A Rarely Reported Crustacean Species, Rissoides pallidus (Giesbrecht, 1910) (Stomatopoda, Squillidae), Caught in the Strait of Sicily Waters (Central Mediterranean Sea)
Abstract
1. Introduction
2. Materials and Methods
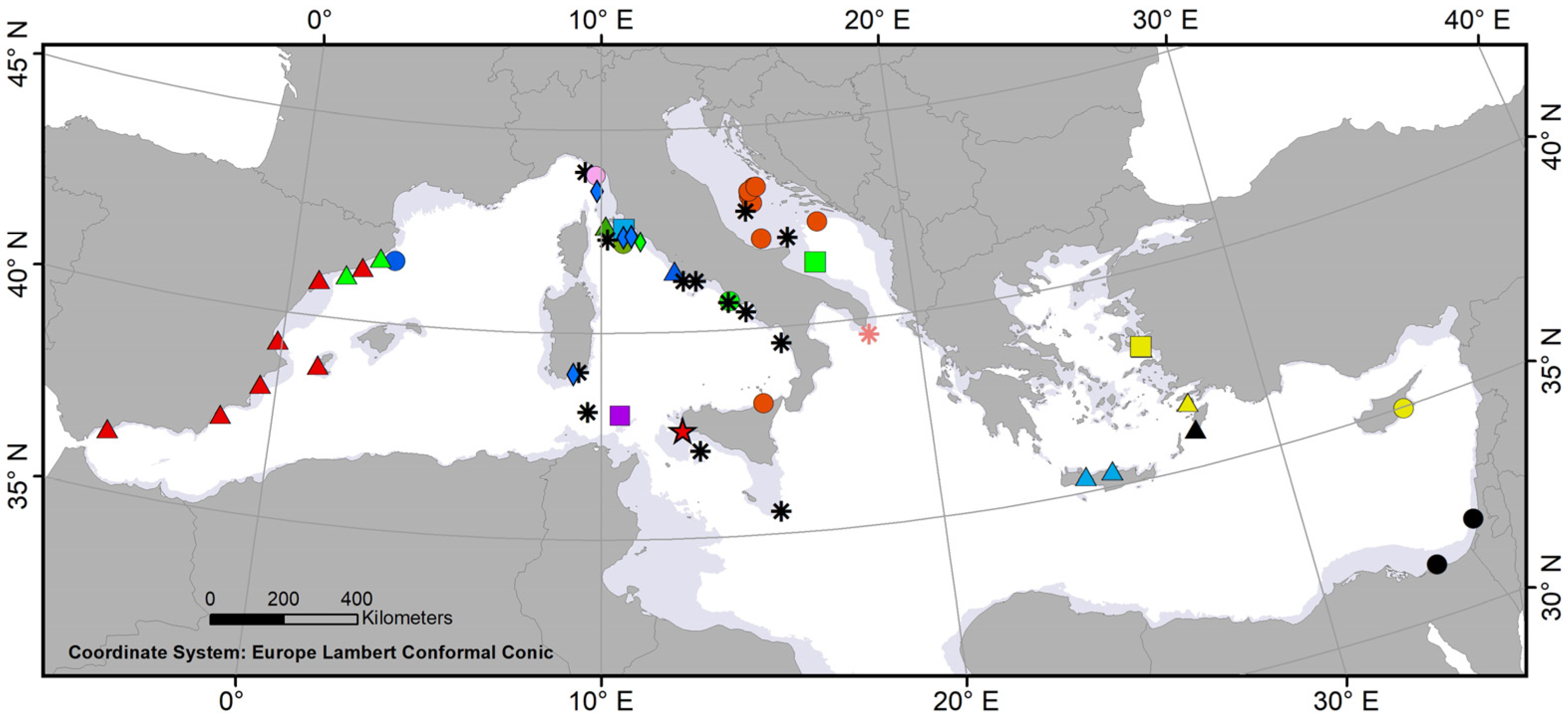
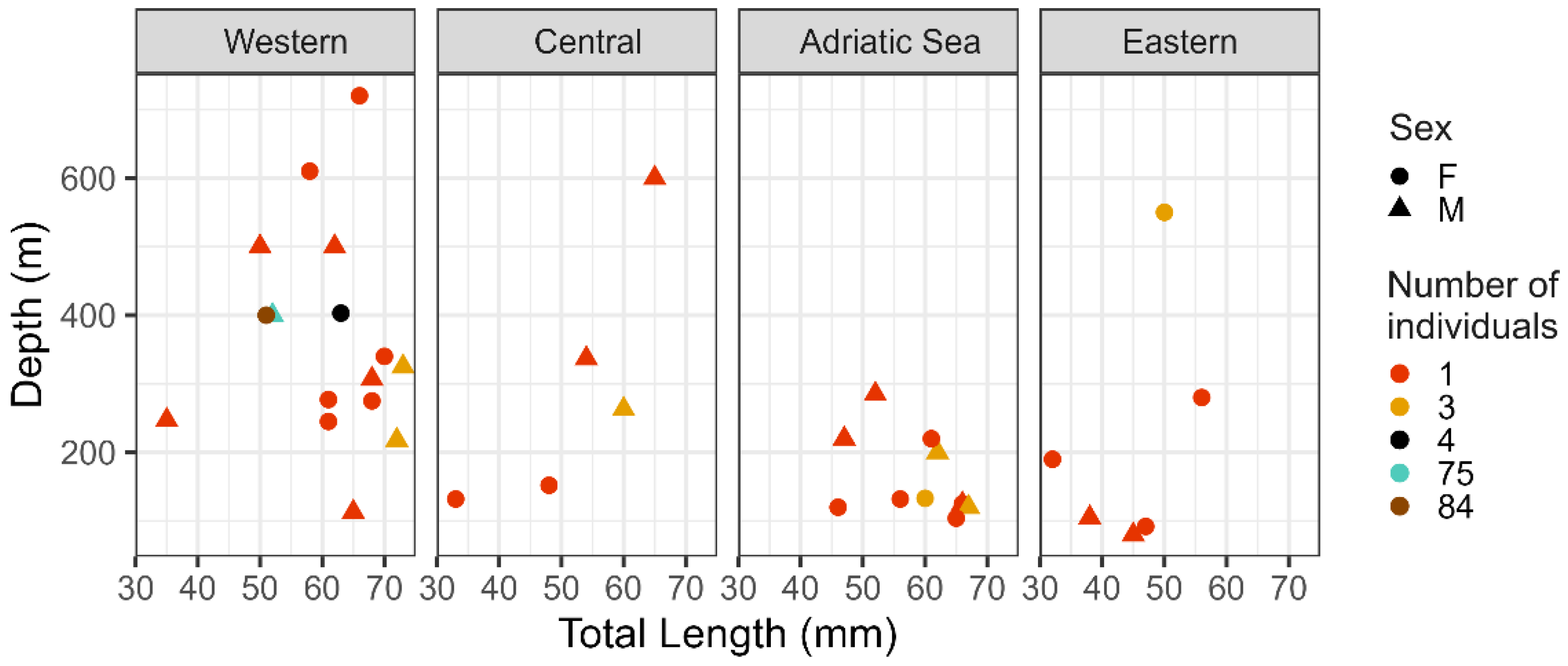
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Sardo, G.; Geraci, M.L.; Falsone, F.; Gancitano, S.; Gancitano, V.; Massi, D.; Okpala, C.O.R.; Scannella, D.; Titone, A.; Vitale, S.; et al. First Records with biological notes of Umbrina ronchus, Valenciennes, 1843 (Osteichthyes, Sciaenidae) in the Strait of Sicily (Central Mediterranean Sea). Fishes 2023, 8, 434. [Google Scholar] [CrossRef]
- Quignard, J.P.; Tomasini, J.A. Mediterranean fish biodiversity. Biol. Mar. Mediterr. 2000, 7, 1–66. [Google Scholar]
- Geraci, M.L.; Scannella, D.; Falsone, F.; Colloca, F.; Vitale, S.; Rizzo, P.; Di Maio, F.; Milisenda, G.; Fiorentino, F. Preliminary study on the biological traits of the Por’s goatfish Upeneus pori (Chordata: Actinopterygii) off the southern coast of Lampedusa Island (Central Mediterranean). Eur. Zool. J. 2018, 85, 231–241. [Google Scholar] [CrossRef]
- Scannella, D.; Falsone, F.; Geraci, M.L.; Froglia, C.; Fiorentino, F.; Giusto, G.B.; Zava, B.; Insacco, G.; Colloca, F. Westward expansion of the Northern brown shrimp Penaeus aztecus Ives, 1891 (Crustacea, Penaeidae) in the Mediterranean Sea: First records in the Strait of Sicily. BioInvasions Rec. 2017, 6, 67–72. [Google Scholar] [CrossRef]
- Falsone, F.; Scannella, D.; Geraci, M.L.; Vitale, S.; Sardo, G.; Fiorentino, F. Further records of Callinectes sapidus (Rathbun, 1896) (Decapoda, Brachyura, Portunidae) in the Strait of Sicily. Mar. Biodivers. Rec. 2020, 13, 8. [Google Scholar] [CrossRef]
- Geraci, M.L.; Falsone, F.; Scannella, D.; Vitale, S. An additional record of the non-indigenous species (NIS) Seriola fasciata from the southern coast of Sicily (Central Mediterranean Sea). Acta Adriat. 2020, 61, 223–230. [Google Scholar] [CrossRef]
- Falsone, F.; Scannella, D.; Geraci, M.L.; Vitale, S.; Colloca, F.; Di Maio, F.; Milisenda, G.; Gancitano, V.; Bono, G.; Fiorentino, F. Identification and characterization of trammel net métiers: A case study from the southwestern Sicily (Central Mediterranean). Reg. Stud. Mar. Sci. 2020, 39, 101419. [Google Scholar] [CrossRef]
- Geraci, M.L.; Falsone, F.; Gancitano, V.; Scannella, D.; Fiorentino, F.; Vitale, S. Assessing cephalopods fisheries in the Strait of Sicily by using poor data modelling. Front. Mar. Sci. 2021, 8, 1757. [Google Scholar] [CrossRef]
- Di Maio, F.; Geraci, M.L.; Scannella, D.; Russo, T.; Fiorentino, F. Evaluation of the Economic Performance of Coastal Trawling off the Southern Coast of Sicily (Central Mediterranean Sea). Sustainability 2022, 14, 4743. [Google Scholar] [CrossRef]
- Falsone, F.; Gancitano, V.; Geraci, M.L.; Sardo, G.; Scannella, D.; Serena, F.; Vitale, S.; Fiorentino, F. Assessing the Stock Dynamics of Elasmobranchii off the Southern Coast of Sicily by Using Trawl Survey Data. Fishes 2022, 7, 136. [Google Scholar] [CrossRef]
- WoRMS. 2021. Rissoides Manning & Lewinsohn. 1982. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=136112 (accessed on 1 June 2021).
- Ahyong, S.T.; Harling, C. The phylogeny of the stomatopod Crustacea. Aust. J. Zool. 2000, 48, 607–642. [Google Scholar] [CrossRef]
- Colmenero, A.I.; Raso, J.G.; Abelló, P. New records of Parasquilla ferussaci (Roux, 1830) (Crustacea, Stomatopoda) from the Eastern Atlantic and Western Mediterranean. Arx. Misc. Zool. 2009, 7, 72–77. [Google Scholar] [CrossRef]
- Froglia, C. Crustacea Malacostraca Stomatopoda. In Checklist of the Italian Fauna; Bologna, M.A., Zapparoli, M., Oliverio, M., Minelli, A., Bonato, L., Cianferoni, F., Stoch, F., Eds.; Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Verona, Italy, 2021; Version 1.0. Last Update: 31 May 2021. [Google Scholar]
- Abelló, P.; Carbonell, A.; Torres, P. Biogeography of epibenthic crustaceans on the shelf and upper slope off the Iberian Peninsula Mediterranean coasts: Implications for the establishment of natural management areas. Sci. Mar. 2002, 66, 183–198. [Google Scholar] [CrossRef]
- Dounas, C.; Steudel, C. Stomatopod Crustacea from the Island of Crete. Crustaceana 1994, 66, 252–254. [Google Scholar] [CrossRef]
- Lewinsohn, C.; Manning, R.B. Stomatopod Crustacea from the Eastern Mediterranean; Smithsonian Contributions to Zoology: Washington, DC, USA, 1980; p. 22. [Google Scholar]
- Manning, R.B. A monograph of the West African Stomatopod Crustacea. Atlantide Rep. 1977, 12, 25–181. [Google Scholar]
- Biscoito, M.J. An account on the stomatopod crustaceans of Madeira. Bol. Mus. Munic. Funchal 1985, 37, 158–174. [Google Scholar]
- Moro, L.; Herrera, R.; Ortea, J.; Riera, R.; Bacallado, J.J.; Martín, J. Aportaciones al conocimiento y distribución de los decápodos y estomatópodos (Crustacea: Malacostraca) de las Islas Canarias (Contributions to the knowledge and distribution of decapods and stomatopods (Crustacea: Malacostraca) of the Canary Islands). Rev. Acad. Canar. Cienc. 2014, 26, 33–82. [Google Scholar]
- Geraci, M.L.; Sardo, G.; Scannella, D.; Falsone, F.; Di Maio, F.; Gancitano, V.; Fiorentino, F.; Chirco, P.; Massi, D.; Vitale, S. Exploring the feasibility of technological transfers of two by-catch reduction devices in the crustacean bottom trawling of the central Mediterranean. Front. Mar. Sci. 2023, 10, 1011605. [Google Scholar] [CrossRef]
- Geraci, M.L.; Sardo, G.; Falsone, F.; Scannella, D.; Breen, M.; Fiorentino, F.; Sala, A.; Vitale, S. Escape Survival and Scale Damage Assessment of Red Mullet (Mullus barbatus Linnaeus, 1758) during Bottom Trawling in the Central Mediterranean Sea. Biology 2023, 12, 649. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. Available online: http://qgis.osgeo.org (accessed on 20 July 2023).
- Giesbrecht, W. Stomatopoden. Erster Theil. In Fauna Flora Golf Neapel; 1910; Volume 33, pp. 1–239. [Google Scholar]
- Demetropoulos, A.; Neocleous, D. The Fishes and Crustaceans of Cyprus; Fisheries Bulletin; Ministry of Agriculture and Natural Resources, Fisheries Department, Republic of Cyprus: Nicosia, Cyprus, 1969; Volume 1, pp. 1–21.
- Massi, D.; Zamboni, A.; Bellingeri, M.; Fiorentino, F. Recenti Reperti di Crostacei Stomatopodi del Genere Rissoides (Squillidae) in Mar Ligure. Biol. Mar. Mediterr. 1995, 2, 301–302. [Google Scholar]
- Manning, R.B.; Froglia, C. Description of a new Allosquilla with notes on other Adriatic Stomatopoda Crustacea. Quad. Del Lab. Di Tecnol. Della Pesca 1979, 2, 177–190. [Google Scholar]
- Valladares, F.J. Presencia de Rissoides pallidus (Giesbrecht) y otros crustaceos estomatopodos en la costa mediterranea espanola (Presence of Rissoides pallidus (Giesbrecht) and other stomatopod crustaceans on the Spanish Mediterranean coast). Misc. Zool. 1987, 11, 373–377. [Google Scholar]
- De Ranieri, S.; Mori, M. Stomatopoda squillidae collected in the northern Tyrrhenian Sea. Crustaceana 1991, 60, 218–222. [Google Scholar] [CrossRef]
- Froglia, C. Stomatopod Crustacea of the Ligurian Sea. Doriana 1992, 6, 1–10. [Google Scholar]
- Pipitone, C.; Tumbiolo, M.L. Decapod and stomatopod crustaceans from the trawlable bottoms of the Sicilian Channel (Central Mediterranean Sea). Crustaceana 1993, 65, 358–364. [Google Scholar] [CrossRef]
- Abelló, P.; Pretus, J.; Corbera, J. Occurrence and distribution of some stomatopod crustaceans in the westem Mediterranean. Misc. Zool. 1993, 17, 107–113. [Google Scholar]
- Kocataş, A.; Kataǧan, T. On Stomatopoda from Turkey with the first record of Rissoides pallidus for the Turkish fauna. Crustaceana 1995, 68, 649–652. [Google Scholar] [CrossRef]
- Biagi, F.; Sartor, P.; Ardizzone, G.D.; Belcari, P.; Belluscio, A.; Serena, F. Analysis of demersal fish assemblages of the Tuscany and Latium coasts (north-western Mediterranean). Sci. Mar. 2002, 66, 233–242. [Google Scholar] [CrossRef]
- D’onghia, G.; Mastrototaro, F.; Matarrese, A.; Politou, C.; Mytilineou, C. Biodiversity of the upper slope demersal community in the eastern Mediterranean: Preliminary comparison between two areas with and without trawl fishing. J. Northwest Atl. Fish. Sci. 2003, 31, 263. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Galil, B.S. Decapoda and Stomatopoda (Crustacea) of Rodos island (Greece) and the Erythrean expansion NW of the Levantine Sea. Med. Mar. Sci. 2003, 4, 57–66. [Google Scholar] [CrossRef][Green Version]
- Sartor, P.; Sbrana, M.; Reale, B.; Belcari, P. Impact of the deep sea trawl fishery on demersal communities of the northern Tyrrhenian Sea (Western Mediterranean). J. Northwest Atl. Fish. Sci. 2003, 31, 275–284. [Google Scholar] [CrossRef]
- Colloca, F.; Carpentieri, P.; Balestri, E.; Ardizzone, G.D. A critical habitat for Mediterranean fish resources: Shelf–break areas with Leptometra phalangium (Echinodermata: Crinoidea). Mar. Biol. 2004, 145, 1129–1142. [Google Scholar] [CrossRef]
- Ungaro, N.; Marano, C.A.; Ceriola, L.; Martino, M. Distribution of demersal crustaceans in the southern Adriatic Sea. Acta Adriat. 2005, 46, 27–40. [Google Scholar]
- Mori, M.; Mura, M.; De Ranieri, S. Sexual Dimorphism in Rissoides Pallidus (Giesbrecht) (Crustacea, Stomatopoda). Thalass. Salentina 2009, 32, 63–71. [Google Scholar]
- Kocak, C. On the depth of occurrence of Rissoides pallidus (Giesbrecht, 1910) (stomatopoda, Squillidae) in the eastern Mediterranean Sea. Crustaceana 2011, 84, 117–121. [Google Scholar] [CrossRef]
- Innocenti, G. Collections of the Natural History Museum, Zoological Section “La Specola” of the University of Florence. XXIV. Crustacea, class Malacostraca, order Stomatopoda. Atti Della Soc. Toscana Di Sci. Nat. Resid. Pisa. Mem. Ser. B 2006, 113, 13–18. [Google Scholar]
- Garofalo, G.; Gristina, M.; Toccaceli, M.; Giusto, G.B.; Rizzo, P.; Sinacori, G. Geostatistical modelling of biocenosis distribution in the Strait of Sicily. GIS/Spat. Anal. Fish. Aquat. Sci. 2004, 2, 241–250. [Google Scholar]
- Bono, G.; Falsone, F.; Falco, F.; Di Maio, F.; Gabriele, M.; Gancitano, V.; Geraci, M.L.; Scannella, D.; Mancuso, M.; Okpala, C.O.R.; et al. Microplastics and alien black particles as contaminants of deepwater rose shrimp (Parapenaeus longistroris Lucas, 1846) in the central Mediterranean Sea. J. Adv. Biotechnol. Bioeng. 2020, 8, 23–28. [Google Scholar] [CrossRef]
- Massi, D.; Titone, A.; Gristina, M.; Garofalo, G.; Lauria, V.; Micalizzi, R.; Sinacori, G.; Fiorentino, F. Characterization and biogeographic affinity of megazoobenthos in the Central Mediterranean Sea. Mar. Ecol. 2020, 42, e12627. [Google Scholar] [CrossRef]
- Ramsay, K.; Holt, R.H.F. Mantis shrimp Rissoides desmaresti in Tremadog Bay, North Wales. J. Mar. Biol. Assoc. UK 2001, 81, 695–696. [Google Scholar] [CrossRef]
- Laban, C.; Lindeboom, H.J. Penetration depth of beam trawl gear. BEON Rapp. 1991, 13, 37–52. [Google Scholar]
- Raso, J.E.G.; Muñoz, V.C.; Muñoz, J.E.G. Additional records of two rare crustaceans from southern Spain (Western Mediterranean Sea): Platysquilla eusebia (Stomatopoda) and Automate branchialis (Decapoda). Mar. Biodivers. Rec. 2010, 3, e29. [Google Scholar] [CrossRef]
- Sardo, G.; Geraci, M.L.; Scannella, D.; Falsone, F.; Vitale, S. New records of two uncommon species, Calappa tuerkayana Pastore, 1995 (Decapoda, Calappidae) and Parasquilla ferrussaci (Roux, 1828) (Stomatopoda, Parasquillidae), from the Strait of Sicily (central Mediterranean Sea). Arx. Misc. Zool. 2020, 18, 113–121. [Google Scholar] [CrossRef]
- Scannella, D.; Geraci, M.L.; Falsone, F.; Colloca, F.; Zava, B.; Serena, F.; Di Maio, F.; Vitale, S. A new record of a great white shark, Carcharodon carcharias (Chondrichthyes: Lamnidae) in the Strait of Sicily, Central Mediterranean Sea. Acta Adriat. 2020, 61, 231–238. [Google Scholar] [CrossRef]
- Geraci, M.L.; Ragonese, S.; Scannella, D.; Falsone, F.; Gancitano, V.; Mifsud, J.; Gambin, M.; Said, A.; Vitale, S. Batoid Abundances, Spatial Distribution and Life History Traits in the Strait of Sicily (Central Mediterranean Sea): Bridging a Knowledge Gap through Three Decades of Survey. Animals 2021, 11, 2189. [Google Scholar] [CrossRef]
- Falsone, F.; Geraci, M.L.; Scannella, D.; Okpala, C.O.R.; Giusto, G.B.; Bosch–Belmar, M.; Bono, G. Occurrence of two rare species from order Lampriformes: Crestfish Lophotus lacepede (Giorna, 1809) and scalloped ribbonfish Zu cristatus (Bonelli, 1819) in the northern coast of Sicily, Italy. Acta Adriat. 2017, 58, 137–146. [Google Scholar] [CrossRef]
- Sardo, G.; Geraci, M.L.; Falsone, F.; Gancitano, S.; Gancitano, V.; Scannella, D.; Okpala, C.O.R.; Titone, A.; Vitale, S. First record and otolith morphometric description of an adult lightfish, Ichthyococcus ovatus (Actinopterygii: Stomiiformes: Phosichthyidae), caught in the Strait of Sicily (central Mediterranean Sea). Acta Ichthyol. Piscat. 2022, 52, 159–166. [Google Scholar] [CrossRef]
- Geraci, M.L.; Di Lorenzo, M.; Falsone, F.; Scannella, D.; Di Maio, F.; Colloca, F.; Vitale, S.; Serena, F. The occurrence of Norwegian skate, Dipturus nidarosiensis (Elasmobranchii: Rajiformes: Rajidae), in the Strait of Sicily, central Mediterranean. Acta Ichthyol. Piscat. 2019, 49, 203–208. [Google Scholar] [CrossRef]
- Geraci, M.L.; Falsone, F.; Scannella, D.; Sardo, G.; Vitale, S. Dolphin-Fisheries Interactions: An Increasing Problem for Mediterranean Small-Scale Fisheries. Examines Mar. Biol. Oceanogr. 2019, 3, 1–2. [Google Scholar] [CrossRef]


| Coefficients: | Estimate | Std. Error | t Value | p-Value | R2 |
|---|---|---|---|---|---|
| Intercept | 2.36 | 6.54 | 0.36 | 0.74 | 0.91 |
| TL | 4.27 | 0.61 | 7.03 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardo, G.; Geraci, M.L.; Falsone, F.; Okpala, C.O.R.; Scannella, D.; Titone, A.; Vitale, S. A Rarely Reported Crustacean Species, Rissoides pallidus (Giesbrecht, 1910) (Stomatopoda, Squillidae), Caught in the Strait of Sicily Waters (Central Mediterranean Sea). Hydrobiology 2023, 2, 575-582. https://doi.org/10.3390/hydrobiology2040038
Sardo G, Geraci ML, Falsone F, Okpala COR, Scannella D, Titone A, Vitale S. A Rarely Reported Crustacean Species, Rissoides pallidus (Giesbrecht, 1910) (Stomatopoda, Squillidae), Caught in the Strait of Sicily Waters (Central Mediterranean Sea). Hydrobiology. 2023; 2(4):575-582. https://doi.org/10.3390/hydrobiology2040038
Chicago/Turabian StyleSardo, Giacomo, Michele Luca Geraci, Fabio Falsone, Charles Odilichukwu R. Okpala, Danilo Scannella, Antonino Titone, and Sergio Vitale. 2023. "A Rarely Reported Crustacean Species, Rissoides pallidus (Giesbrecht, 1910) (Stomatopoda, Squillidae), Caught in the Strait of Sicily Waters (Central Mediterranean Sea)" Hydrobiology 2, no. 4: 575-582. https://doi.org/10.3390/hydrobiology2040038
APA StyleSardo, G., Geraci, M. L., Falsone, F., Okpala, C. O. R., Scannella, D., Titone, A., & Vitale, S. (2023). A Rarely Reported Crustacean Species, Rissoides pallidus (Giesbrecht, 1910) (Stomatopoda, Squillidae), Caught in the Strait of Sicily Waters (Central Mediterranean Sea). Hydrobiology, 2(4), 575-582. https://doi.org/10.3390/hydrobiology2040038







