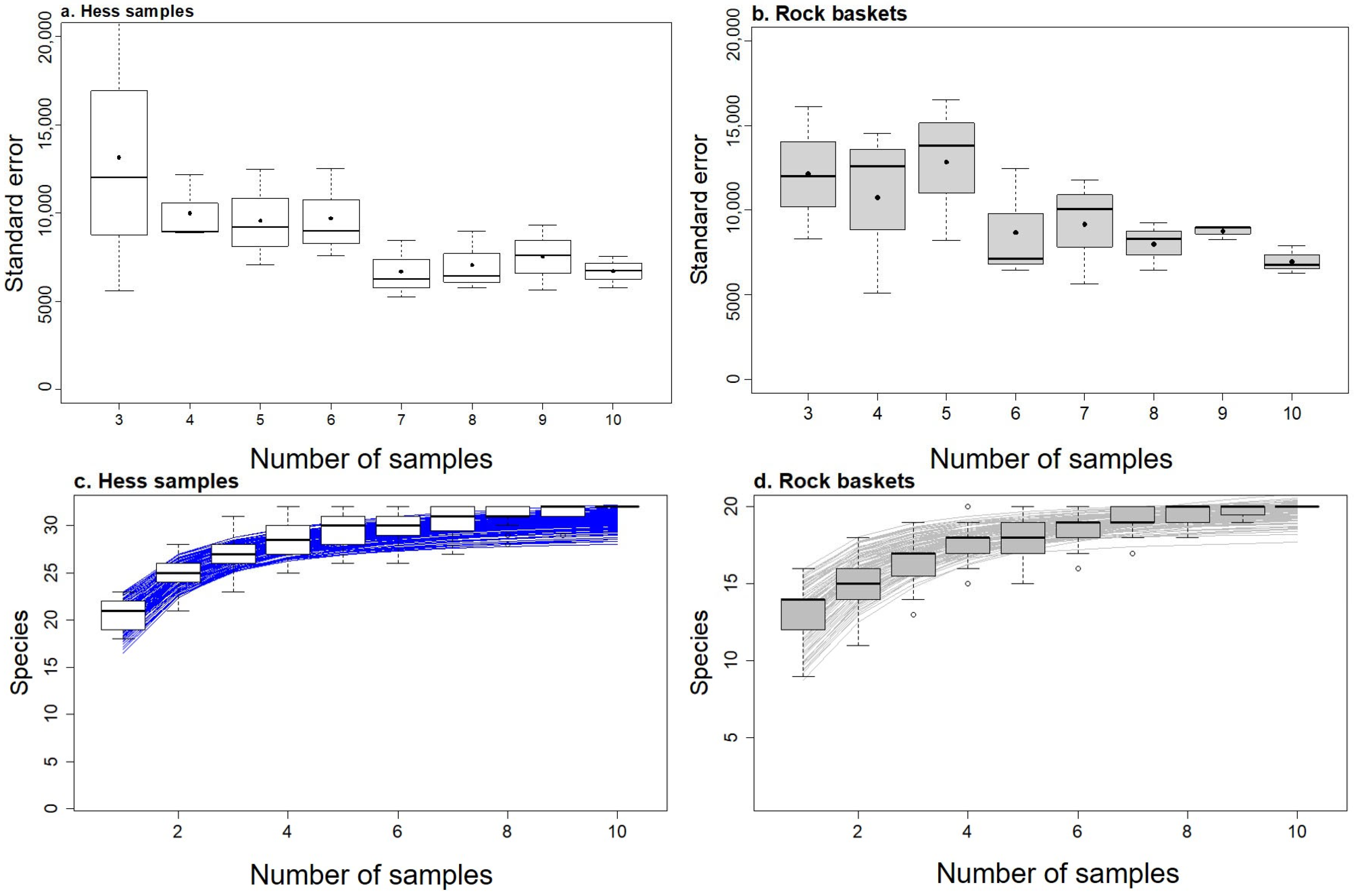
2.5. Variance and Species Accumulation Curves
3. Results
3.1. Water Chemistry
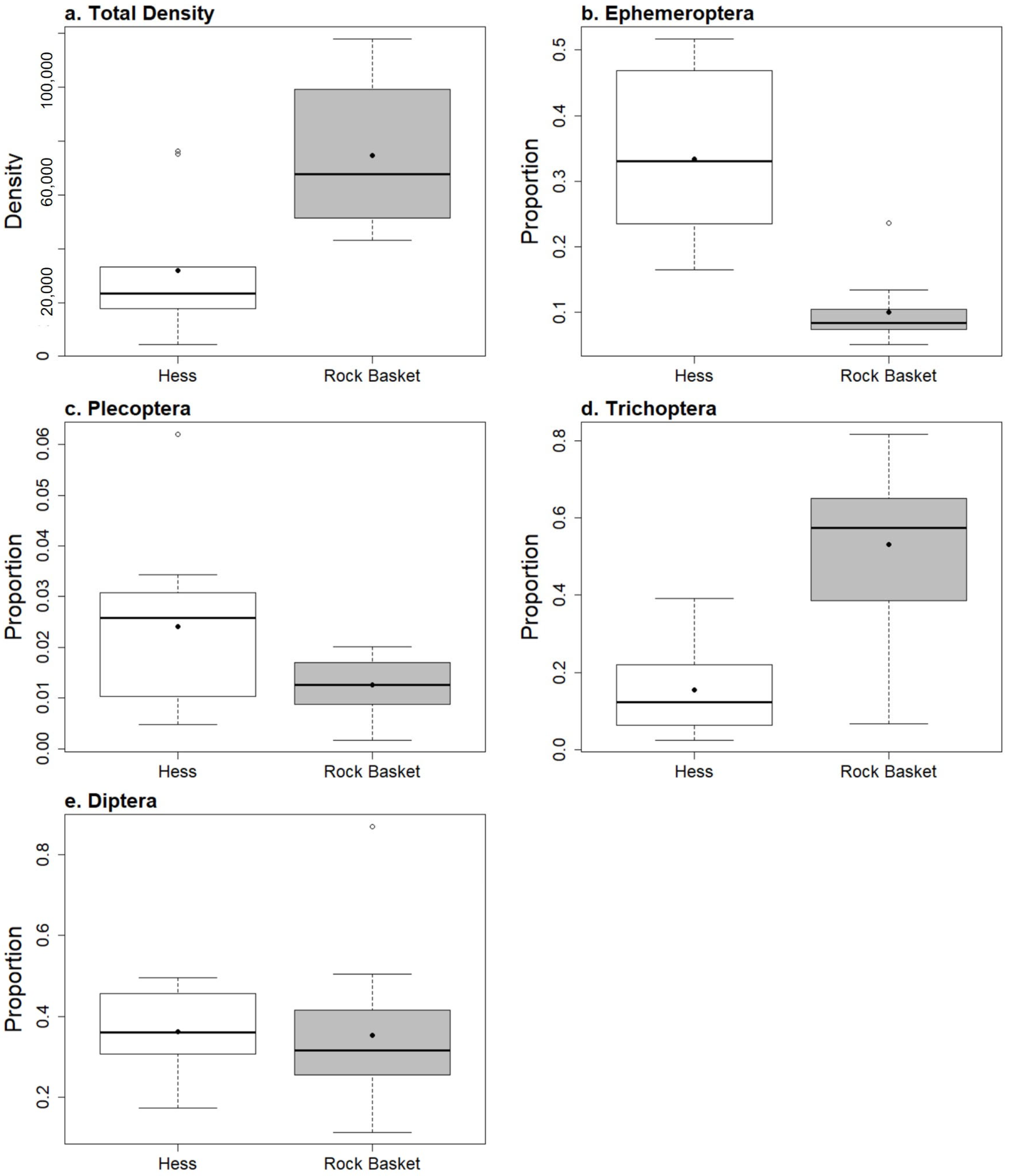
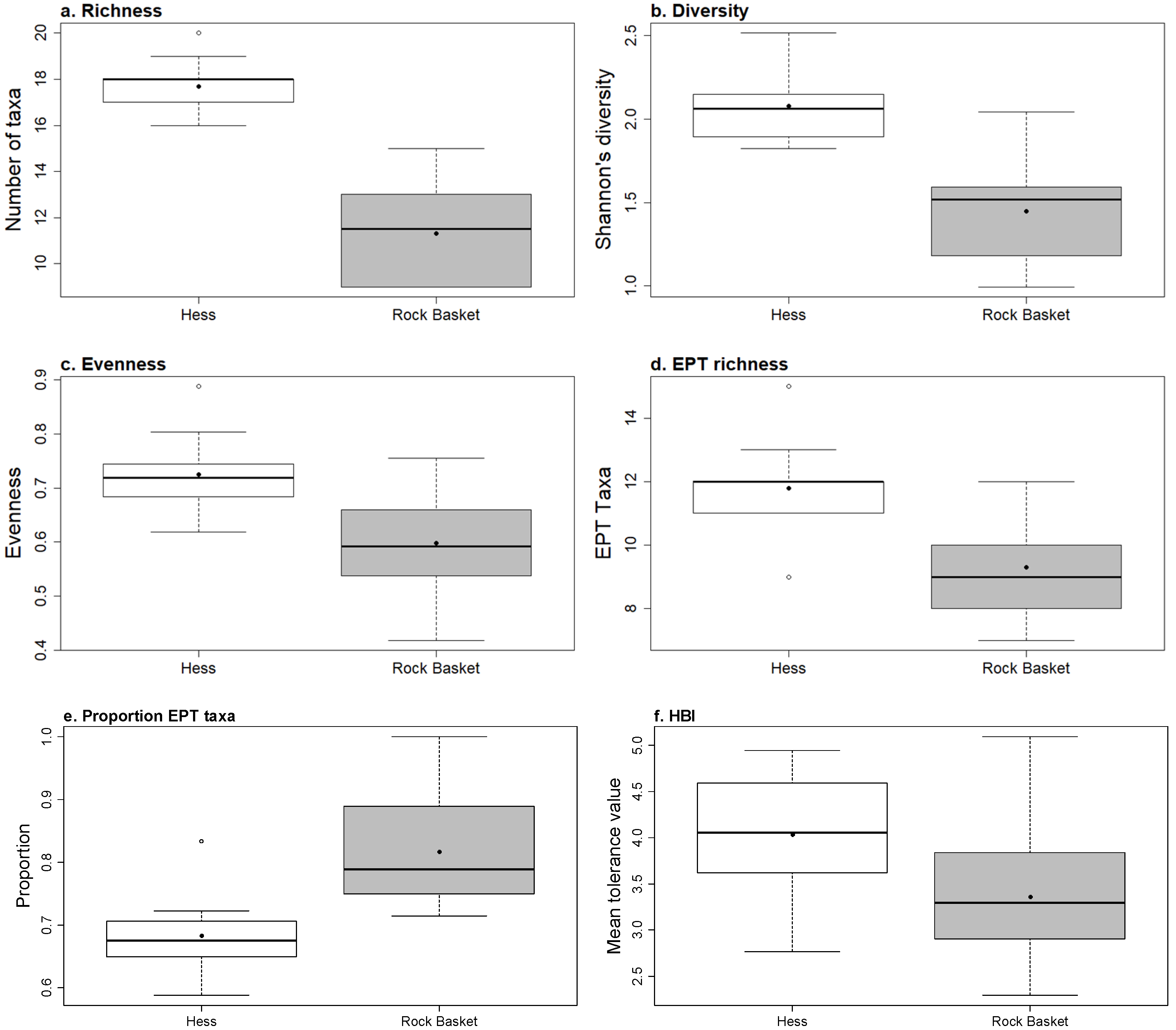
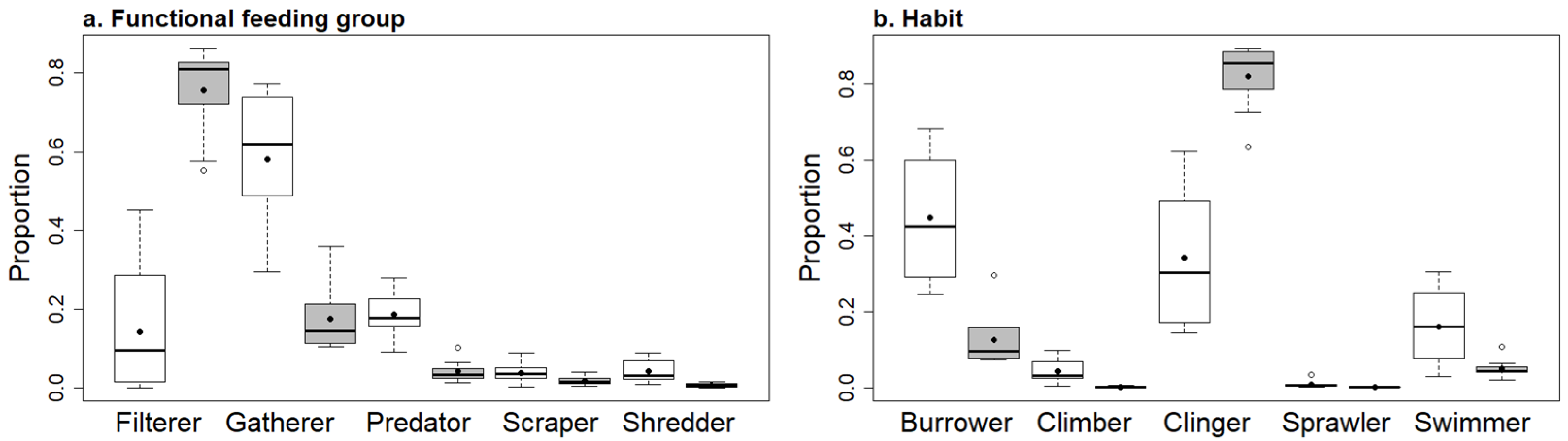
3.2. Hess Samples
3.3. Rock Baskets
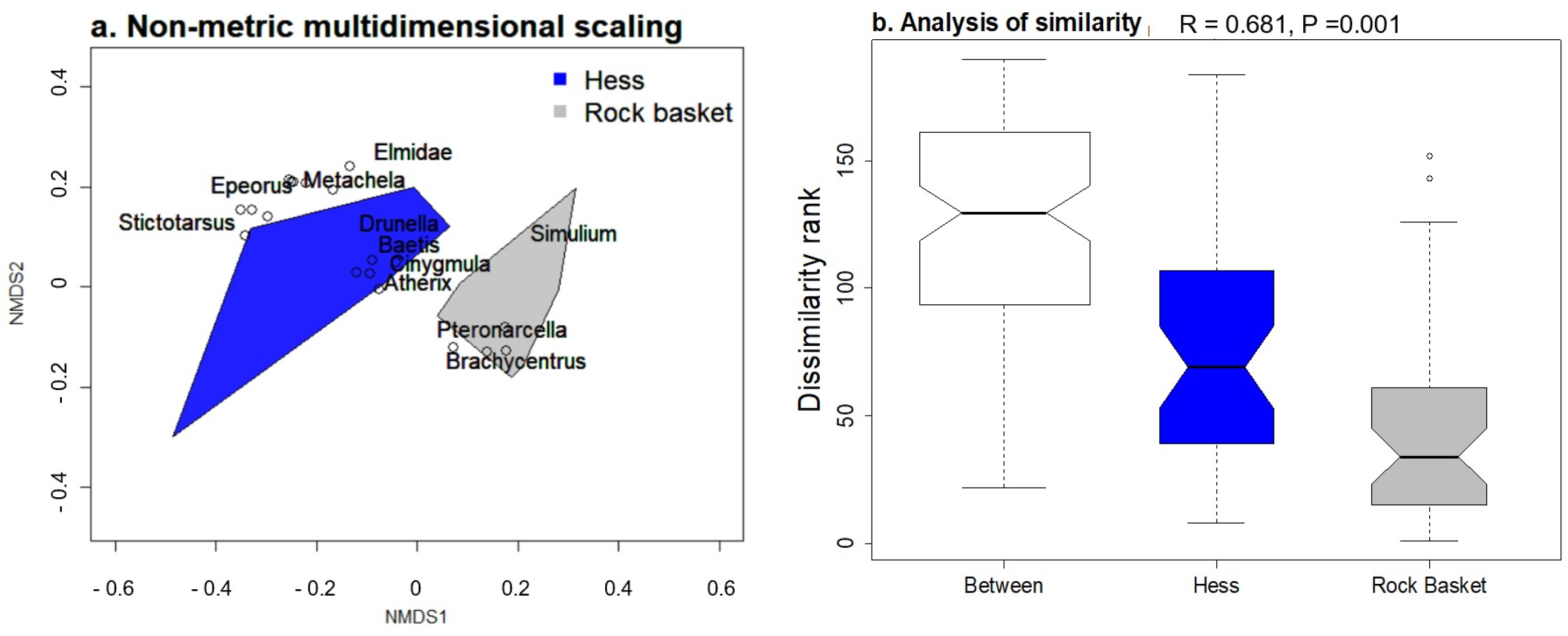
3.4. Comparing Samplers
3.5. Variance and Species Accumulation with Sample Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxa | Hess | Rock Basket |
|---|---|---|
| Arthropoda | ||
| Arachnida | ||
| Acari * | 548 ± 226 | |
| Insecta | ||
| Coleoptera | ||
| Dytiscidae | ||
| Stictotarsus * | 30 ± 8 | |
| Elmidae | 372 ± 42 | |
| Ampumixis | 86 ± 86 | |
| Diptera | ||
| Athericidae | ||
| Atherix | 161 ± 50 | 114 ± 15 |
| Ceratopogonidae | ||
| Probezzia * | 108 ± 22 | |
| Chironomidae (pupae) | 329 ± 69 | 272 ± 40 |
| Non-Tanypodinae | 8462 ± 1358 | 8054 ± 757 |
| Tanypodinae | 51 ± 12 | 86 ± 0 |
| Empididae | 23 ± 23 | |
| Hemerodromia * | 93 ± 93 | |
| Metachela * | 113 ±33 | |
| Neoplasta ‡ | 86 ± 86 | |
| Limoniidae | ||
| Hexatoma * | 146 ± 29 | |
| Simuliidae (pupae) | 51 ± 13 | 886 ± 363 |
| Simulium | 1499 ± 504 | 18,262 ± 7643 |
| Ephemeroptera | ||
| Baetidae | ||
| Baetis | 4353 ± 1512 | 3367 ± 359 |
| Ephemerellidae | ||
| Drunella | 5181 ± 1938 | 2223 ± 417 |
| Heptageniidae | ||
| Cinygmula | 1014 ± 408 | 1281 ± 227 |
| Epeorus * | 332 ± 90 | |
| Rhithrogena * | 579 ± 185 | |
| Leptophlebiidae | 120 ± 15 | |
| Neoleptophlebia * | 630 ± 154 | |
| Paraleptophlebia | 35 ± 35 | 86 ± 0 |
| Hemiptera ‡ | 86 ± 86 | |
| Plecoptera | 204 ± 75 | |
| Chloroperlidae | ||
| Plumiperla * | 235 ± 55 | |
| Perlidae | ||
| Claassenia | 42 ± 7 | 535 ± 79 |
| Perlodidae * | 336 ± 153 | |
| Pteronarcyidae | ||
| Pteronarella | 247 ± 126 | 459 ± 93 |
| Trichoptera | 93 ± 0 | |
| Brachycentridae | ||
| Brachycentrus | 3511 ± 1809 | 34,262 ± 7186 |
| Glossosomatidae | ||
| Glossosoma * | 12 ± 12 | |
| Hydropsychidae | ||
| Arctopsyche | 103 ± 28 | 314 ± 66 |
| Hydropsyche | 760 ± 185 | 4429 ± 613 |
| Lepidostomatidae | ||
| Lepidostoma | 1358 ± 483 | 201 ± 16 |
| Leptoceridae | ||
| Oecetis | 139 ± 19 | 95 ± 15 |
| Annelida | ||
| Oligochaeta | 3136 ± 1222 | 86 ± 86 |
| Nematoda * | 179 ± 55 | |
| Crustacea | ||
| Ostracoda * | 93 ± 93 | |
| Molluska | ||
| Gastropoda | ||
| Physa gyrina ‡ | 16 ± 2 |
References
- Petts, G.; Moller, H.; Roux, A. Historical Change in Large Alluvial Rivers: Western Europe; Wiley: Chichester, UK, 1993. [Google Scholar]
- Leitner, P.; Borgwardt, F.; Birk, S.; Graf, W. Multiple stressor effects on benthic macroinvertebrates in very large European rivers—A typology-based evaluation of faunal responses as a basis for future bioassessment. Sci. Total Environ. 2021, 756, 143472. [Google Scholar] [CrossRef] [PubMed]
- Best, J. Anthropogenic stresses on the world’s big rivers. Nat. Geosci. 2018, 12, 7–21. [Google Scholar] [CrossRef]
- Johnson, B.L.; Richardson, W.B.; Naimo, T.J. Past, present, and future concepts in large river ecology. BioScience 1995, 45, 134–141. [Google Scholar] [CrossRef]
- Czuba, J.A.; Allen, G.H. When does a stream become a river? River Res. Appl. 2023, 39, 1925–1929. [Google Scholar] [CrossRef]
- Hynes, H.B.N. Keynote address. In Proceedings of the International Large River Symposium, Honey Harbour, ON, Canada, 14–21 September 1986; pp. 5–10. [Google Scholar]
- Dodds, W. Nutrients and the “dead zone”: The link between nutrient ratios and dissolved oxygen in the northern Gulf of Mexico. Front. Ecol. Environ. 2006, 4, 211–217. [Google Scholar] [CrossRef]
- Rabalais, N.; Turner, R.; Wiseman, W. Gulf of Mexico hypoxia, aka “the dead zone”. Annu. Rev. Ecol. Syst. 2002, 33, 235–263. [Google Scholar] [CrossRef]
- Tank, J.L.; Rosi-Marshall, E.J.; Baker, M.A.; Hall, R.O., Jr. Are rivers just big streams? A pulse method to quantify nitrogen demand in a large river. Ecology 2008, 89, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.M.; Resh, V.H. (Eds.) Freshwater Biomonitoring and Benthic Macroinvertebrates; Chapman and Hall: New York, NY, USA, 1993; 512p. [Google Scholar]
- Flotemersch, J.; Stribling, J.B.; Paul, M. Concepts and Approaches for the Bioassessment of Non-Wadeable Streams and Rivers; US Environmental Protection Agency: Cincinnati, OH, USA, 2006. [Google Scholar]
- Neto, J.M.; Feio, M.J.; Teixeira, H.; Patricio, J.; Serra, S.R.Q.; Franco, J.N.; Calapez, A.R.; Constantino, E. Transitional and freshwater bioassessments: One site, two perspectives? Mar. Pollut. Bull. 2014, 78, 153–164. [Google Scholar] [CrossRef]
- Hughes, R.M.; Herlihy, A.T.; Gerth, W.J.; Pan, Y. Estimating vertebrate, benthic macroinvertebrate, and diatom taxa richness in raftable Pacific Northwest rivers for bioassessment purposes. Environ. Monit. Assess. 2012, 184, 3185–3198. [Google Scholar] [CrossRef]
- Bartsch, L.A.; Richardson, W.B.; Naimo, T.J. Sampling benthic macroinvertebrates in a large flood-plain river: Considerations of study design, sample size, and cost. Environ. Monit. Assess. 1998, 52, 425–439. [Google Scholar] [CrossRef]
- Weigel, B.M.; Dimick, J.J. Development, validation, and application of a macroinvertebrate-based Index of Biotic Integrity for nonwadeable rivers of Wisconsin. J. N. Am. Benthol. Soc. 2011, 30, 665–679. [Google Scholar] [CrossRef]
- Szekeres, J.; Borza, P.; Csnyi, B.; Graf, W.; Huber, T.; Leitner, P.; Milosevic, D.; Paunovic, M.; Pavelescu, C.; Eros, T. Comparison of littoral and deep water sampling methods for assessing macroinvertebrate assemblages along the longitudinal profile of a very large river (the Danube River, Europe). River Res. Appl. 2019, 35, 989–998. [Google Scholar] [CrossRef]
- Blocksom, K.A.; Flotemersch, J.E. Comparison of macroinvertebrate sampling methods for nonwadeable streams. Environ. Monit. Assess. 2005, 102, 243–262. [Google Scholar] [CrossRef]
- Roby, K.B.; Newbold, J.D.; Erman, D.C. Effectiveness of an artificial substrate for sampling macroinvertebrates in small streams. Freshw. Biol. 1978, 8, 1–8. [Google Scholar] [CrossRef]
- Czerniawska-Kusza, I. Use of Artificial Substrates for Sampling Benthic Macroinvertebrates in the Assessment of Water Quality of Large Lowland Rivers. Pol. J. Environ. Stud. 2004, 13, 579–584. [Google Scholar]
- Letovsky, E.; Myers, I.E.; Canepa, A.; McCabe, D.J. Differences between kick sampling techniques and short-term Hester-Dendy sampling for stream macroinvertebrates. Bios 2012, 83, 47–55. [Google Scholar] [CrossRef]
- Tronstad, L.M.; Hotaling, S. Long-term trends in aquatic ecosystem bioassessment metrics are not influenced by sampling method: Empirical evidence from the Niobrara River. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 28. [Google Scholar] [CrossRef]
- Tronstad, L.M.; Wilmot, O.; Thornbrugh, D.; Hotaling, S. To composite or replicate: How sampling method and protocol differences alter collected stream invertebrates and associated bioassessment metrics. Environ. Monit. Assess. 2020, 192, 531. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J.A.; Thompson, A.; Asher, E.; Gregory, S.V.; Reeves, G.; Ratliff, D.; Bouwes, N.; Frissell, C.; Williams, R.N. Columbia River Basin. In Rivers of North America; Elsevier: Amsterdam, The Netherlands, 2023; pp. 558–615. [Google Scholar]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to the Aquatic Insect of North America; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 2019; 1498p. [Google Scholar]
- Thorp, J.H.; Covich, A.P. (Eds.) Ecology and Classification of North American Freshwater Invertebrates, 3rd ed.; Elsevier: New York, NY, USA, 2010; 1021p. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish; EPA 841-B-99-002; U.S. Environmental Protection Agency: Washington, DC, USA, 1999. [Google Scholar]
- Hilsenhoff, W.L. An improved biotic index of organic stream pollution. Great Lakes Entomologist 1987, 20, 31–39. [Google Scholar] [CrossRef]
- Delignette-Muller, M.; Dutang, C. fitdistrplus: An R package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Lenth, R. emmeans: Estimated Mearginal Means, aka Least-Squares Means. Comprehensive R Archive Network. 2021. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 15 July 2023).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, H.; et al. vegan: Community Ecology Package. 2013. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 15 July 2023).
- DeBruine, L. faux: Simulation for Factorial Designs. R Package Version 1.2.1. Zenodo. 2023. Available online: https://debruine.github.io/faux/ (accessed on 15 July 2023).
- Wickham, H. The Split-Apply_Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Sanders, L.; Bingham, C.; Beckett, D. Macroinvertebrate Gear Evaluation; US Army Engineer Waterways Experiment Station: Vicksburg, MS, USA, 1986. [Google Scholar]
- Rabeni, C.F.; Gibbs, K. Comparison of two methods used by divers for sampling benthic invertebrates in deep rivers. J. Fish. Res. Board Can. 1978, 35, 332–336. [Google Scholar] [CrossRef]
- McCabe, D.J.; Hayes-Pontius, E.M.; Canepa, A.; Berry, K.S.; Levine, B.C. Measuring standardized effect size improves interpretation of biomonitoring studies and facilitates meta-analysis. Freshw. Sci. 2012, 31, 800–812. [Google Scholar] [CrossRef]
- Turner, A.M.; Trexler, J.C. Sampling aquatic invertebrates from marshes: Evaluating the options. J. N. Am. Benthol. Soc. 1997, 16, 694–709. [Google Scholar] [CrossRef]
- Canton, S.P.; Chadwick, J.W. Aquatic Insect Communities of Natural and Artificial Substrates in a Montane Stream. J. Freshw. Ecol. 1983, 2, 153–158. [Google Scholar] [CrossRef]
- Flotemersch, J.E.; Blocksom, K.; Hutchens, J.J.; Autrey, B.C. Development of a standardized Large River Bioassessment Protocol (LR-BP) for macroinvertebrate assemblages. River Res. Appl. 2006, 22, 775–790. [Google Scholar] [CrossRef]
- Rabeni, C.F.; Minshall, G.W. Factors Affecting Microdistribution of Stream Benthic Insects. Oikos 1977, 29, 33–43. [Google Scholar] [CrossRef]
- Bevilacqua, M.S.; Vitório, A.; Felix, R.W.; Ribeiro, E.G.; Bozelli, R.L.; Figueiredo-Barros, M.P.; de Assis Esteves, F. Small is better: Comparing the efficiency of two Surber samplers’ sizes for biomonitoring programs in tropical headwater streams in the Amazon region. Environ. Monit. Assess. 2020, 192, 708. [Google Scholar] [CrossRef]


| Parameter | Measurement | Units |
|---|---|---|
| Dissolved oxygen | 100.3 | % saturation |
| 9.37 | mg O2/L | |
| Water temperature | 18.6 | °C |
| Specific conductivity | 168.5 | µS/cm |
| pH | 8.62 | |
| Oxidation-reduction potential | 86.7 | mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tronstad, L.M.; Tronstad, B.P. Assessing Methods to Monitor Aquatic Invertebrates in a Large River: Comparing Rock Baskets and Hess Samplers in the Snake River, Wyoming, USA. Hydrobiology 2024, 3, 209-223. https://doi.org/10.3390/hydrobiology3030014
Tronstad LM, Tronstad BP. Assessing Methods to Monitor Aquatic Invertebrates in a Large River: Comparing Rock Baskets and Hess Samplers in the Snake River, Wyoming, USA. Hydrobiology. 2024; 3(3):209-223. https://doi.org/10.3390/hydrobiology3030014
Chicago/Turabian StyleTronstad, Lusha M., and Bryan P. Tronstad. 2024. "Assessing Methods to Monitor Aquatic Invertebrates in a Large River: Comparing Rock Baskets and Hess Samplers in the Snake River, Wyoming, USA" Hydrobiology 3, no. 3: 209-223. https://doi.org/10.3390/hydrobiology3030014
APA StyleTronstad, L. M., & Tronstad, B. P. (2024). Assessing Methods to Monitor Aquatic Invertebrates in a Large River: Comparing Rock Baskets and Hess Samplers in the Snake River, Wyoming, USA. Hydrobiology, 3(3), 209-223. https://doi.org/10.3390/hydrobiology3030014






