Effects of Leaf Species and Conditioning State of Fresh Leaves on Colonization by Stream and Pond Macroinvertebrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Data Analysis
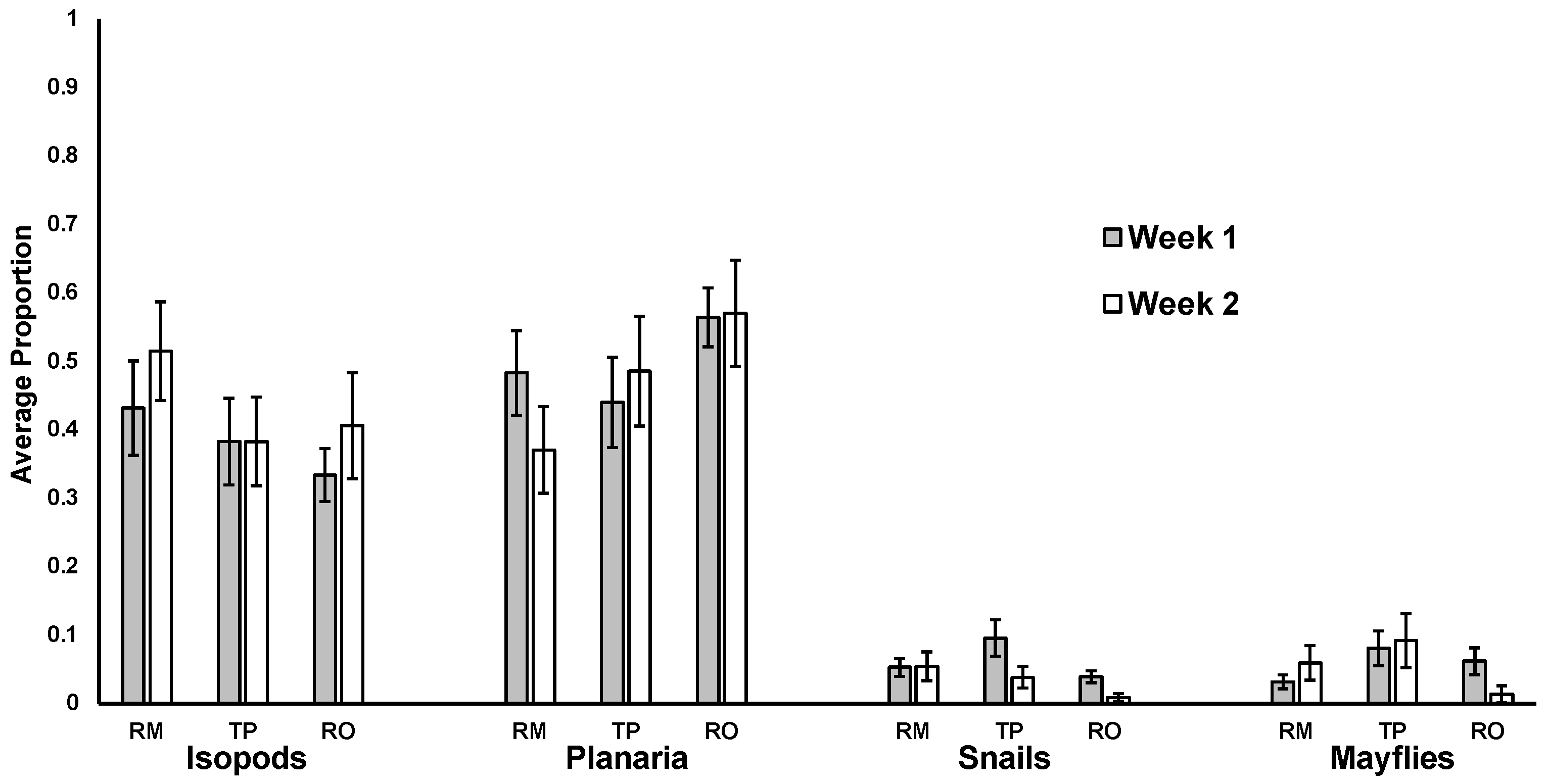
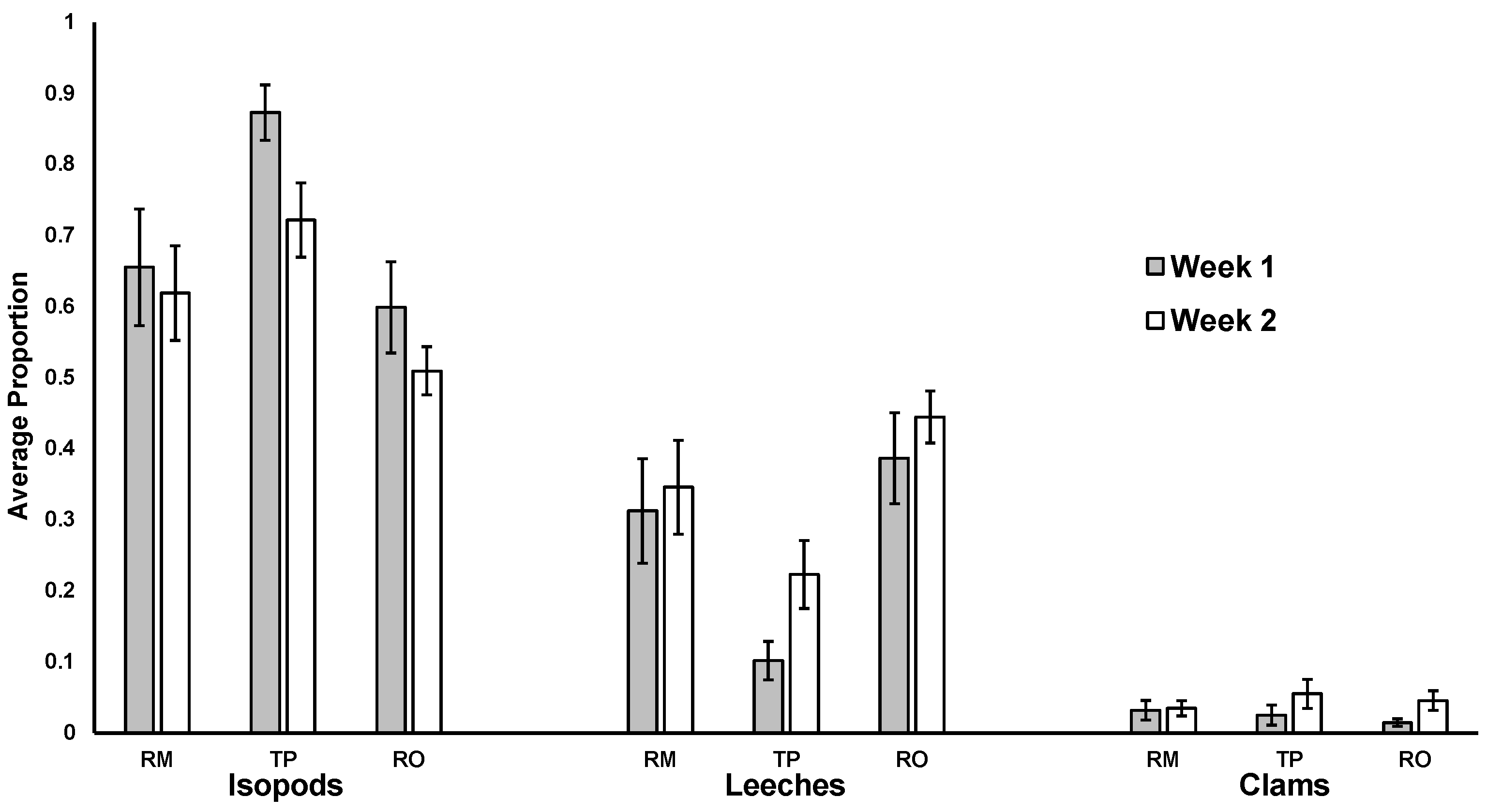
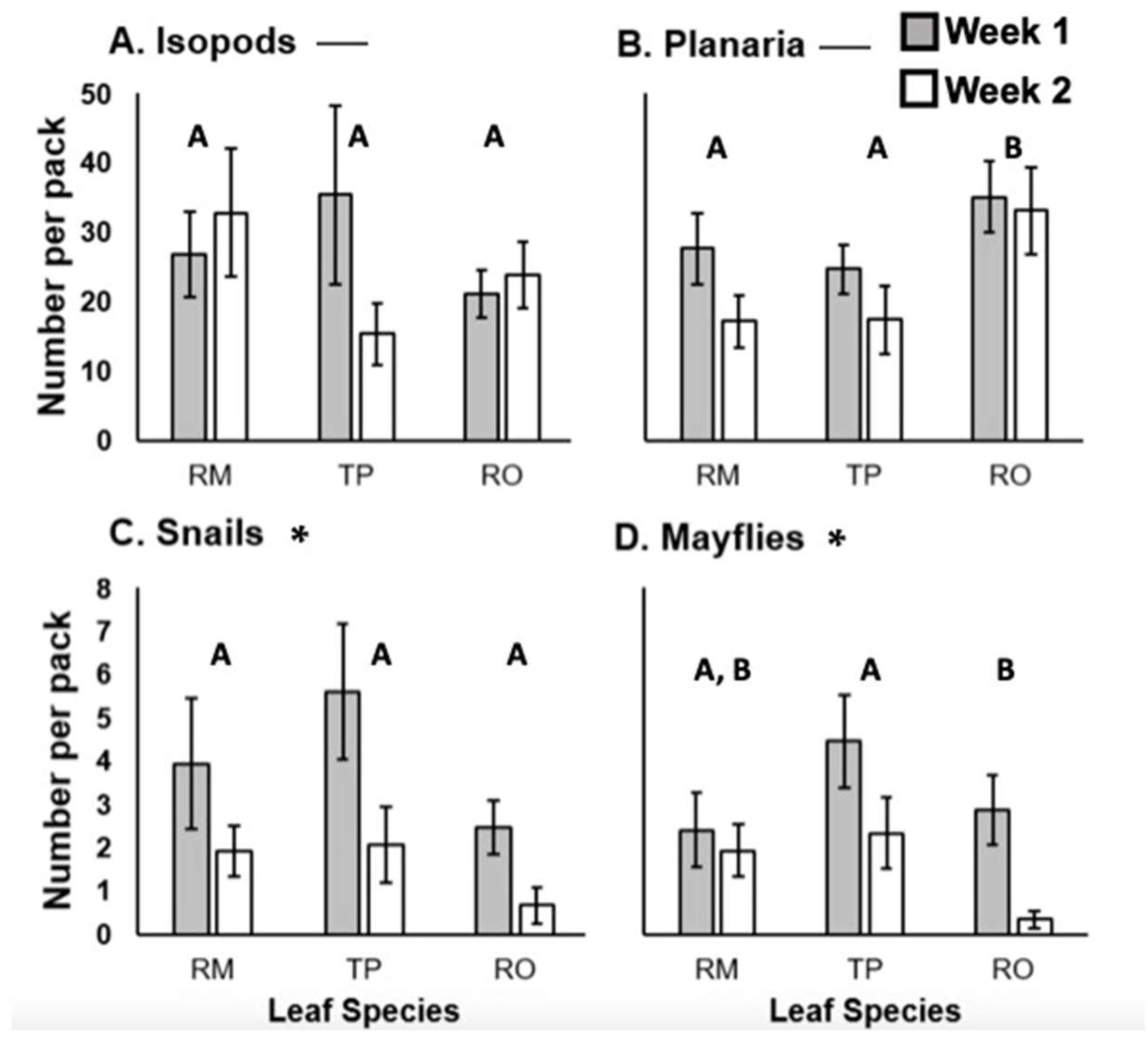
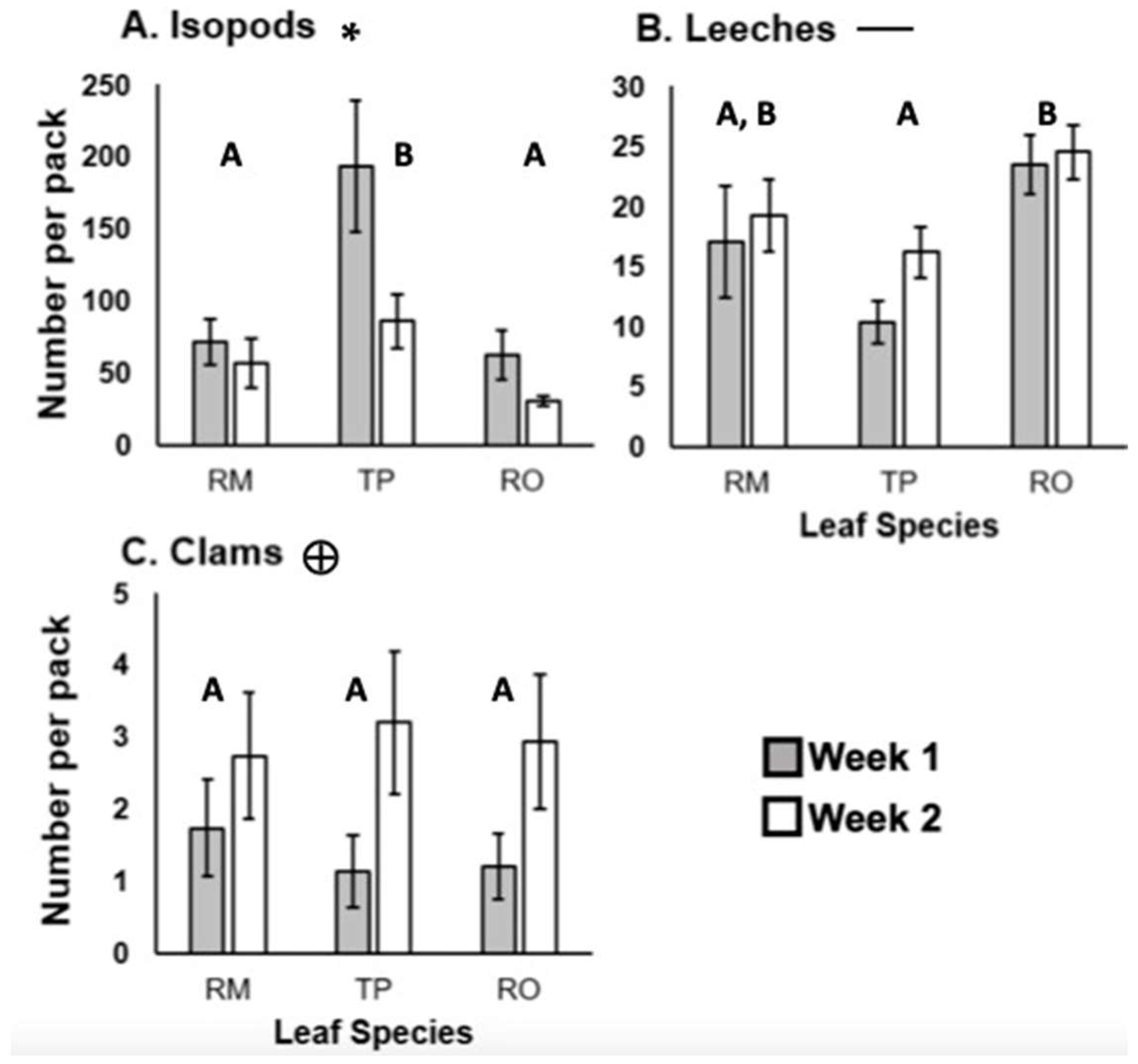
3. Results
4. Discussion
4.1. Effect of Leaf Species
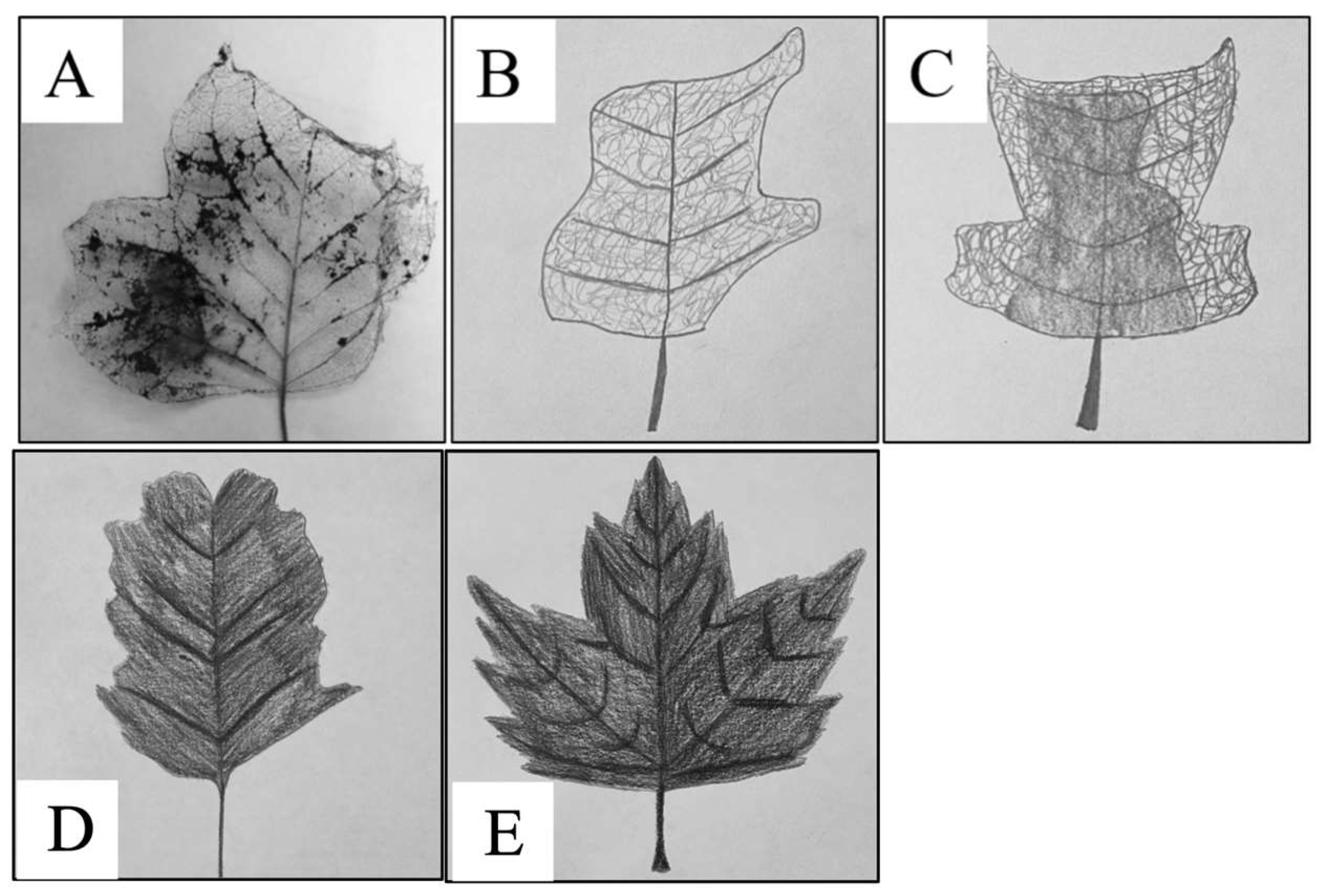
4.2. No Impact of Leaf Skeletonization
4.3. Importance of These Studies
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Reference | Ecosystem | Invertebrates Studied | Leaves Are Congeners of the Current Study? |
|---|---|---|---|
| [16] | lotic | N/A | no |
| [19] | (lotic) | Amphipoda, Trichoptera | no |
| [63] | lotic | N/A | no |
| [13] | lotic | N/A | no |
| [9] | (lotic) | Trichoptera | Yes, maple and oak |
| [64] | lotic | Amphipoda, Trichoptera | Yes, maple |
| [12] | lotic | Amphipoda, Trichoptera | Yes, maple |
| [52] | lotic | N/A | Yes, maple and oak |
| [65] | lotic | wide variety of shredders | no |
| [66] | lotic | aggregated functional feeding groups | Yes, red maple |
| [67] | lotic | N/A | Yes, oak |
| [51] | lotic | Amphipoda, Coleoptera, Diptera, Ephemeroptera, Plecoptera, Trichoptera | Yes, maple |
| [68] | lotic | aggregated functional feeding groups | Yes, red maple |
| [20] | lotic | Diptera, Ephemeroptera, Plecoptera, Trichoptera | no |
| [61] | (lotic) | Gastropoda, Ephemeroptera, Trichoptera | no |
References
- Wallace, J.B.; Webster, J.R. The role of macroinvertebrates in stream ecosystem function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems. BioScience 1999, 49, 119–127. [Google Scholar] [CrossRef]
- Frainer, A.; Mckie, B.G. Shifts in the diversity and composition of consumer traits constrain the effects of land use on stream ecosystem functioning. Adv. Ecol. Res. 2015, 52, 169–199. [Google Scholar] [CrossRef]
- Boyero, L.; López-Rojo, N.; Tonin, A.M.; Pérez, J.; Correa-Araneda, F.; Pearson, R.G.; Bosch, J.; Albariño, R.J.; Anbalagan, S.; Barmuta, L.A.; et al. Impacts of detritivore diversity loss on instream decomposition are greatest in the tropics. Nat. Commun. 2021, 12, 3700. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.C. Revisiting the fates of dead leaves that fall into streams. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 547–568. [Google Scholar] [CrossRef]
- Bird, G.A.; Kaushik, N.K. Coarse particulate organic matter in streams. In Perspectives in Running Water Ecology; Lock, M.A., Williams, D.D., Eds.; Springer: Boston, MA, USA, 1981; pp. 41–68. [Google Scholar]
- Hagen, E.M.; McCluney, K.E.; Wyant, K.A.; Soykan, C.U.; Keller, A.C.; Luttermoser, K.C.; Holmes, E.J.; Moore, J.C.; Sabo, J.L. A meta-analysis of the effects of detritus on primary producers and consumers in marine, freshwater, and terrestrial ecosystems. Oikos 2012, 121, 1507–1515. [Google Scholar] [CrossRef]
- Brett, M.T.; Bunn, S.E.; Chandra, S.; Galloway, A.W.E.; Guo, F.; Kainz, M.J.; Kankaala, P.; Lau, D.C.P.; Moulton, T.P.; Power, M.E.; et al. How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshw. Biol. 2017, 62, 833–853. [Google Scholar] [CrossRef]
- Kochi, K.; Kagaya, T. Green leaves enhance the growth and development of a stream macroinvertebrate shredder when senescent leaves are available. Freshw. Biol. 2005, 50, 656–667. [Google Scholar] [CrossRef]
- Willoughby, L.G.; Earnshaw, R. Gut passage time in Gammarus pulex (Crustacea, Amphipoda) and aspects of summer feeding in a stony stream. Hydrobiologia 1982, 97, 105–117. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances: Climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. Bioscience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Kochi, K.; Kagaya, T.; Kusumoto, D. Does mixing of senescent and green leaves result in nonadditive effects on leaf decomposition? J. N. Am. Benthol. Soc. 2010, 29, 454–464. [Google Scholar] [CrossRef]
- Jackrel, S.L.; Wootton, J.T. Local adaptation of stream communities to intraspecific variation in a terrestrial ecosystem subsidy. Ecology 2014, 95, 37–43. [Google Scholar] [CrossRef]
- Danger, M.; Funck, J.A.; Devin, S.; Heberle, J.; Felten, V. Phosphorus content in detritus controls life-history traits of a detritivore. Funct. Ecol. 2013, 27, 807–815. [Google Scholar] [CrossRef]
- Stoler, A.B.; Relyea, R.A. Leaf litter quality induces morphological and developmental changes in larval amphibians. Ecology 2013, 94, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Martins, R.T.; Couceiro, S.R.M. Breakdown of green and senescent leaves in Amazonian streams: A case study. Limnology 2021, 22, 27–34. [Google Scholar] [CrossRef]
- Potter, C.S.; Ragsdale, H.L.; Berish, C.W. Resorption of foliar nutrients in a regenerating southern Appalachian forest. Oecologia 1987, 73, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U.; Tamm, U. Species differences in timing of leaf fall and foliage chemistry modify nutrient resorption efficiency in deciduous temperate forest stands. Tree Physiol. 2005, 25, 1001–1014. [Google Scholar] [CrossRef]
- Friberg, N.; Jacobsen, D. Feeding plasticity of two detritivore-shredders. Freshw. Biol. 1994, 32, 133–142. [Google Scholar] [CrossRef]
- Stout, R.J.; Taft, W.H.; Merrit, R.W. Patterns of macroinvertebrate colonization on fresh and senescent alder leaves in two Michigan streams. Freshw. Biol. 1985, 15, 573–580. [Google Scholar] [CrossRef]
- Bärlocher, F.; Kendrick, B. Leaf-conditioning by microorganisms. Oecologia 1975, 20, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Gessner, M.O.; Chauvet, E. Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 1994, 75, 1807–1817. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Maltby, L.; Calow, P. Importance of fungi in the diet of Gammarus pulex and Asellus aquaticus I: Feeding strategies. Oecologia 1993, 93, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.A.S.; Maltby, L.; Calow, P. Importance of fungi in the diet of Gammarus pulex and Asellus aquaticus II: Effects on growth, reproduction, and physiology. Oecologia 1993, 96, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Cararo, E.R.; Bernardi, J.P.; Lima-Rezende, C.A.; Magro, J.D.; Rezende, R.S. Chemistry Matters: High leaf litter consumption does not represent a direct increase in shredders’ biomass. Neotrop. Entomol. 2023, 52, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Cummins, K.W. Structure and function of stream ecosystems. BioScience 1974, 24, 631–641. [Google Scholar] [CrossRef]
- Faber, P.M.; Keller, E.; Sands, A.; Massey, B.M. The Ecology of Riparian Habitats of the Southern California Coastal Region: A Community Profile; Biological Report 85(7.27); Fish and Wildlife Service, U.S. Department of the Interior: Washington, DC, USA, 1989. [Google Scholar]
- Anderson, N.H.; Sedell, J.R. Detritus processing by macroinvertebrates in stream ecosystems. Annu. Rev. Entomol. 1979, 24, 351–377. [Google Scholar] [CrossRef]
- Brinson, M.M.; Lugo, A.E.; Brown, S. Primary productivity, decomposition and consumer activity in freshwater wetlands. Annu. Rev. Ecol. Syst. 1981, 12, 123–161. [Google Scholar] [CrossRef]
- Abrams, M.D. The red maple paradox. BioScience 1998, 48, 355–364. [Google Scholar] [CrossRef]
- Abrams, M.D. Where has all the white oak gone? BioScience 2003, 53, 927–939. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Hynes, H.B.N. The fate of dead leaves that fall into streams. Arch. Hydrobiol. 1971, 68, 465–515. [Google Scholar]
- Webster, J.R.; Benfield, E.F. Vascular plant breakdown in freshwater ecosystems. Annu. Rev. Ecol. Syst. 1986, 17, 567–594. [Google Scholar] [CrossRef]
- Lecerf, A.; Dobson, M.; Dang, C.K.; Chauvet, E. Riparian plant species loss alters trophic dynamics in detritus-based stream ecosystems. Oecologia 2005, 146, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, E.V.; Schwartz, C.I.; Davidson, A.T. Long-term maintenance requirements of the riparian isopod, Lirceus sp. Hydrobiologia 2017, 802, 53–69. [Google Scholar] [CrossRef]
- Pennsylvania Department of Environmental Protection. Integrated Water Quality Report. Assessment ID 15100. 2020. Available online: https://gis.dep.pa.gov/IRViewer2020/ (accessed on 5 April 2022).
- Harrelson, C.C.; Rawlins, C.L.; Potyondy, J.P. Stream Channel Reference Sites: An Illustrated Guide to Field Technique; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1994. [Google Scholar]
- Shapas, T.J.; Hilsenhoff, W.L. Feeding habits of Wisconsin’s predominant lotic Plecoptera, Ephemeroptera, and Trichoptera. Great Lakes Entomol. 1976, 9, 175–188. [Google Scholar] [CrossRef]
- Marcus, J.H.; Sutcliffe, D.W.; Willoughby, L.G. Feeding and growth of Asellus aquaticus (Isopoda) on food items from the littoral of Windermere, including green leaves of Elodea canadensis. Freshw. Biol. 1978, 8, 505–519. [Google Scholar] [CrossRef]
- McCullough, D.A.; Minshall, G.W.; Cushing, C.E. Bioenergetics of a stream “collector” organism, Tricorythodes minutus (Insecta: Ephemeroptera). Limnol. Oceanogr. 1979, 24, 45–58. [Google Scholar] [CrossRef]
- Zaranko, D.T.; Farara, D.G.; Thompson, F.G. Another exotic mollusc in the Laurentian Great Lakes: The New Zealand native Potamopyrgus antipodarum (Gray 1843) (Gastropoda, Hydroibiidae). Can. J. Fish. Aquat. Sci. 1997, 54, 809–814. [Google Scholar] [CrossRef]
- Hoddle, M.S. New Zealand Mud Snail. Center for Invasive Species Research, University of California Riverside. Available online: https://cisr.ucr.edu/invasive-species/new-zealand-mud-snail (accessed on 12 April 2024).
- Ostrofsky, M.L. Relationship between chemical characteristics of autumn-shed leaves and aquatic processing rates. J. N. Am. Benthol. Soc. 1997, 16, 750–759. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jones, C.P.; Karonen, M.; Salminen, J.P. Tannin composition affects the oxidative activities of tree leaves. J. Chem. Ecol. 2006, 32, 2235–2251. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.M.; Finzi, A.C. Differential effects of sugar maple, red oak, and hemlock tannins on carbon and nitrogen cycling in temperate forest soils. Oecologia 2008, 155, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Hynes, H.B.N. Experimental study on the role of autumn-shed leaves in aquatic environments. J. Ecol. 1968, 56, 229–243. [Google Scholar] [CrossRef]
- Frost, P.C.; Elser, J.J. Growth responses of littoral mayflies to the phosphorus content of their food. Ecol. Lett. 2002, 5, 232–240. [Google Scholar] [CrossRef]
- Davies, R.W.; Wrona, F.J.; Linton, L. A serological study of prey selection by Helobdella stagnalis (Hirudinoidea). J. Anim. Ecol. 1979, 48, 181–194. [Google Scholar] [CrossRef]
- Young, J.O.; Martin, A.J.; Seaby, R.M.H. Competitive interactions between the lake-dwelling leeches Glossiphonia complanata and Helobdella stagnalis: An experimental investigation of the significance of a food refuge. Oecologia 1993, 93, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Vila-Farré, M.; Rink, J.C. The Ecology of Freshwater Planarians. In Planarian Regeneration: Methods and Protocols; Methods in Molecular Biology; Rink, J.C., Ed.; Humana Press: New York, NY, USA; Springer Nature: New York, NY, USA, 2018; Volume 1774, pp. 173–205. [Google Scholar] [CrossRef]
- Maloney, D.C.; Lamberti, G.A. Rapid decomposition of summer-input leaves in a Northern Michigan stream. Am. Midl. Nat. 1995, 133, 184–195. [Google Scholar] [CrossRef]
- Kochi, K.; Mishima, Y.; Nagasaka, A. Lateral input of particulate organic matter from bank slopes surpasses direct litter fall in the uppermost reaches of a headwater stream in Hokkaido, Japan. Limnology 2010, 11, 77–84. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Holomuzki, J.; Short, T. Habitat use and fish avoidance behaviors by the stream-dwelling isopod Lirceus fontinalis. Oikos 1988, 52, 79–86. [Google Scholar] [CrossRef]
- Odabaşi, D.A.; Kenan, I.; Odabaşi, S. Distribution of Sphaeriidae (Mollusca: Bivalvia) in relation to environmental variables in northwestern basin streams in Türkiye: Ecology of the Sphaeriidae in streams of Türkiye. Aquat. Anim. Rep. 2024, 2, 22–30. [Google Scholar]
- Way, C.M. Dynamics of filter-feeding in Musculium transversum (Bivalvia: Sphaeriidae). J. N. Am. Benthol. Soc. 1989, 8, 243–249. [Google Scholar] [CrossRef]
- Arsuffi, T.L.; Suberkropp, K. Selective feeding by shredders on leaf-colonizing stream fungi: Comparison of macroinvertebrate taxa. Oecologia 1989, 79, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.E.; Stouffer, A.N.; Iyengar, E.V. Effects of species of leaves and conditioning time on vernal colonization by temperate riparian isopods (Lirceus sp.). Hydrobiology 2024, 3, 63–73. [Google Scholar] [CrossRef]
- Styron, C.E. Taxonomy of two populations of an aquatic isopod, Lirceus fontinalis Raf. Am. Midl. Nat. 1969, 82, 402–416. [Google Scholar] [CrossRef]
- Layer, K.; Hildrew, A.G.; Woodward, G. Grazing and detritivory in 20 stream food webs across a broad pH gradient. Oecologia 2013, 171, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Yeates, L.V.; Barmuta, L.A. The effects of willow and eucalypt leaves on feeding preference and growth of some Australian aquatic macroinvertebrates. Aust. J. Ecol. 1999, 24, 593–598. [Google Scholar] [CrossRef]
- Ligeiro, R.; Moretti, M.S.; Gonçalves, J.F.; Callisto, M. What is more important for invertebrate colonization in a stream with low-quality litter inputs: Exposure time or leaf species? Hydrobiologia 2010, 654, 125–136. [Google Scholar] [CrossRef]
- Gessner, M.O. Difference in processing dynamics of fresh and dried leaf litter in a stream ecosystem. Freshw. Biol. 1991, 26, 387–398. [Google Scholar] [CrossRef]
- Kochi, K.; Yanai, S. Shredder colonization and decomposition of green and senescent leaves during summer in a headwater stream in northern Japan. Ecol. Res. 2006, 21, 544–550. [Google Scholar] [CrossRef]
- Landeiro, V.L.; Hamada, N.; Godoy, B.S.; Melo, A.S. Effects of litter patch area on macroinvertebrate assemblage structure and leaf breakdown in Central Amazonian streams. Hydrobiologia 2010, 649, 355–363. [Google Scholar] [CrossRef]
- Leff, L.G.; McArthur, J.V. Effect of nutrient content on leaf decomposition in a coastal plain stream: A comparison of green and senescent leaves. J. Freshw. Ecol. 1990, 5, 269–277. [Google Scholar] [CrossRef]
- López, E.S.; Pardo, I.; Felpeto, N. Seasonal differences in green leaf breakdown and nutrient content of deciduous and evergreen tree species and grass in a granitic headwater stream. Hydrobiologia 2001, 464, 51–61. [Google Scholar] [CrossRef]
- McArthur, J.V.; Leff, L.G.; Kovacic, D.A.; Jaroscak, J. Green leaf decomposition in coastal plain streams. J. Freshw. Ecol. 1986, 3, 553–558. [Google Scholar] [CrossRef]
| Site | Mat Location | Water Depth (cm) | Stream Width (m) | Sediment Average Size (mm) | Sediment Size Range (mm) |
|---|---|---|---|---|---|
| CC | 1 | 29 | 5.4 | 9.71 | 1 to 39 |
| 2 | 10 | 3.6 | 2.95 | 1 to 8 | |
| 3 | 9 | 3.6 | 6.33 | 1 to 19 | |
| 4 | 15 | 7.5 | 4.29 | 1 to 8 | |
| 5 | 15 | 7.5 | 6.00 | 1 to 25 | |
| SP | 1 | 20 | |||
| 2 | 21 | ||||
| 3 | 30 | ||||
| 4 | 25 | ||||
| 5 | 33 |
| A. | Skeletonization Rating (Low to High) | |||||
|---|---|---|---|---|---|---|
| Field Site | Leaf Species | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 |
| Cedar Creek | red maple | 0 | 3 | 5 | 6 | 1 |
| tulip poplar | 0 | 2 | 3 | 6 | 4 | |
| red oak | 14 | 1 | 0 | 0 | 0 | |
| Scout Pond | red maple | 0 | 0 | 3 | 5 | 7 |
| tulip poplar | 0 | 0 | 2 | 5 | 8 | |
| red oak | 15 | 0 | 0 | 0 | 0 | |
| B. | Remaining Surface Area Quantile | |||||
| Field Site | Leaf Species | 0% | 25% | 50% | 75% | 100% |
| Scout Pond | red maple | 0 | 3 | 10 | 2 | 0 |
| tulip poplar | 0 | 6 | 7 | 2 | 0 | |
| red oak | 0 | 0 | 1 | 11 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, A.R.; Iyengar, E.V. Effects of Leaf Species and Conditioning State of Fresh Leaves on Colonization by Stream and Pond Macroinvertebrates. Hydrobiology 2024, 3, 85-99. https://doi.org/10.3390/hydrobiology3020007
Hoffman AR, Iyengar EV. Effects of Leaf Species and Conditioning State of Fresh Leaves on Colonization by Stream and Pond Macroinvertebrates. Hydrobiology. 2024; 3(2):85-99. https://doi.org/10.3390/hydrobiology3020007
Chicago/Turabian StyleHoffman, Austin R., and Erika V. Iyengar. 2024. "Effects of Leaf Species and Conditioning State of Fresh Leaves on Colonization by Stream and Pond Macroinvertebrates" Hydrobiology 3, no. 2: 85-99. https://doi.org/10.3390/hydrobiology3020007
APA StyleHoffman, A. R., & Iyengar, E. V. (2024). Effects of Leaf Species and Conditioning State of Fresh Leaves on Colonization by Stream and Pond Macroinvertebrates. Hydrobiology, 3(2), 85-99. https://doi.org/10.3390/hydrobiology3020007






