The Devil Firefish Pterois miles (Bennett, 1828): Life History Traits of a Potential Fishing Resource in Rhodes (Eastern Mediterranean)
Abstract
1. Introduction
2. Materials and Methods
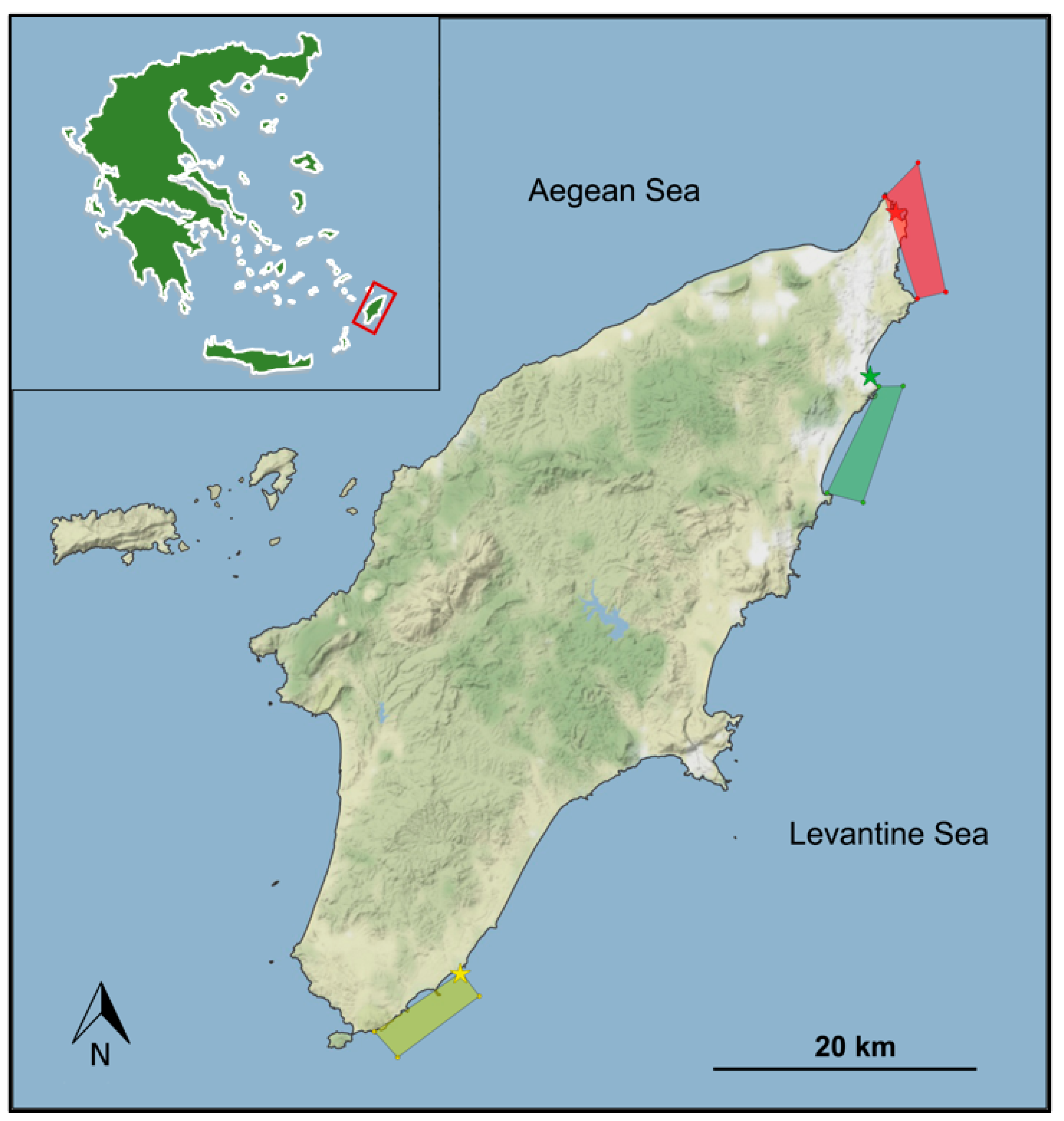
2.1. Study Area and Sampling Methodology
2.2. Statistical Analysis
2.3. Growth
2.4. Mortality and the Exploitation Rate
2.5. Reproduction
2.6. Relative Y/R and B/R Analysis: Knife-Edge Selection
3. Results
3.1. The Sex Ratio
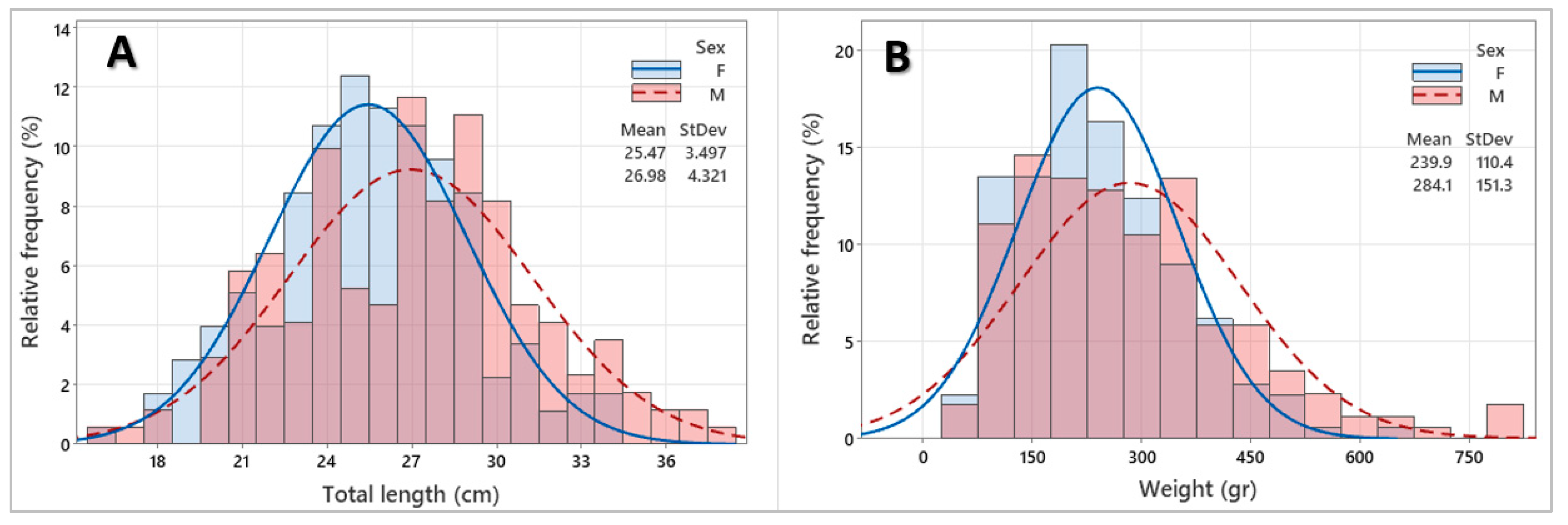
3.2. Population Structure
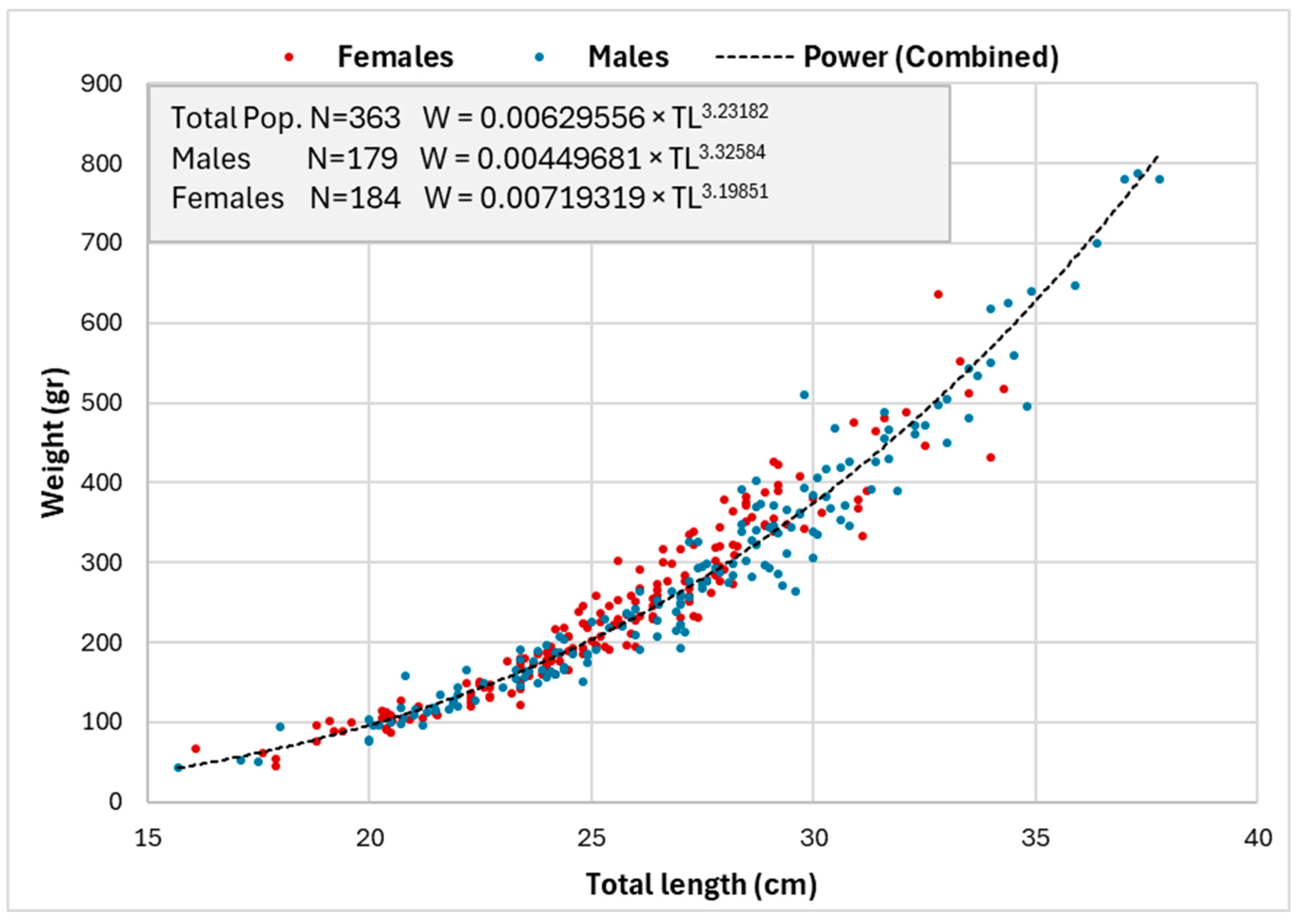
3.3. Length–Weight Relationship
3.4. Age Composition
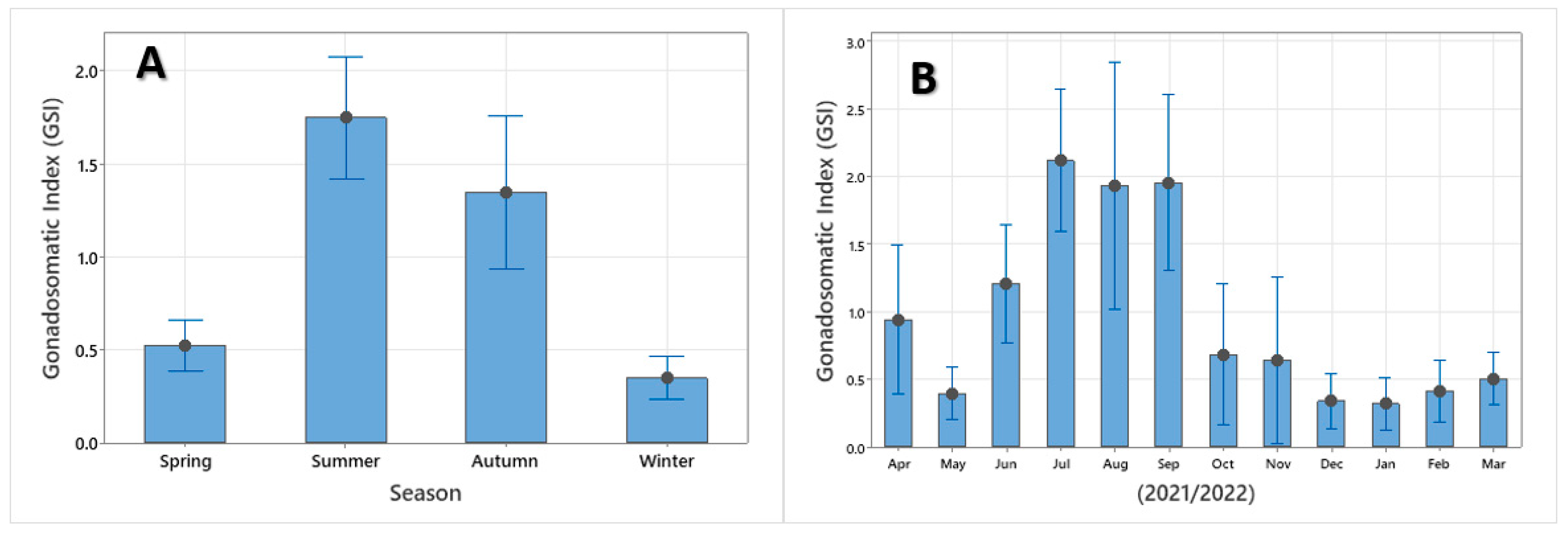
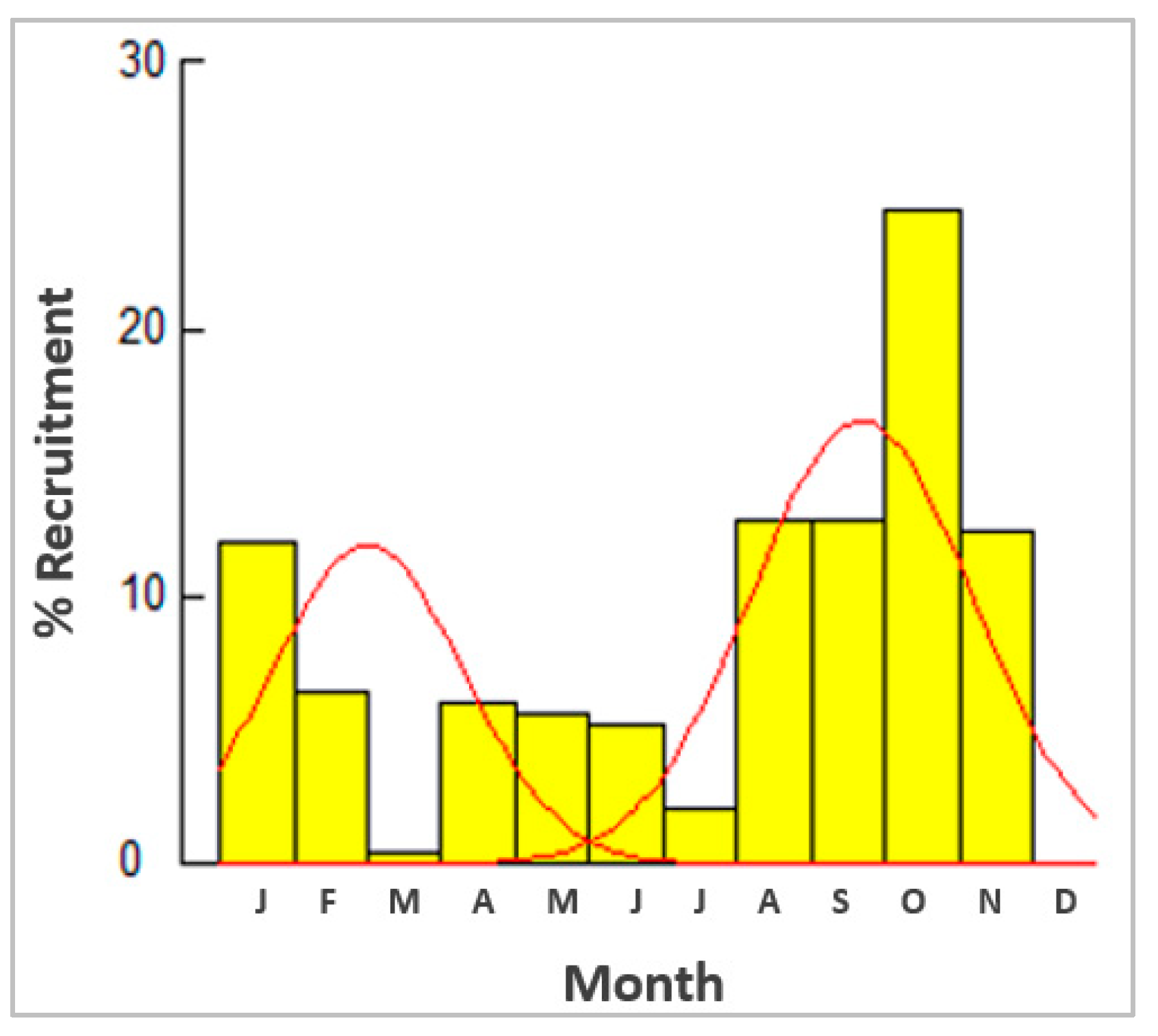
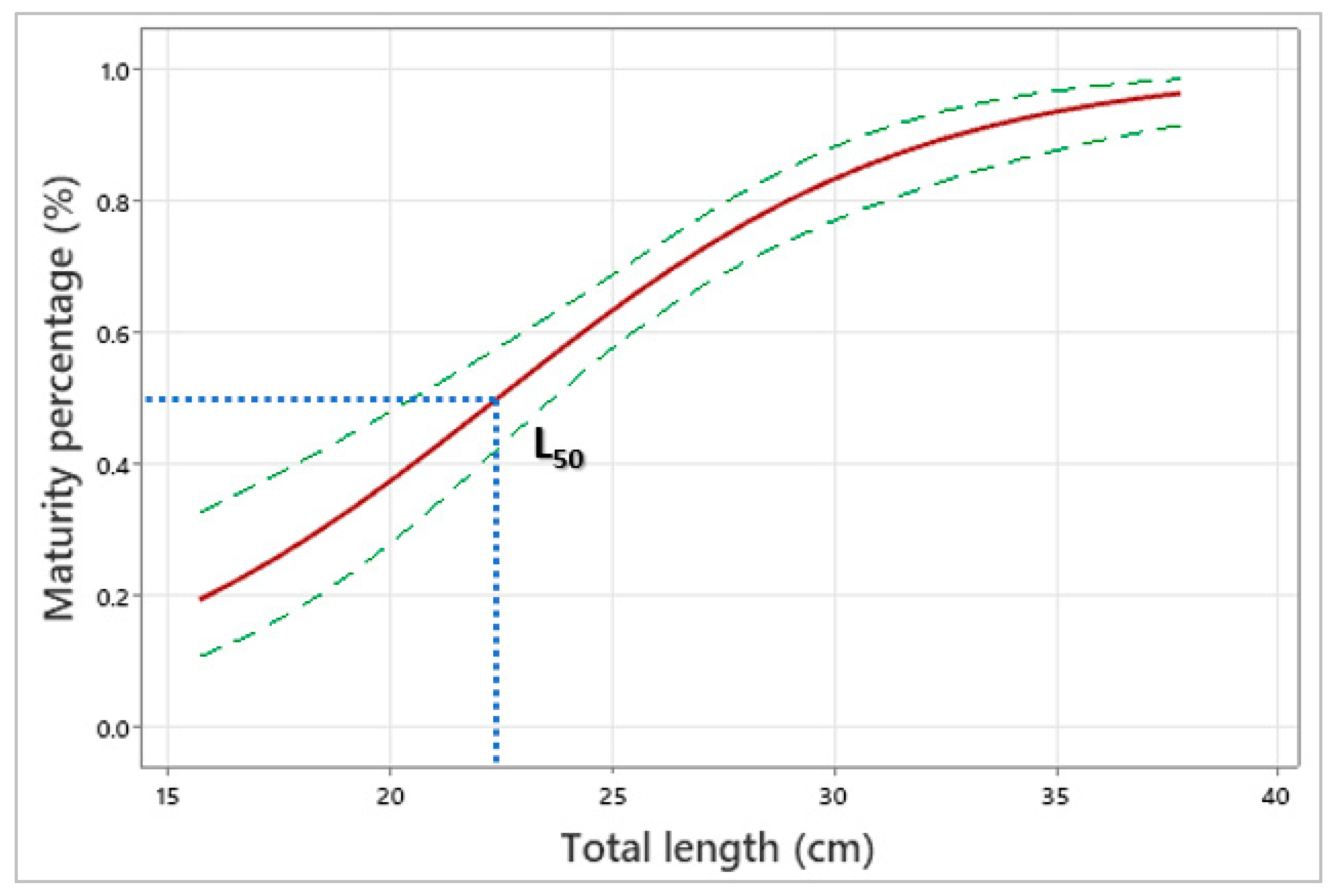
3.5. Reproduction
3.6. Condition Factor
3.7. Growth and Mortality
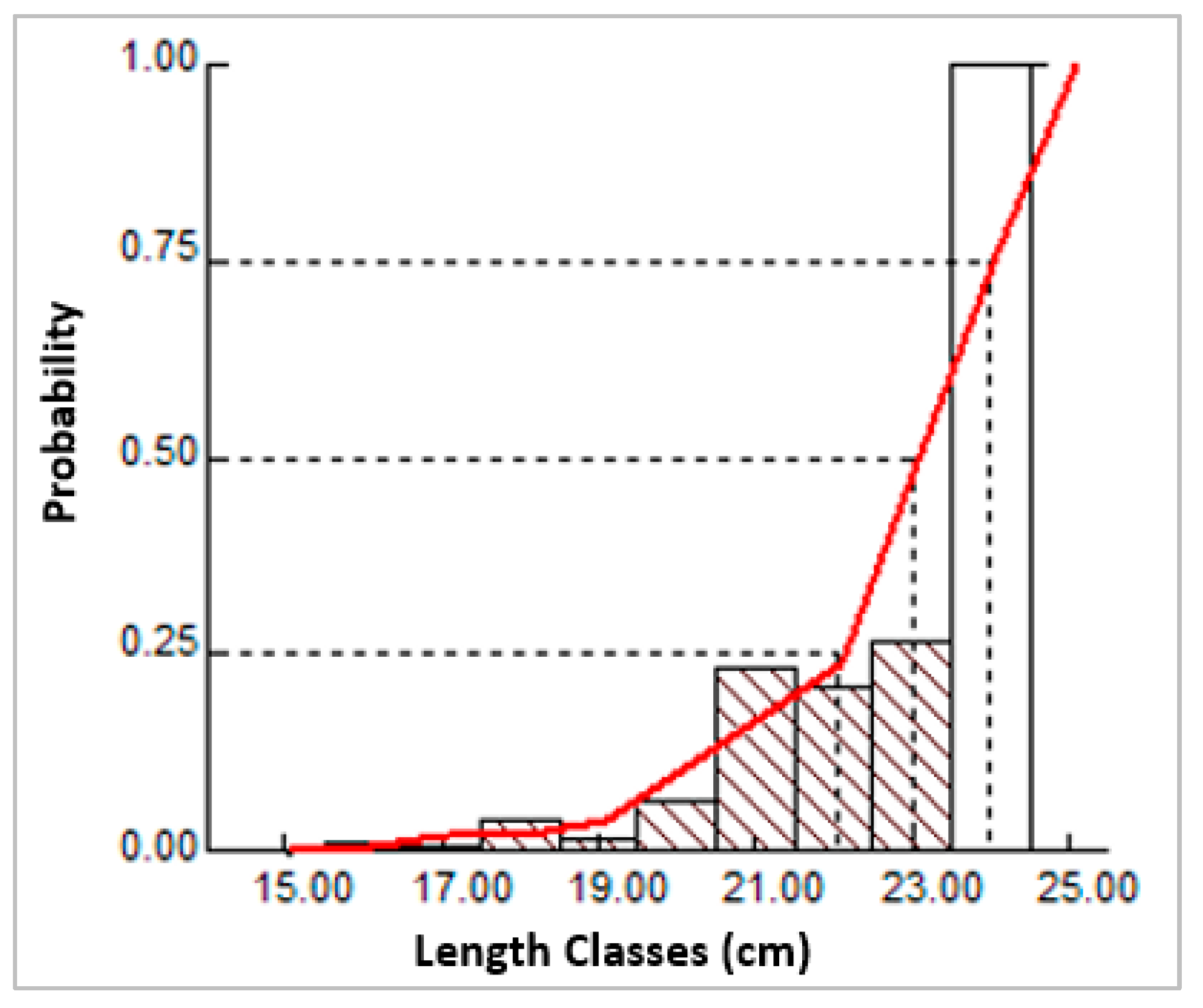
3.8. The Probability of Capture
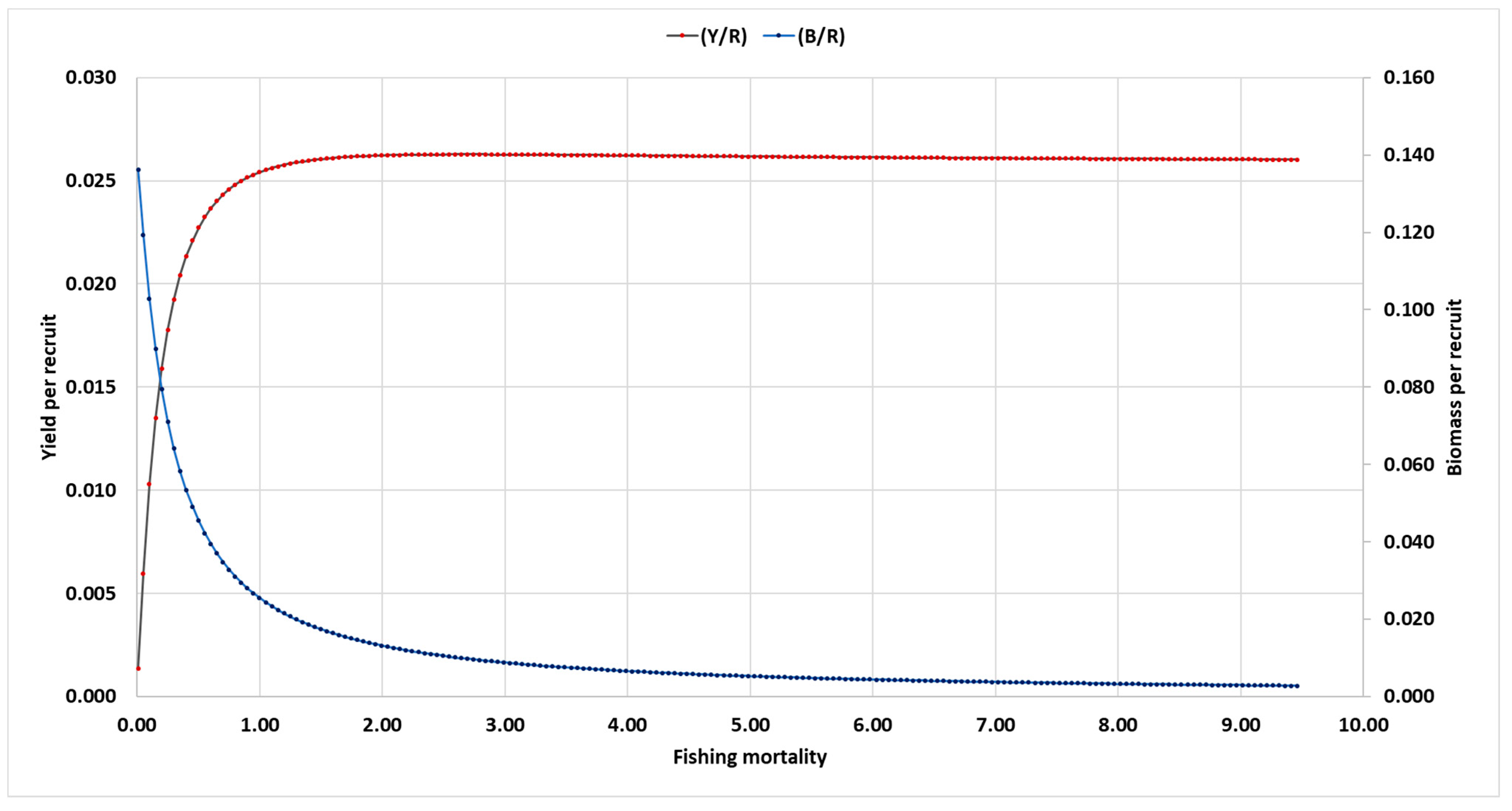
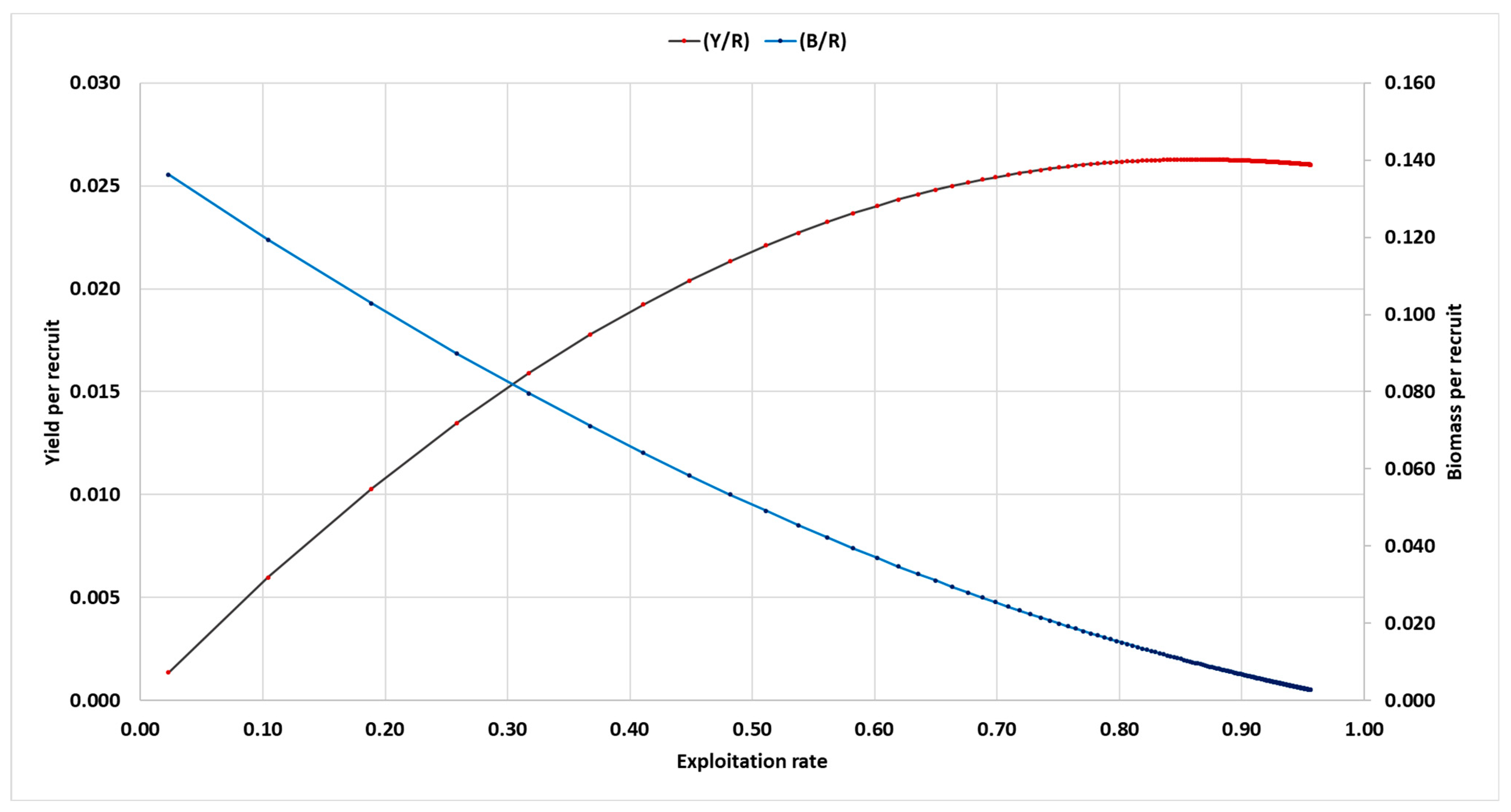
3.9. Relative Y/R and B/R Analysis: Knife-Edge Selection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Arndt, E.; Givan, O.; Edelist, D.; Sonin, O.; Belmaker, J. Shifts in eastern Mediterranean fish communities: Abundance changes, trait overlap, and possible competition between native and non-native species. Fishes 2018, 3, 19. [Google Scholar] [CrossRef]
- Givan, O.; Edelist, D.; Sonin, O.; Belmaker, J. Thermal affinity as the dominant factor changing Mediterranean fish abundances. Glob. Chang. Biol. 2018, 24, e80–e89. [Google Scholar] [CrossRef]
- Kleitou, P.; Hall-Spencer, J.; Rees, S.; Sfenthourakis, S.; Demetriou, A.; Chartosia, N.; Jimenez, C.; Hadjioannou, L.; Petrou, A.; Christodoulides, Y. Tackling the Lionfish Invasion in the Mediterranean. The EU-LIFE RELIONMED Project: Progress and Results. In 1st Mediterranean Symp Non-Indigenous Species; Langar, H., Ouerghi, A., Eds.; SPA/RAC: Antalya, Turkey, 2019; pp. 65–70. [Google Scholar]
- Kleitou, P.; Crocetta, F.; Giakoumi, S.; Giovos, I.; Hall-Spencer, J.M.; Kalogirou, S.; Kletou, D.; Moutopoulos, D.K.; Rees, S. Fishery reforms for the management of non-indigenous species. J. Environ. Manag. 2021, 280, 111690. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Galanidi, M. Mediterranean non indigenous species at the start of the 2020s: Recent changes. Mar. Biodivers. Rec. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196. [Google Scholar] [CrossRef]
- Golani, D.; Azzurro, E.; Dulčić, J.; Massutí, E.; Orsi-Relini, L. Atlas of Exotic Fishes in the Mediterranean Sea; CIESM Publishers: Monte Carlo, Monaco, 2021. [Google Scholar]
- Azzurro, E.; Smeraldo, S.; Minelli, A.; D’Amen, M. ORMEF: A Mediterranean database of exotic fish records. Sci. Data 2022, 9, 363. [Google Scholar] [CrossRef]
- Galil, B.; Marchini, A.; Occhipinti-Ambrogi, A.; Ojaveer, H. The enlargement of the Suez Canal—Erythraean introductions and management challenges. Manag. Biol. Invasions 2017, 8, 141–152. [Google Scholar] [CrossRef]
- Zenetos, A.; Çinar, M.E.; Crocetta, F.; Golani, D.; Rosso, A.; Servello, G.; Shenkar, N.; Turon, X.; Verlaque, M. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuar. Coast. Shelf Sci. 2017, 191, 171–187. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Courchamp, F.; Woodroffe, R.; Roemer, G. Removing protected populations to save endangered species. Science 2003, 302, 1532. [Google Scholar] [CrossRef] [PubMed]
- Olden, J.D.; Poff, N.L.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Rilov, G.; Edelist, D. Impacts of marine invasive alien species on European fisheries and aquaculture–plague or boon? CIESM Monogr. 2018, 50, 125–132. [Google Scholar]
- Kleitou, P.; Savva, I.; Kletou, D.; Hall-Spencer, J.M.; Antoniou, C.; Christodoulides, Y.; Chartosia, N.; Hadjioannou, L.; Dimitriou, A.C.; Jimenez, C. Invasive lionfish in the Mediterranean: Low public awareness yet high stakeholder concerns. Mar. Policy 2019, 104, 66–74. [Google Scholar] [CrossRef]
- Albano, P.G.; Steger, J.; Bošnjak, M.; Dunne, B.; Guifarro, Z.; Turapova, E.; Hua, Q.; Kaufman, D.S.; Rilov, G.; Zuschin, M. Native biodiversity collapse in the eastern Mediterranean. Proc. R. Soc. B 2021, 288, 20202469. [Google Scholar] [CrossRef] [PubMed]
- Meinesz, A.; Belsher, T.; Thibaut, T.; Antolic, B.; Ben Mustapha, K.; Boudouresque, C.-F.; Chiaverini, D.; Cinelli, F.; Cottalorda, J.-M.; Djellouli, A. The introduced green alga Caulerpa taxifolia continues to spread in the Mediterranean. Biol. Invasions 2001, 3, 201–210. [Google Scholar] [CrossRef]
- Panayotidis, P. On the enigmatic origin of the Mediterranean invasive Caulerpa racemosa (Caulerpales, Chlorophyta). Mediterr. Mar. Sci. 2006, 7, 119–121. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Marín-Guirao, L.; Bernardeau-Esteller, J.; Ramos-Segura, A.; García-Muñoz, R.; Sandoval-Gil, J.M. Spread of the invasive alga Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) along the Mediterranean Coast of the Murcia region (SE Spain). Anim. Biodivers. Conserv. 2011, 34, 73–82. [Google Scholar] [CrossRef]
- Morris, J.A., Jr.; Whitfield, P.E. Biology, Ecology, Control and Management of the Invasive Indo-Pacific lionfish: An Updated Integrated Assessment; NOAA/National Ocean Service/Center for Coastal Fisheries and Habitat Research: Seldovia, Alaska, 2009. [Google Scholar]
- Sutherland, W.J.; Clout, M.; Côté, I.M.; Daszak, P.; Depledge, M.H.; Fellman, L.; Fleishman, E.; Garthwaite, R.; Gibbons, D.W.; De Lurio, J.; et al. A horizon scan of global conservation issues for 2010. Trends Ecol. Evol. 2010, 25, 1–7. [Google Scholar] [CrossRef]
- Kletou, D.; Hall-Spencer, J.M.; Kleitou, P. A lionfish (Pterois miles) invasion has begun in the Mediterranean Sea. Mar. Biodivers. Rec. 2016, 9, 46. [Google Scholar] [CrossRef]
- Dimitriou, A.C.; Chartosia, N.; Hall-Spencer, J.M.; Kleitou, P.; Jimenez, C.; Antoniou, C.; Hadjioannou, L.; Kletou, D.; Sfenthourakis, S. Genetic data suggest multiple introductions of the lionfish (Pterois miles) into the Mediterranean Sea. Diversity 2019, 11, 149. [Google Scholar] [CrossRef]
- Kulbicki, M.; Beets, J.; Chabanet, P.; Cure, K.; Darling, E.; Floeter, S.R.; Galzin, R.; Green, A.; Harmelin-Vivien, M.; Hixon, M. Distributions of Indo-Pacific lionfishes Pterois spp. in their native ranges: Implications for the Atlantic invasion. Mar. Ecol. Prog. Ser. 2012, 446, 189–205. [Google Scholar] [CrossRef]
- Eschmeyer, W.N. Scorpaenidae: Smiths’ Sea Fishes; Smith, M.M., Heemstra, P.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Golani, D.; Sonin, O. New records of the Red Sea fishes, Pterois miles (Scorpaenidae) and Pteragogus pelycus (Labridae) from the eastern Mediterranean Sea. Jpn. J. Ichthyol. 1992, 39, 167–169. [Google Scholar] [CrossRef]
- Bariche, M.; Kajajian, A.; Azzurro, E. Reproduction of the invasive bluespotted cornetfish Fistularia commersonii (Teleostei, Fistulariidae) in the Mediterranean Sea. Mar. Biol. Res. 2013, 9, 169–180. [Google Scholar] [CrossRef]
- Turan, C.; Ergüden, D.; Gürlek, M.; Yağlioğlu, D.; Ali, U.; Uygur, N. First record of the Indo-Pacific lionfish Pterois miles (Bennett, 1828) (Osteichthyes: Scorpaenidae) for the Turkish marine waters. J. Black Sea/Mediterr. Environ. 2014, 20, 158–163. [Google Scholar]
- Crocetta, F.; Agius, D.; Balistreri, P.; Bariche, M.; Bayhan, Y.; Çakir, M.; Ciriaco, S.; Corsini-Foka, M.; Deidun, A.; El Zrelli, R. New mediterranean biodiversity records (October 2015). Mediterr. Mar. Sci. 2015, 16, 682. [Google Scholar] [CrossRef]
- Iglésias, S.; Frotté, L. Alien marine fishes in Cyprus: Update and new records. Aquat. Invasions 2015, 10, 425–438. [Google Scholar] [CrossRef]
- Jimenez, C.; Petrou, A.; Andreou, V.; Hadjioannou, L.; Wolf, W.; Koutsoloukas, N.; Alhaija, R.A. Veni, vidi, vici: The successful establishment of the lionfish Pterois miles in Cyprus (Levantine Sea). Rapp. Comm. Int. Mer Mediterr. 2016, 41, 417. [Google Scholar]
- Crocetta, F.; Al Mabruk, S.A.A.; Azzurro, E.; Bakiu, R.; Bariche, M.; Batjakas, I.E.; Bejaoui, T.; Ben Souissi, S.J.; Cauchi, J.; Corsini-Foka, M. New Alien Mediterranean Biodiversity Records (November 2021); Hellenic Centre for Marine Research: Héraklion, Greece, 2021. [Google Scholar]
- Dailianis, T.; Akyol, O.; Babali, N.; Bariche, M.; Crocetta, F.; Gerovasileiou, V.; Ghanem, R.; Gökoğlu, M.; Hasiotis, T.; Izquierdo-Munoz, A. New mediterranean biodiversity records (July 2016). Mediterr. Mar. Sci. 2016, 17, 608. [Google Scholar] [CrossRef]
- Azzurro, E.; Stancanelli, B.; Di Martino, V.; Bariche, M. Range expansion of the common lionfish Pterois miles (Bennett, 1828) in the Mediterranean Sea: An unwanted new guest for Italian waters. BioInvasions Rec. 2017, 6, 95–98. [Google Scholar] [CrossRef]
- Stern, N.; Rothman, S.B.S.; Hüseyinoglu, M.F.; Öztürk, B. Iron Lion Zion: The successful, albeit lingered, invasion of the lionfish in the Israeli Mediterranean Sea. In Lionfish Invasion and Its Management in the Mediterranean Sea; Turkish Marine Research Foundation Publication: Istanbul, Turkey, 2018; Volume 49, pp. 51–56. [Google Scholar]
- Dimitriadis, C.; Galanidi, M.; Zenetos, A.; Corsini-Foka, M.; Giovos, I.; Karachle, P.K.; Fournari–Konstantinidou, I.; Kytinou, E.; Issaris, Y.; Azzurro, E. Updating the occurrences of Pterois miles in the Mediterranean Sea, with considerations on thermal boundaries and future range expansion. Mediterr. Mar. Sci. 2020, 21, 62–69. [Google Scholar] [CrossRef]
- Poursanidis, D.; Kalogirou, S.; Azzurro, E.; Parravicini, V.; Bariche, M.; Zu Dohna, H. Habitat suitability, niche unfilling and the potential spread of Pterois miles in the Mediterranean Sea. Mar. Pollut. Bull. 2020, 154, 111054. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
- Kleitou, P.; Hall-Spencer, J.M.; Savva, I.; Kletou, D.; Hadjistylli, M.; Azzurro, E.; Katsanevakis, S.; Antoniou, C.; Hadjioannou, L.; Chartosia, N. The case of lionfish (Pterois miles) in the Mediterranean Sea demonstrates limitations in EU legislation to address marine biological invasions. J. Mar. Sci. Eng. 2021, 9, 325. [Google Scholar] [CrossRef]
- Harris, H.E.; Fogg, A.Q.; Allen, M.S.; Ahrens, R.N.M.; Patterson III, W.F. Precipitous declines in northern Gulf of Mexico invasive lionfish populations following the emergence of an ulcerative skin disease. Sci. Rep. 2020, 10, 1934. [Google Scholar] [CrossRef]
- Schultz, E.T. Pterois volitans and Pterois miles: Two valid species. Copeia 1986, 1986, 686–690. [Google Scholar] [CrossRef]
- Gress, E.; Andradi-Brown, D.A.; Woodall, L.; Schofield, P.J.; Stanley, K.; Rogers, A.D. Lionfish (Pterois spp.) invade the upper-bathyal zone in the western Atlantic. PeerJ 2017, 5, e3683. [Google Scholar] [CrossRef] [PubMed]
- Orejas, C.; Jiménez, C.; Gori, A.; Rivera, J.; Lo Iacono, C.; Aurelle, D.; Hadjioannou, L.; Petrou, A.; Achilleos, K. 23 Corals of Aphrodite: Dendrophyllia ramea Populations of Cyprus. In Mediterranean Cold-Water Corals: Past, Present and Future Understanding the Deep-Sea Realms of Coral; Springer: Berlin/Heidelberg, Germany, 2019; pp. 257–260. [Google Scholar]
- Savva, I.; Chartosia, N.; Antoniou, C.; Kleitou, P.; Georgiou, A.; Stern, N.; Hadjioannou, L.; Jimenez, C.; Andreou, V.; Hall-Spencer, J.M. They are here to stay: The biology and ecology of lionfish (Pterois miles) in the Mediterranean Sea. J. Fish Biol. 2020, 97, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.L.; Andradi-Brown, D.A.; Hudson, C.J.; Bennett-Williams, J.; Noades, F.; Curtis-Quick, J.; Lewis, O.T.; Exton, D.A. Shelter use interactions of invasive lionfish with commercially and ecologically important native invertebrates on Caribbean coral reefs. PLoS ONE 2020, 15, e0236200. [Google Scholar] [CrossRef]
- Kondylatos, G.; Vagenas, G.; Kalaentzis, K.; Mavrouleas, D.; Conides, A.; Karachle, P.K.; Corsini-Foka, M.; Klaoudatos, D. Exploring the Structure of Static Net Fisheries in a Highly Invaded Region: The Case of Rhodes Island (Eastern Mediterranean). Sustainability 2023, 15, 14976. [Google Scholar] [CrossRef]
- Morris, J.A. Invasive lionfish: A Guide to Control and Management; Gulf and Caribbean Fisheries Institute: Marathon, FL, USA, 2012. [Google Scholar]
- Farquhar, S.D. A brief review of fishing gears associated with invasive lionfish removals. SDRP J. Aquac. Fish. Fish Sci. 2017, 1, 37–40. [Google Scholar]
- García-Berthou, E. The characteristics of invasive fishes: What has been learned so far? J. Fish Biol. 2007, 71, 33–55. [Google Scholar] [CrossRef]
- Sih, A.; Bolnick, D.I.; Luttbeg, B.; Orrock, J.L.; Peacor, S.D.; Pintor, L.M.; Preisser, E.; Rehage, J.S.; Vonesh, J.R. Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 2010, 119, 610–621. [Google Scholar] [CrossRef]
- Côté, I.M.; Green, S.J.; Hixon, M.A. Predatory fish invaders: Insights from Indo-Pacific lionfish in the western Atlantic and Caribbean. Biol. Conserv. 2013, 164, 50–61. [Google Scholar] [CrossRef]
- Gardner, P.G.; Frazer, T.K.; Jacoby, C.A.; Yanong, R.P.E. Reproductive biology of invasive lionfish (Pterois spp.). Front. Mar. Sci. 2015, 2, 7. [Google Scholar] [CrossRef]
- Fogg, A.Q.; Brown-Peterson, N.J.; Peterson, M.S. Reproductive life history characteristics of invasive red lionfish (Pterois volitans) in the northern Gulf of Mexico. Bull. Mar. Sci. 2017, 93, 791–813. [Google Scholar] [CrossRef]
- Côté, I.M.; Smith, N.S. The lionfish Pterois sp. invasion: Has the worst-case scenario come to pass? J. Fish Biol. 2018, 92, 660–689. [Google Scholar] [CrossRef]
- Fogg, A.Q.; Evans, J.T.; Peterson, M.S.; Brown-Peterson, N.; Hoffmayer, E.R.; Ingram, G.W., Jr. Comparison of age and growth parameters of invasive red lionfish (Pterois volitans) across the northern Gulf of Mexico. Fish. Bull. 2019, 117, 1. [Google Scholar] [CrossRef]
- Albins, M.A.; Hixon, M.A. Worst case scenario: Potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ. Biol. Fishes 2013, 96, 1151–1157. [Google Scholar] [CrossRef]
- Green, S.J.; Dulvy, N.K.; Brooks, A.M.L.; Akins, J.L.; Cooper, A.B.; Miller, S.; Côté, I.M. Linking removal targets to the ecological effects of invaders: A predictive model and field test. Ecol. Appl. 2014, 24, 1311–1322. [Google Scholar] [CrossRef]
- Zannaki, K.; Corsini-Foka, M.; Kampouris, T.E.; Batjakas, I.E. First results on the diet of the invasive Pterois miles (Actinopterygii: Scorpaeniformes: Scorpaenidae) in the Hellenic waters. Acta Ichthyol. Piscat. 2019, 49, 311–317. [Google Scholar] [CrossRef]
- Agostino, D.D.; Jimenez, C.; Reader, T.; Hadjioannou, L.; Heyworth, S.; Aplikioti, M.; Argyrou, M.; Feary, D.A. Behavioural traits and feeding ecology of Mediterranean lionfish and naiveté of native species to lionfish predation. Mar. Ecol. Prog. Ser. 2020, 638, 123–135. [Google Scholar] [CrossRef]
- Batjakas, I.E.; Evangelopoulos, A.; Giannou, M.; Pappou, S.; Papanikola, E.; Atsikvasi, M.; Poursanidis, D.; Gubili, C. Lionfish Diet Composition at Three Study Sites in the Aegean Sea: An Invasive Generalist? Fishes 2023, 8, 314. [Google Scholar] [CrossRef]
- Crocetta, F.; Shokouros-Oskarsson, M.; Doumpas, N.; Giovos, I.; Kalogirou, S.; Langeneck, J.; Tanduo, V.; Tiralongo, F.; Virgili, R.; Kleitou, P. Protect the natives to combat the aliens: Could Octopus vulgaris Cuvier, 1797 be a natural agent for the control of the lionfish invasion in the Mediterranean Sea? J. Mar. Sci. Eng. 2021, 9, 308. [Google Scholar] [CrossRef]
- Ulman, A.; Harris, H.E.; Doumpas, N.; Deniz Akbora, H.; Mabruk, A.; Azzurro, E.; Bariche, M.; Çiçek, B.A.; Deidun, A.; Demirel, N. Low pufferfish and lionfish predation in their native and invaded ranges suggests human control mechanisms may be necessary to control their Mediterranean abundances. Front. Mar. Sci. 2021, 8, 868. [Google Scholar] [CrossRef]
- Roy, H.E.; Rabitsch, W.; Scalera, R. Study on Invasive Alien Species–Development of Risk Assessments to Tackle Priority Species and Enhance Prevention; Final report; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Pollard, D.A.; Francour, P.; Fennessy, S. Epinephelus aeneus. The IUCN Red List of Threatened Species 2018, e.T132722A100459597; IUCN Red List: Cambridge, UK, 2018. [Google Scholar]
- Pollard, D.A.; Afonso, P.; Bertoncini, A.A.; Fennessy, S.; Francour, P.; Barreiros, J. Epinephelus marginatus. The IUCN Red List of Threatened Species 2018, e.T7859A100467602; IUCN Red List: Cambridge, UK, 2018. [Google Scholar]
- Kimball, M.E.; Miller, J.M.; Whitfield, P.E.; Hare, J.A. Thermal tolerance and potential distribution of invasive lionfish (Pterois volitans/miles complex) on the east coast of the United States. Mar. Ecol. Prog. Ser. 2004, 283, 269–278. [Google Scholar] [CrossRef]
- Whitfield, P.E.; Muñoz, R.C.; Buckel, C.A.; Degan, B.P.; Freshwater, D.W.; Hare, J.A. Native fish community structure and Indo-Pacific lionfish Pterois volitans densities along a depth-temperature gradient in Onslow Bay, North Carolina, USA. Mar. Ecol. Prog. Ser. 2014, 509, 241–254. [Google Scholar] [CrossRef]
- Barker, B.D.; Horodysky, A.Z.; Kerstetter, D.W. Hot or not? Comparative behavioral thermoregulation, critical temperature regimes, and thermal tolerances of the invasive lionfish Pterois sp. versus native western North Atlantic reef fishes. Biol. Invasions 2018, 20, 45–58. [Google Scholar] [CrossRef]
- Schofield, P.J. Update on geographic spread of invasive lionfishes (Pterois volitans [Linnaeus, 1758] and P. miles [Bennett, 1828]) in the Western North Atlantic Ocean, Caribbean Sea and Gulf of Mexico. Aquat. Invasions 2010, 5, S117–S122. [Google Scholar] [CrossRef]
- Côté, I.M.; Akins, L.; Underwood, E.; Curtis-Quick, J.; Green, S.J. Setting the record straight on invasive lionfish control: Culling works. PeerJ Prepr. 2014, 2, e398v1. [Google Scholar]
- Frazer, T.K.; Jacoby, C.A.; Edwards, M.A.; Barry, S.C.; Manfrino, C.M. Coping with the lionfish invasion: Can targeted removals yield beneficial effects? Rev. Fish. Sci. 2012, 20, 185–191. [Google Scholar] [CrossRef]
- Graham, R. A Comparison of Eight Country Plans for the Invasive Indo-Pacific Lionfish in the Wider Caribbean. MSc Thesis, Dalhousie University, Halifax, Nova Scotia, 2016. [Google Scholar]
- FAO. Mid-Term Strategy (2017–2020) towards the Sustainability of Mediterranean and Black Sea Fisheries; FAO: Rome, Italy, 2017. [Google Scholar]
- Graham, R.E.; Fanning, L.M. A comparison of eight country plans for the invasive Indo-Pacific lionfish in the wider Caribbean. Glob. Ecol. Conserv. 2017, 12, 253–262. [Google Scholar] [CrossRef]
- Peiffer, F.; Bejarano, S.; de Witte, G.P.; Wild, C. Ongoing removals of invasive lionfish in Honduras and their effect on native Caribbean prey fishes. PeerJ 2017, 5, e3818. [Google Scholar] [CrossRef] [PubMed]
- Hüseyinoğlu, M.F.; Öztürk, B. Lionfish Invasion and Its Management in the Mediterranean Sea; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2018. [Google Scholar]
- Kleitou, P.; Rees, S.; Cecconi, F.; Kletou, D.; Savva, I.; Cai, L.L.; Hall-Spencer, J.M. Regular monitoring and targeted removals can control lionfish in Mediterranean Marine Protected Areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2870–2882. [Google Scholar] [CrossRef]
- Kleitou, P.; Hall-Spencer, J.; Rees, S.; Kletou, D. Guide to Lionfish Management in the Mediterranean; University of Plymouth: Plymouth, UK, 2022. [Google Scholar]
- Harris, H.E.; Fogg, A.Q.; Yanong, R.P.; Frasca, S., Jr.; Cody, T.; Waltzek, T.B.; Patterson, W.F. First Report of an Emerging Ulcerative Skin Disease in Invasive Lionfish: FA209/FA209, 9/2018. EDIS 2018, 5, 1–7. [Google Scholar]
- Rosenthal, E. Answer for invasive species: Put it on a plate and eat it. New York Times, 9 July 2011. [Google Scholar]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, M.A.; Kuebbing, S.; Dimarco, R.D.; Simberloff, D. Invasive species: To eat or not to eat, that is the question. Conserv. Lett. 2012, 5, 334–341. [Google Scholar] [CrossRef]
- Rapoport, E.H.; Raffaele, E.; Ghermandi, L.; Margutti, L. Edible weeds: A scarcely used resource. Bull. Ecol. Soc. Am. 1995, 76, 163–166. [Google Scholar]
- de León, R.; Vane, K.; Bertuol, P.; Chamberland, V.C.; Simal, F.; Imms, E.; Vermeij, M.J.A. Effectiveness of lionfish removal efforts in the southern Caribbean. Endanger. Species Res. 2013, 22, 175–182. [Google Scholar] [CrossRef]
- Alemu, I.J.B. The status and management of the lionfish, Pterois sp. in Trinidad and Tobago. Mar. Pollut. Bull. 2016, 109, 402–408. [Google Scholar] [CrossRef]
- Edwards, M.A.; Frazer, T.K.; Jacoby, C.A. Age and growth of invasive lionfish (Pterois spp.) in the Caribbean Sea, with implications for management. Bull. Mar. Sci. 2014, 90, 953–966. [Google Scholar] [CrossRef]
- Pasko, S.; Goldberg, J. Review of harvest incentives to control invasive species. Manag. Biol. Invasions 2014, 5, 263. [Google Scholar] [CrossRef]
- Chagaris, D.; Binion-Rock, S.; Bogdanoff, A.; Dahl, K.; Granneman, J.; Harris, H.; Mohan, J.; Rudd, M.B.; Swenarton, M.K.; Ahrens, R. An ecosystem-based approach to evaluating impacts and management of invasive lionfish. Fisheries 2017, 42, 421–431. [Google Scholar] [CrossRef]
- Eddy, C.; Pitt, J.; Oliveira, K.; Morris, J.A.; Potts, J.; Bernal, D. The life history characteristics of invasive lionfish (Pterois volitans and P. miles) in Bermuda. Environ. Biol. Fishes 2019, 102, 887–900. [Google Scholar] [CrossRef]
- Frid, O.; Belmaker, J. Catch dynamics of set net fisheries in Israel. Fish. Res. 2019, 213, 1–11. [Google Scholar] [CrossRef]
- Kondylatos, G.; Mavrouleas, D.; Gratsia, E.; Kasapidis, P.; Corsini-Foka, M.; Klaoudatos, D. First record of Arcania brevifrons Chen, 1989 (Decapoda; Leucosiidae) and further record of Macrophthalmus (Macrophthalmus) indicus Davie, 2012 (Decapoda; Macrophthalmidae) in Hellenic waters. BioInvasions Rec. 2023, 12, 234–244. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Lu, F.; Mathew, T. A parametric bootstrap approach for ANOVA with unequal variances: Fixed and random models. Comput. Stat. Data Anal. 2007, 51, 5731–5742. [Google Scholar] [CrossRef]
- Hampton, R.E.; Havel, J.E. Introductory Biological Statistics; Waveland Press: Long Grove, IL, USA, 2006; ISBN 1577663802. [Google Scholar]
- Rolke, W.; Gongora, C.G. A chi-square goodness-of-fit test for continuous distributions against a known alternative. Comput. Stat. 2021, 36, 1885–1900. [Google Scholar] [CrossRef]
- Şahin, M.D.; Aybek, E.C. Jamovi: An easy-to-use statistical software for the social scientists. Int. J. Assess. Tools Educ. 2019, 6, 670–692. [Google Scholar] [CrossRef]
- Munro, J.L.; Pauly, D. A simple method for comparing the growth of fishes and invertebrates. Fishbyte 1983, 1, 5–6. [Google Scholar]
- Bhattacharya, C.G. A simple method of resolution of a distribution into Gaussian components. Biometrics 1967, 23, 115–135. [Google Scholar] [CrossRef]
- Pauly, D.; Caddy, J.F. A modification of Bhattacharya’s Method for the Analysis of Mixtures of Normal Distributions; FAO: Rome, Italy, 1985; Volume 781. [Google Scholar]
- Sparre, P.; Venema, S.C. Introduction to fish stock assessment. Part 1: Manual; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1998; p. 306. [Google Scholar]
- Von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Froese, R.; Binohlan, C. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Ricker, W.E. Growth rates and models. Fish Physiol. 1979, 8, 677–743. [Google Scholar]
- Then, A.Y.; Hoenig, J.M.; Hall, N.G.; Hewitt, D.A.; Jardim, E. Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci. 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 11. [Google Scholar]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; FAO: Rome, Italy, 1983. [Google Scholar]
- Beverton, R.J.H. Spatial limitation of population size; the concentration hypothesis. Neth. J. Sea Res. 1995, 34, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.; Paloheimo, J.E. Estimating fish length and age at 50% maturity using a logistic type model. Aquat. Sci. 1994, 56, 206–219. [Google Scholar] [CrossRef]
- Ulman, A.; Yildiz, T.; Demirel, N.; Canak, O.; Yemişken, E.; Pauly, D. The biology and ecology of the invasive silver-cheeked toadfish (Lagocephalus sceleratus), with emphasis on the Eastern Mediterranean. NeoBiota 2021, 68, 145–175. [Google Scholar] [CrossRef]
- Moreau, J.; Cuende, F.X. On improving the resolution of the recruitment patterns of fishes. Fishbyte 1991, 9, 45–46. [Google Scholar]
- Fulton, T.W. The rate of growth of fishes. Twenty-Second Annu. Rep. 1904, 3, 141–241. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. On the dynamics of exploited fish populations. Fishery Investigations 1957, 19, 1–533. [Google Scholar]
- Gulland, J.A. Manual of methods for fish stock assessment. Part 1: Fish population analysis. FAO Man Fish. Sci. 1969, 4, 154. [Google Scholar]
- Gulland, J.A. The Fish Resources of the Ocean; Fishing News Books: West Byfleet, UK, 1971; pp. 307–319. [Google Scholar]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Edelist, D.; Rilov, G.; Golani, D.; Carlton, J.T.; Spanier, E. Restructuring the Sea: Profound shifts in the world’s most invaded marine ecosystem. Divers. Distrib. 2013, 19, 69–77. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Kourantidou, M.; Cuthbert, R.N.; Haubrock, P.J.; Novoa, A.; Taylor, N.G.; Leroy, B.; Capinha, C.; Renault, D.; Angulo, E.; Diagne, C. Economic costs of invasive alien species in the Mediterranean basin. NeoBiota 2021, 67, 427–458. [Google Scholar] [CrossRef]
- Rajendiran, P.; Jaafar, F.; Kar, S.; Sudhakumari, C.; Senthilkumaran, B.; Parhar, I.S. Sex determination and differentiation in teleost: Roles of genetics, environment, and Brain. Biology 2021, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Fogg, A.Q.; Hoffmayer, E.R.; Driggers III, W.B.; Campbell, M.D.; Pellegrin, G.J.; Stein, W. Distribution and length frequency of invasive lionfish (Pterois sp.) in the northern Gulf of Mexico. Gulf Caribb. Res. 2013, 25, 111–115. [Google Scholar] [CrossRef]
- Gardner, P.G. Reproductive Biology of Invasive Lionfish (Pterois volitans/miles complex) from Little Cayman Island. MSc Thesis, University of Florida, Gainesville, FL, USA, 2012. [Google Scholar]
- Farquhar, S. Age and Growth of Invasive Lionfish: North Carolina, USA vs. Bonaire, Dutch Caribbean. Explorations 2016, 11, 12–20. [Google Scholar]
- Mouchlianitis, F.A.; Kalaitzi, G.; Kleitou, P.; Savva, I.; Kletou, D.; Ganias, K. Reproductive dynamics of the invasive lionfish (Pterois miles) in the Eastern Mediterranean Sea. J. Fish Biol. 2022, 100, 574–581. [Google Scholar] [CrossRef]
- Morris, J.A., Jr. The Biology and Ecology of the Invasive Indo-Pacific Lionfish; North Carolina State University: Raleigh, NC, USA, 2009. [Google Scholar]
- Stergiou, K.I.; Moutopoulos, D.K. A review of length-weight relationships of fishes from Greek marine waters. Naga ICLARM Q. 2001, 24, 23–39. [Google Scholar]
- Sabido-Itzá, M.M.; Aguilar-Perera, A.; Medina-Quej, A. Length–weight and length–length relations, and relative condition factor of red lionfish, Pterois volitans (Actinopterygii: Scorpaeniformes: Scorpaenidae), from two natural protected areas in the Mexican Caribbean. Acta Ichthyol. Piscat. 2016, 46, 279–285. [Google Scholar] [CrossRef]
- Dağhan, H.; Demirhan, S.A. Some bio-ecological characteristics of the lionfish Pterois miles (Bennett, 1828) distributed in the Gulf of Iskenderun. Mar. Life Sci. 2020, 2, 28–40. [Google Scholar]
- Sandel, V.; Martínez-Fernández, D.; Wangpraseurt, D.; Sierra, L. Ecology and management of the invasive lionfish Pterois volitans/miles complex (Perciformes: Scorpaenidae) in Southern Costa Rica. Rev. Biol. Trop. 2015, 63, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Hernández, C.; Vélez-Zuazo, X.; Ruiz-Diaz, C.P.; Patricio, A.R.; Mege, P.; Navarro, M.; Sabat, A.M.; Betancur-R, R.; Papa, R. Population ecology and genetics of the invasive lionfish in Puerto Rico. Aquat. Invasions 2014, 9, 227–237. [Google Scholar] [CrossRef]
- Ricker, W.E. Effects of size-selective mortality and sampling bias on estimates of growth, mortality, production, and yield. J. Fish. Board Can. 1969, 26, 479–541. [Google Scholar] [CrossRef]
- Bagenal, T.B.; Tesch, F.W. Age and growth. In Methods for Assessment of Fish Production in Fresh Waters; Bagenal, T., Ed.; Blackwell Scientific Publications: Oxford, UK, 1978. [Google Scholar]
- Recasens, L.; Lombarte, A.; Morales-Nin, B.; Tores, G.J. Spatiotemporal variation in the population structure of the European hake in the NW Mediterranean. J. Fish Biol. 1998, 53, 387–401. [Google Scholar] [CrossRef]
- Basilone, G.; Guisande, C.; Patti, B.; Mazzola, S.; Cuttitta, A.; Bonanno, A.; Vergara, A.R.; Maneiro, I. Effect of habitat conditions on reproduction of the European anchovy (Engraulis encrasicolus) in the Strait of Sicily. Fish. Oceanogr. 2006, 15, 271–280. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Domínguez-Petit, R.; Saborido-Rey, F. New bioenergetic perspective of European hake (Merluccius merluccius L.) reproductive ecology. Fish. Res. 2010, 104, 83–88. [Google Scholar] [CrossRef]
- Ralston, S.V.; Williams, H.A. Depth Distributions, Growth, and Mortality of Deep Slope Fishes from the Mariana Archipelago; NOAA Technical Memorandum, NMFS 113; NMFS Scientific Publications Office: Seattle, WA, USA, 1988; 47p. [Google Scholar]
- Jimenez, C.; Andreou, V.; Hadjioannou, L.; Petrou, A.; Alhaija, R.A.; Patsalou, P. Not everyone’s cup of tea: Public perception of culling invasive lionfish in Cyprus. J. Black Sea/Mediterr. Environ. 2017, 23, 38–47. [Google Scholar]
- Azzurro, E.; Bariche, M. Local knowledge and awareness on the incipient lionfish invasion in the eastern Mediterranean Sea. Mar. Freshw. Res. 2017, 68, 1950–1954. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Tsiamis, K.; Deriu, I.; D’Amico, F.; Gervasini, E. EU Regulation 1143/2014: Assessment of Invasive Alien Species of Union Concern Distribution; Publications Office of the European Union: Luxembourg, 2021; p. 173. [Google Scholar]

| Age Group | Mean Total Length (cm) | Standard Deviation | Population % |
|---|---|---|---|
| 1 | 18.12 | 0.59 | 2.79 |
| 2 | 21.32 | 0.84 | 14.90 |
| 3 | 25.01 | 1.39 | 41.49 |
| 4 | 28.35 | 1.20 | 32.40 |
| 5 | 33.52 | 1.16 | 8.42 |
| E | Y/R | B/R | |
|---|---|---|---|
| 0.01 | 0.006 | 0.852 | |
| 0.20 | 0.011 | 0.714 | |
| 0.30 | 0.015 | 0.585 | |
| 0.40 | 0.019 | 0.467 | |
| 0.50 | 0.022 | 0.360 | |
| 0.60 | 0.024 | 0.264 | |
| 0.70 | 0.025 | 0.180 | |
| 0.80 | 0.026 | 0.108 | |
| 0.90 | 0.026 | 0.048 | |
| 0.99 | 0.026 | 0.004 | |
| Biological reference points | Y/R | B/R | |
| Emax | 0.862 | 0.023 | 0.009 |
| E0.1 | 0.707 | 0.025 | 0.024 |
| E0.5 | 0.371 | 0.017 | 0.017 |
| Fopt | 0.424 | 0.021 | 0.051 |
| Flim | 0.553 | 0.023 | 0.042 |
| Eopt | 0.500 | 0.022 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondylatos, G.; Theocharis, A.; Mandalakis, M.; Avgoustinaki, M.; Karagyaurova, T.; Koulocheri, Z.; Vardali, S.; Klaoudatos, D. The Devil Firefish Pterois miles (Bennett, 1828): Life History Traits of a Potential Fishing Resource in Rhodes (Eastern Mediterranean). Hydrobiology 2024, 3, 31-50. https://doi.org/10.3390/hydrobiology3010003
Kondylatos G, Theocharis A, Mandalakis M, Avgoustinaki M, Karagyaurova T, Koulocheri Z, Vardali S, Klaoudatos D. The Devil Firefish Pterois miles (Bennett, 1828): Life History Traits of a Potential Fishing Resource in Rhodes (Eastern Mediterranean). Hydrobiology. 2024; 3(1):31-50. https://doi.org/10.3390/hydrobiology3010003
Chicago/Turabian StyleKondylatos, Gerasimos, Alexandros Theocharis, Manolis Mandalakis, Maria Avgoustinaki, Teodora Karagyaurova, Zoi Koulocheri, Sofia Vardali, and Dimitris Klaoudatos. 2024. "The Devil Firefish Pterois miles (Bennett, 1828): Life History Traits of a Potential Fishing Resource in Rhodes (Eastern Mediterranean)" Hydrobiology 3, no. 1: 31-50. https://doi.org/10.3390/hydrobiology3010003
APA StyleKondylatos, G., Theocharis, A., Mandalakis, M., Avgoustinaki, M., Karagyaurova, T., Koulocheri, Z., Vardali, S., & Klaoudatos, D. (2024). The Devil Firefish Pterois miles (Bennett, 1828): Life History Traits of a Potential Fishing Resource in Rhodes (Eastern Mediterranean). Hydrobiology, 3(1), 31-50. https://doi.org/10.3390/hydrobiology3010003








