Acute Toxicity of Malathion, Permethrin, and Roundup on the Tropical Freshwater Shrimp Xiphocaris elongata (Guérin-Méneville, 1855)
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Acclimation of Freshwater Shrimp
2.2. Acute Toxicity Tests
2.3. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schäfer, R.B.; Caquet, T.; Siimes, K.; Mueller, R.; Lagadic, L.; Liess, M. Effects of pesticides on community structure and ecosystem functions in agricultural streams of three biogeographical regions in Europe. Sci. Total Environ. 2007, 382, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Liess, M.; Von Der Ohe, P.C. Analyzing effects of pesticides on invertebrate communities in streams. Environ. Toxicol. Chem. 2005, 24, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Schiff, K.; Sutula, M. Organophosphorus pesticides in storm-water runoff from southern California (USA). Environ. Toxicol. Chem. 2004, 23, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ye, Y.; Hu, D.; Ou, L.; Wang, X. Characteristics and transport of organochlorine pesticides in urban environment: Air, dust, rain, canopy throughfall, and runoff. J. Environ. Monit. 2010, 12, 2153–2160. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Thurman, E.M.; Linhart, S.M. The environmental occurrence of herbicides: The importance of degradates in ground water. Arch. Environ. Contam. Toxicol. 1998, 35, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.H.; McDowell, L.L. Pesticides in agricultural runoff and their effects on downstream water quality. Environ. Toxicol. Chem. 1982, 1, 267–279. [Google Scholar] [CrossRef]
- Daam, M.A.; Van den Brink, P.J. Implications of differences between temperate and tropical freshwater ecosystems for the ecological risk assessment of pesticides. Ecotoxicology 2009, 19, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, J.S.; Castillo, L.E. Ecotoxicology in tropical regions. Environ. Sci. Pollut. Res. 2018, 25, 13203–13206. [Google Scholar] [CrossRef] [PubMed]
- Buttermore, E.; Cope, G.W.; Kwak, T.J.; Cooney, P.B.; Shea, D.; Lazaro, P.R. Contaminants in tropical island streams and their biota. Environ. Res. 2018, 161, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Santiago, X.B.; Rivera, D.; Pabón, A.; García, A. An examination of the use of pesticides in Puerto Rican agriculture. Rurals 2016, 10, 1–10. [Google Scholar]
- van Bruggen AH, C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Mensah, P.K.; Muller, W.J.; Palmer, C.G. Acute toxicity of Roundup®® herbicide to three life stages of the freshwater shrimp Caridina nilotica (Decapoda: Atyidae). Phys. Chem. Earth 2011, 36, 905–909. [Google Scholar] [CrossRef]
- Kumar, A.; Doan, H.; Barnes, M.; Chapman, J.C.; Kookana, R.S. Response and recovery of acetylcholinesterase activity in freshwater shrimp, Paratya australiensis (Decapoda: Atyidae) exposed to selected anti-cholinesterase insecticides. Ecotoxicol. Environ. Saf. 2010, 73, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Stenersen, J. Chemical Pesticides: Mode of Action and Toxicology; CRC Press: Boca Raton, FL, USA, 2004; p. 296. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Permethrin, EFED Revised Risk Assessment for the Reregistration Eligibility Decision on Permethrin; Docket Number 2004-0385-0014; United States Environmental Protection Agency: Washington, DC, USA, 2005.
- De Oliveira, P.; Gomes, A.Q.; Pacheco, T.R.; Vitorino de Almeida, V.; Saldanha, C.; Calado, A. Cell-specific regulation of acetylcholinesterase expression under inflammatory conditions. Clin. Hemorheol. Microcirc. 2012, 51, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Cox, C. Permethrin insecticide fact sheet. J. Pestic. Reform 1998, 18, 1–20. [Google Scholar]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Schettino, T. Acetylcholinesterase inhibition as a relevant biomarker in environmental biomonitoring: New insights and future perspectives. BioMed Res. Int. 2011, 1, 87–115. [Google Scholar] [CrossRef]
- Printes, L.B.; Callaghan, A. A comparative study on the relationship between acetylcholinesterase activity and acute toxicity in Daphnia magna exposed to anticholinesterase insecticides. Environ. Toxicol. Chem. 2004, 23, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Correll, R.; Grocke, S.; Bajet, C. Toxicity of selected pesticides to freshwater shrimp, Paratya australiensis (Decapoda: Atyidae): Use of time series acute toxicity data to predict chronic lethality. Ecotoxicol. Environ. Saf. 2010, 73, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jawahar, A. Toxic impacts of two organophosphorus pesticides on the acetylcholinesterase activity and biochemical composition of freshwater fairy shrimp Streptocephalus dichotomus. Int. J. Pharma Bio Sci. 2013, 4, 966–972. [Google Scholar]
- Printes, L.B.; Callaghan, A. Intraclonal variability in Daphnia acetylcholinesterase activity: The implications for its applicability as a biomarker. Environ. Toxicol. Chem. 2003, 22, 2042–2047. [Google Scholar] [CrossRef]
- Rinderhagen, M.; Ritteroff, J.; Zauke, G.P. Crustaceans as bioindicators. Environ. Res. 2000, 9, 161–194. [Google Scholar]
- Guérin-Méneville, F.E. Crustáceos. In Historia Física, Político y Natural de la Isla de Cuba; de la Sagra, R., Ed.; Arthus Bertrand: Paris, France, 1856. [Google Scholar]
- Perez-Reyes, O.; Crowl, T.A.; Hernandez-Garcia, P.J.; Ledesma-Fuste, R.; Villar-Fornes, F.A.; Covich, A.P. Freshwater decapods of Puerto Rico: A checklist and reports of new localities. Zootaxa 2013, 3717, 329–344. [Google Scholar] [CrossRef]
- Wehrtmann, I.S.; Ramírez, A.; Pérez-Reyes, O. Freshwater decapod diversity and conservation in Central America and the Caribbean. In A Global Overview of the Conservation of Freshwater Decapod Crustaceans; Springer: Cham, Switzerland, 2016; pp. 267–301. [Google Scholar]
- Pérez-Reyes, O.; Crowl, T.A.; Covich, A.P. Effects of food supplies and water temperature on growth rates of two species of freshwater tropical shrimps. Freshw. Biol. 2015, 60, 1514–1524. [Google Scholar] [CrossRef]
- Pottier, G.; Bargier, N.; Bennevault, Y.; Vigouroux, R.; Azam, D.; Marchand, F.; Nevoux, M.; Roussel, J.M. Optimising electrofishing settings for shrimp and fish in shallow tropical streams. Fish. Res. 2022, 256, 106457. [Google Scholar] [CrossRef]
- SAS Institute, (2023) JMP Statistical Discovery LLC.
- Sánchez-Fortún, S.; Barahona, M.V. Comparative study on the environmental risk induced by several pyrethroids in estuarine and freshwater invertebrate organisms. Chemosphere 2005, 59, 553–559. [Google Scholar] [CrossRef]
- Altshuler, I.; Demiri, B.; Xu, S.; Constantin, A.; Yan, N.D.; Cristescu, M.E. An Integrated Multi-Disciplinary Approach for Studying Multiple Stressors in Freshwater Ecosystems: Daphnia as a Model Organism. Integr. Comp. Biol. 2011, 51, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Seda, J.; Petrusek, A. Daphnia as a model organism in limnology and aquatic biology: Introductory remarks. J. Limnol. 2011, 70, 337–344. [Google Scholar] [CrossRef]
- Satapornvanit, K.; Baird, D.J.; Little, D.C. Laboratory toxicity test and post-exposure feeding inhibition using the giant freshwater prawn Macrobrachium rosenbergii. Chemosphere 2009, 74, 1209–1215. [Google Scholar] [CrossRef]
- Perez-Reyes, O. Population and Community Dynamics of Freshwater Decapods in Response to Ecological and Anthropogenic Factors in Subtropical Streams in the Caribbean. Doctoral Thesis, Utah State University, Logan, UT, USA, 2014. [Google Scholar]
- Pérez-Reyes, O.; Crowl, T.A.; Covich, A.P. Comparison of decapod communities across an urban-forest land use gradient in Puerto Rican streams. Urban Ecosyst. 2016, 19, 181–203. [Google Scholar] [CrossRef]
- Cruz-Rosa, S.; Pérez-Reyes, O. Titanium oxide nanoparticles as emerging aquatic pollutants: An evaluation of the nanotoxicity in the freshwater shrimp larvae Atya lanipes. Ecologies 2023, 4, 141–151. [Google Scholar] [CrossRef]
- Hook, S.E.; Doan, H.; Gonzago, D.; Musson, D.; Du, J.; Kookana, R.; Sellars, M.; Kumar, A. The impacts of modern-use pesticides on shrimp aquaculture: An assessment for northeastern Australia. Ecotoxicol. Environ. Saf. 2018, 148, 770–780. [Google Scholar] [CrossRef]
- Smith, T.M.; Stratton, G.W. Effects of synthetic pyrethroid insecticides on nontarget organisms. Residue Rev. 1986, 97, 93–120. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, M.E.; Serrano, L.; Chung, K.W.; Hoguet, J.; Key, P.B. Effects of the insecticide permethrin on three life stages of the grass shrimp, Palaemonetes pugio. Ecotoxicol. Environ. Saf. 2006, 64, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.; Cappello, S. Cypermethrin Toxicity to Aquatic Life: Bioassays for the Freshwater Prawn Palaemonetes argentinus. Arch. Environ. Contam. Toxicol. 2006, 51, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Key, P.B.; Fulton, M.H.; Scott, G.I.; Layman, S.L.; Wirth, E.F. Lethal and sublethal effects of Malathion on three life stages of the grass shrimp, Palaemonetes pugio. Aquat. Toxicol. 1998, 40, 311–322. [Google Scholar] [CrossRef]
- Yasser, A.G.; Naser, M. Acute toxicity and histopathological effects of Malathion on shrimp Macrobrachium nipponense (De Haan, 1849) (Caridea: Palaemonidae). J. Biol. Stud. 2023, 5, 774–779. [Google Scholar] [CrossRef]
- Saxena, T.; Manohar, S.; Chouhan, R. Intense Toxicity and Effects of Malathion Pesticides to Fresh Water Prawn Macrobrachium dayanum. J. Coast. Life Med. 2022, 10, 681–686. [Google Scholar]
- Rico, A.; Waichman, A.V.; Geber-Corrêa, R.; van den Brink, P.J. Effects of Malathion and carbendazim on Amazonian freshwater organisms: Comparison of tropical and temperate species sensitivity distributions. Ecotoxicology 2011, 20, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yang, X.; Huang, Y.; Yan, G.; Cheng, Y. Assessment of the oxidative and genotoxic effects of the glyphosate-based herbicide roundup on the freshwater shrimp, Macrobrachium nipponensis. Chemosphere 2018, 210, 896–906. [Google Scholar] [CrossRef]
- Fiorino, E.; Sehonova, P.; Plhalova, L.; Blahova, J.; Svobodova, Z.; Faggio, C. Effects of glyphosate on early life stages: Comparison between Cyprinus carpio and Danio rerio. Environ. Sci. Pollut. Res. 2018, 25, 8542–8549. [Google Scholar] [CrossRef]
- Uren WT, M.; Laing, L.V.; Florance, H.; Santos, E.M. Effects of Glyphosate and its Formulation, Roundup, on Reproduction in Zebrafish (Danio rerio). Environ. Sci. Technol. 2014, 48, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Mensah, P.K.; Palmer, C.G.; Odume, O.N. Ecotoxicology of Glyphosate and Glyphosate-Based Herbicides—Toxicity to Wildlife and Humans. In Toxicity and Hazard of Agrochemicals; InTech: Rijeka, Croatia, 2015; pp. 93–112. [Google Scholar] [CrossRef]
- Torres-Pérez, W.X.; Pérez-Reyes, O. Effect of Particle Size and Pesticide Contamination on Preference and Ingestion Rates by the Tropical Freshwater Shrimp Xiphocaris elongata. Open J. Ecol. 2023, 13, 183–198. [Google Scholar] [CrossRef]


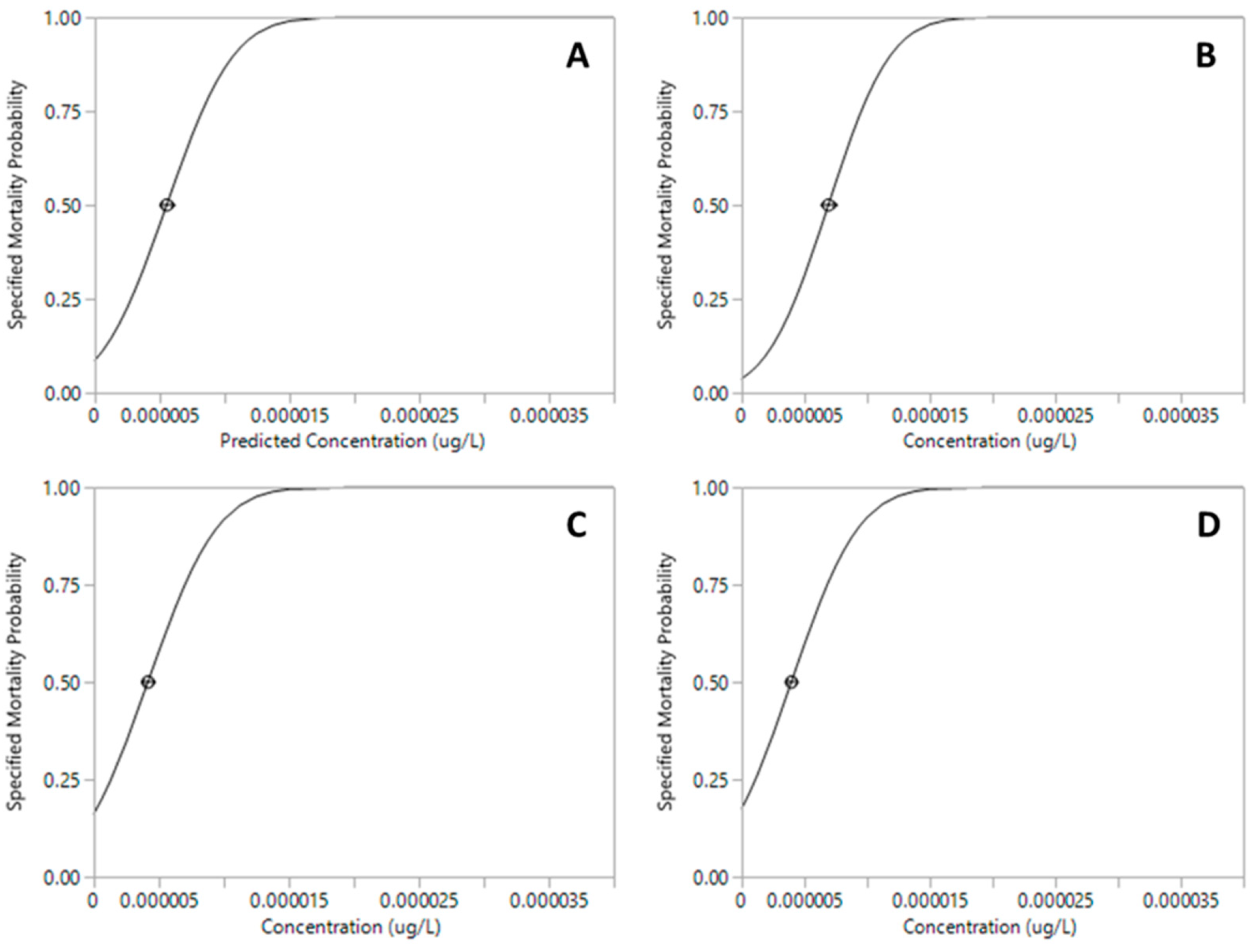
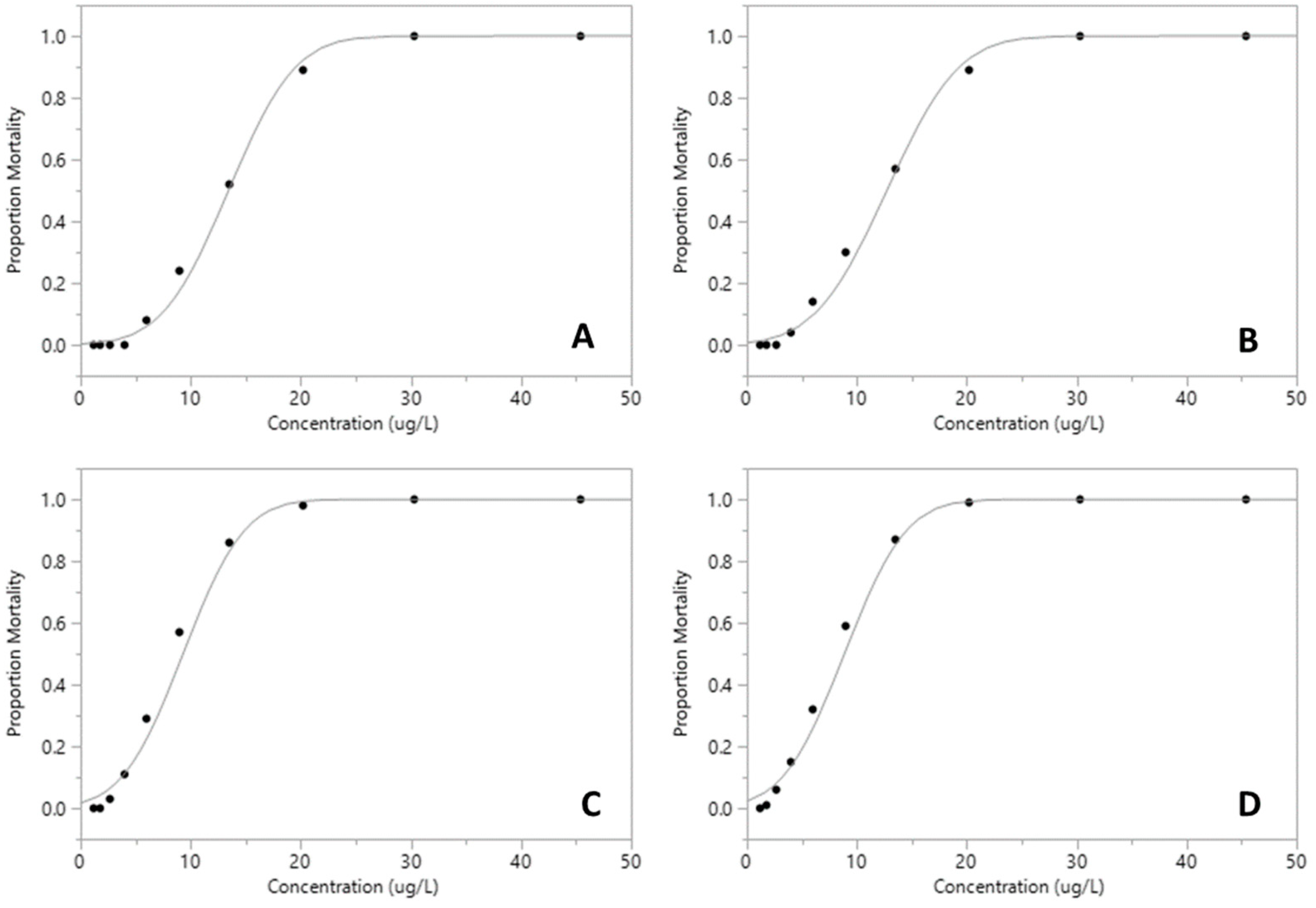
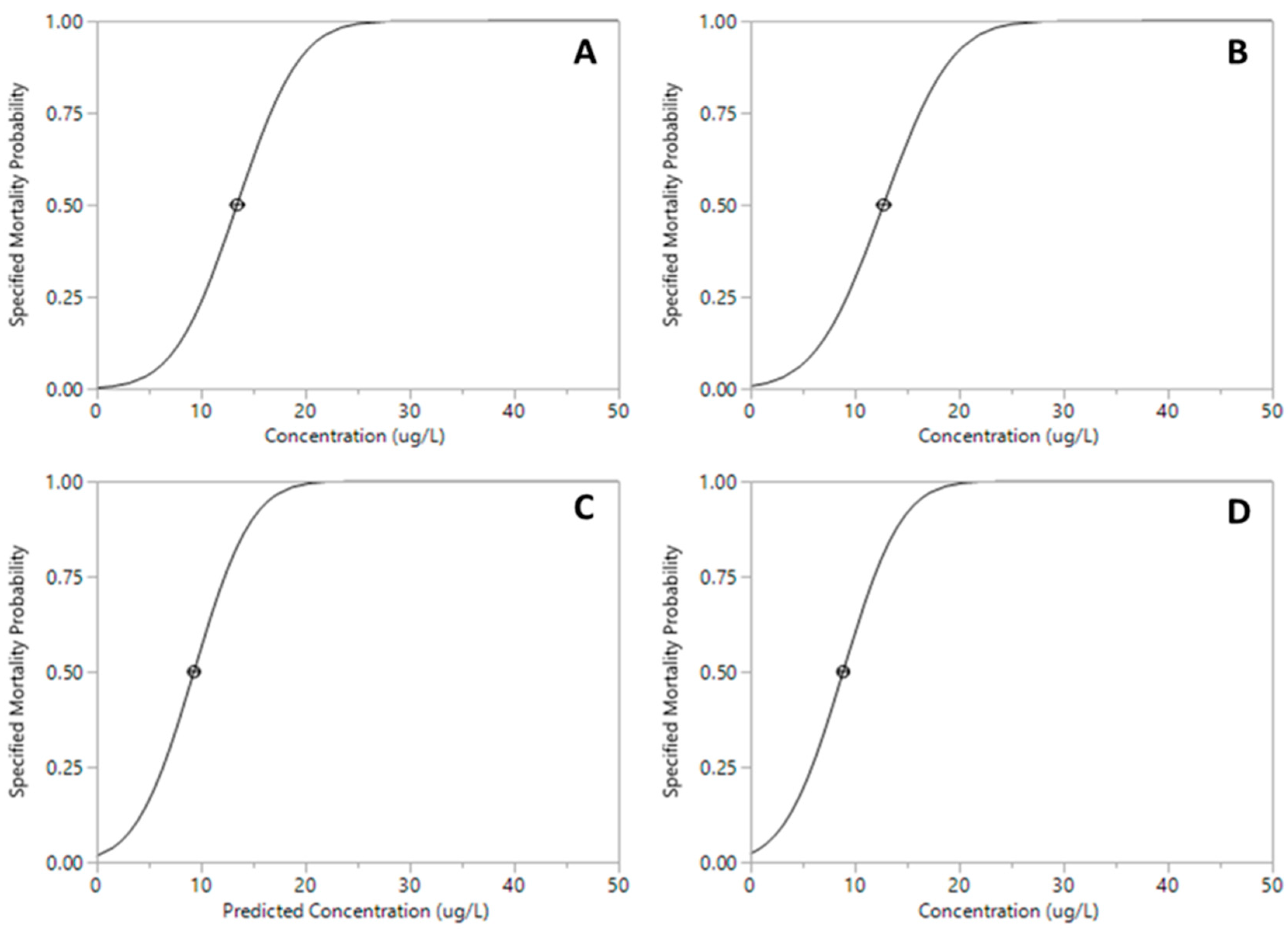
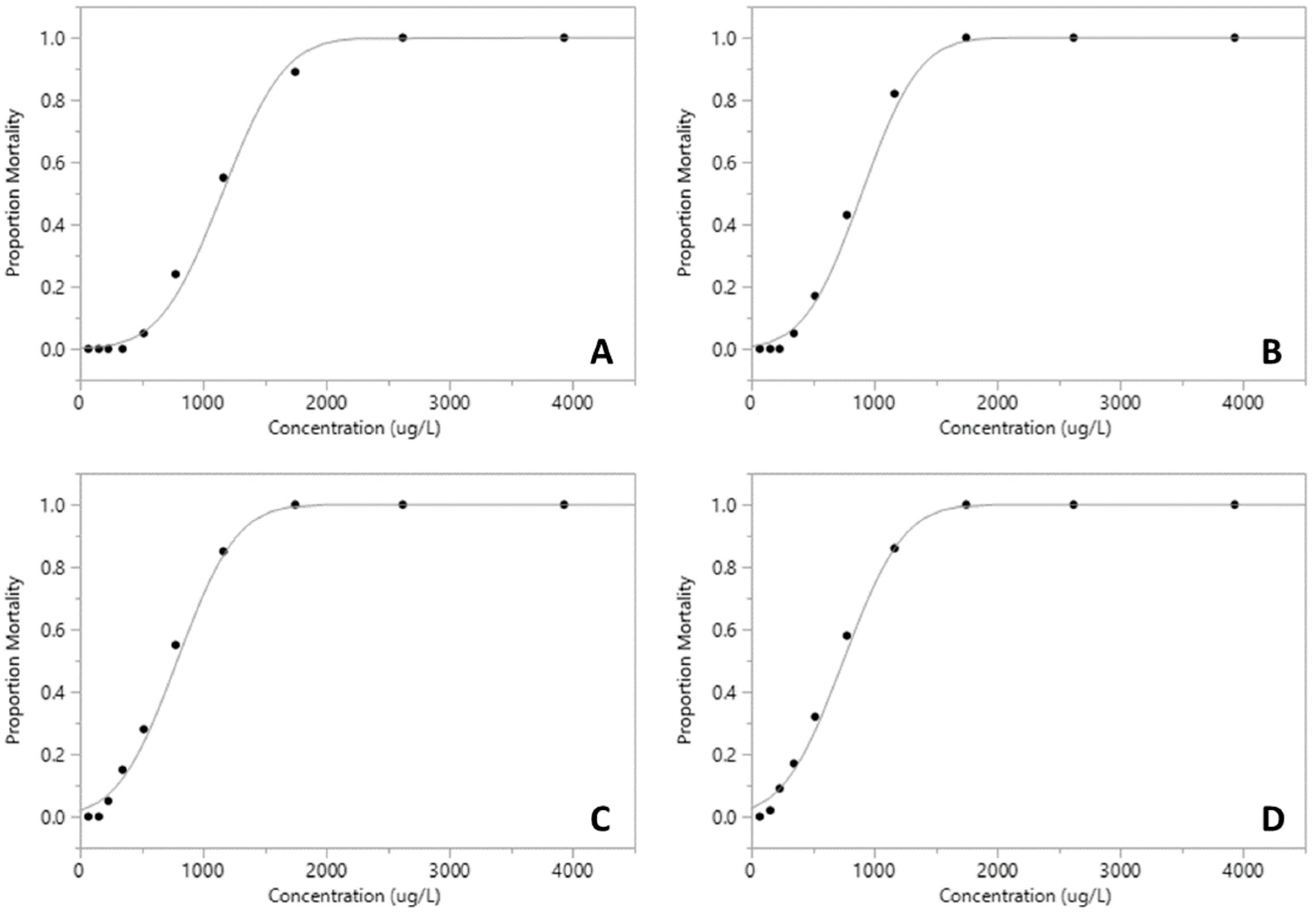
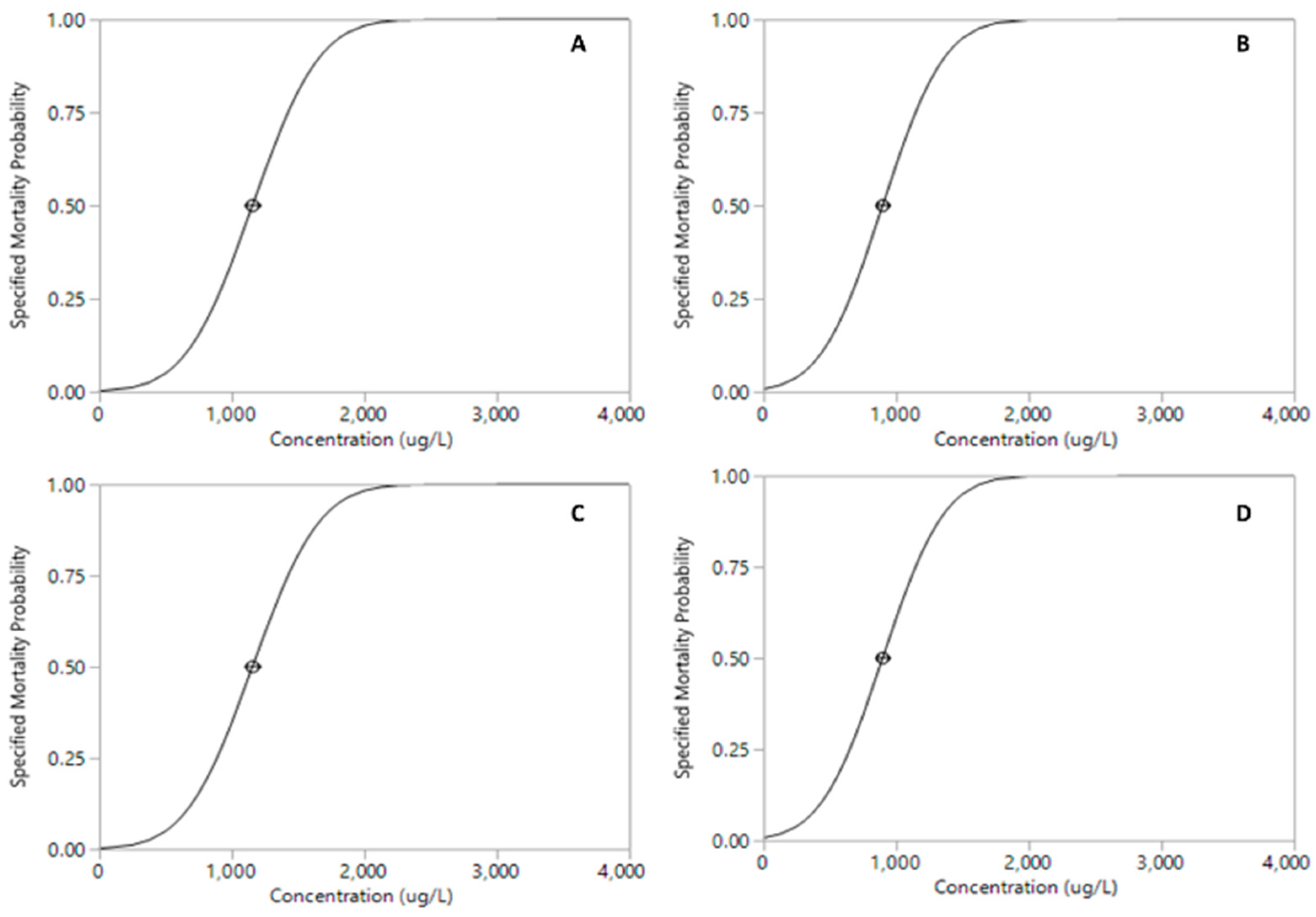
| Pesticide | LC50 µg·L−1 (95% CI) | df | F | p |
|---|---|---|---|---|
| Permethrin | 3.96 × 10−6 (4.49 × 10−6–4.52 × 10−6) | 9 | 66.13 | <0.001 *** |
| Malathion | 8.87 (8.31–9.49) | 9 | 84.35 | <0.001 *** |
| Roundup | 748.92 (701.01–802.45) | 9 | 107.64 | <0.001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Pérez, W.X.; Pérez-Reyes, O. Acute Toxicity of Malathion, Permethrin, and Roundup on the Tropical Freshwater Shrimp Xiphocaris elongata (Guérin-Méneville, 1855). Hydrobiology 2024, 3, 149-158. https://doi.org/10.3390/hydrobiology3030011
Torres-Pérez WX, Pérez-Reyes O. Acute Toxicity of Malathion, Permethrin, and Roundup on the Tropical Freshwater Shrimp Xiphocaris elongata (Guérin-Méneville, 1855). Hydrobiology. 2024; 3(3):149-158. https://doi.org/10.3390/hydrobiology3030011
Chicago/Turabian StyleTorres-Pérez, Wesley X., and Omar Pérez-Reyes. 2024. "Acute Toxicity of Malathion, Permethrin, and Roundup on the Tropical Freshwater Shrimp Xiphocaris elongata (Guérin-Méneville, 1855)" Hydrobiology 3, no. 3: 149-158. https://doi.org/10.3390/hydrobiology3030011
APA StyleTorres-Pérez, W. X., & Pérez-Reyes, O. (2024). Acute Toxicity of Malathion, Permethrin, and Roundup on the Tropical Freshwater Shrimp Xiphocaris elongata (Guérin-Méneville, 1855). Hydrobiology, 3(3), 149-158. https://doi.org/10.3390/hydrobiology3030011






