Abstract
Niphargus carolinensis sp. nov. was sampled from the Carolina Mine located in North Rhine–Westphalia, Germany. The new species is described and compared to phylogenetically related species and species identified in nearby locations. The three phylogenetic markers (i.e., COI, 28S rRNA and ITS2) studied in the examined specimens had different sequences compared to those belonging to species present in locations neighboring the Carolina Mine, i.e., in a radius of 40 km. N. carolinensis sp. nov. is a small-to-medium-sized species that is poorly setose; has a relatively short antenna I, trapezoidal gnathopod propodites, long pereopod VI, and short uropod III; and is not differentiated sexually. The new species described herein is a case of narrow endemism and adds to the diversity of the genus Niphargus in Germany. This work is a contribution to knowledge on groundwater amphipod diversity and the systematics of the genus Niphargus close to the northern border of the distribution of this genus.
Keywords:
Niphargus; new species; the Carolina Mine; Germany; North Rhine—Westphalia; morpho-logy; COI; 28S rRNA; ITS2 1. Introduction
With over 400 species [1], Niphargus is the most speciose subterranean amphipod genus; it is also the genus with the highest number of species in freshwater. It is widely distributed from Spain [2] to Iran [3], being almost completely missing in areas previously covered by the Late Quaternary ice sheets in the northern part of Europe. While it has a large number of species with mostly small distribution areas in the south of its range, the species’ richness decreases toward the north. At the same time, the distribution area of the individual species expands. Genus Niphargus, thus, follows the Rapoport rule [4].
In Germany [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22], approximately 17 species of the Family Niphargidae are known [23], of which 7 are recorded in the federal state of North Rhine–Westphalia, i.e., Niphargellus nolli Schellenberg, 1938, Niphargus aquilex Schiodte, 1855, Niphargus schellenbergi (S. Karaman, 1932), Niphargus puteanus (Koch, 1836), Niphargus fontanus (Bate, 1859), Niphargus kochianus (Bate, 1859), Niphargus stygius (Schiodte, 1847) (its presence being strongly questioned) and Niphargus stadleri S. (Karaman, 1932) [18,20,24]. N. stadleri was synonymized with N. puteanus [20].
Here, we present a new species, which, in spite of intensive searches in the near (circle around the type locality with a radius of 2 km) and far (Germany, Luxembourg, Belgium) surroundings, could only be found in one mine and is, therefore, according to current knowledge, narrowly endemic.
2. Materials and Methods
2.1. Sampling
From 2015 to 2022, 6798 niphargids were collected from 917 locations (caves, artificial cavities, springs, river interstitial sites, wells) in Germany (Figure 1A) and identified morphologically [10,14,23,25]. In total, 824 specimens in 96 locations (5 river interstitial sites, 7 helocrene sites, 3 limnocrene sites, 54 rheocrene springs, 2 spring basins, 13 caves, 11 artificial caverns and 1 shaft well) originated from North Rhine–Westphalia, of which 135 specimens were analyzed in order to determine their COI, 28S rRNA, and ITS2 sequences. The closest caves where we collected Niphargus successfully were Gesshardshöhle (51.26° N, 7.65° E) and Kückelshauser Klutert (51.34° N, 7.44° E).
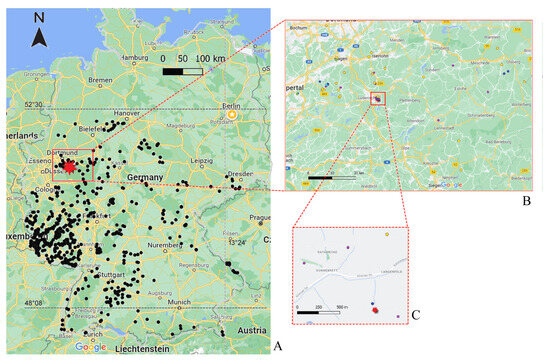
Figure 1.
Sites in Germany where Niphargus specimens were collected. The site of Niphargus carolinensis sp. nov. is marked with a red star (A). The red rectangle represents the area where the Caro-lina Mine is present (red star), with all species identified in the neighboring locations (B), and a depiction of a smaller area (C) indicating the Carolina Mine (red star) and the nearby location with different Niphargus species, i.e., purple dots are the locations where N. schellenbergi is present, the blue dot stands for N. fontanus, and the yellow dot stands for species within the N. aquilex complex. Black dots indicate sites in Germany where Niphargidae were found and sequenced.
2.2. Sampling Site
Niphargus carolinensis sp. nov. was found in the Carolina Mine (51.25 °N, 7.64 °E), which is an abandoned copper ore mine (Figure 2). It consists of a lower adit, which is no longer accessible, and an upper adit [26], where Niphargus carolinensis sp. nov. was found. In 1755, the upper adit was originally 48 m long and re-opened [26]. Soon after 1764, at 71 m from the adit entrance, ore containing rock was reached, and it was excavated up to 8 m above the adit [26]. At various heights, wooden crossbeams were installed between the walls at this time, supporting a platform to enable mining upwards [26]. These beams broke, at least partially, and are now lying in the gently flowing stream of the gallery. After 95 m, the gallery is collapsed. The main waterflow emerges from this collapse, and it cannot be pursued further. A breakthrough to a former daytime shaft and, thus, a ventilation system are, therefore, no longer present [26]. The broken beams can well be seen via this link: https://www.youtube.com/watch?v=Znkw4sbR7qA (accessed on 22 October 2023). The German hardness of the water of the Carolina Mine is 6°, and its temperature is 8.5 °C.
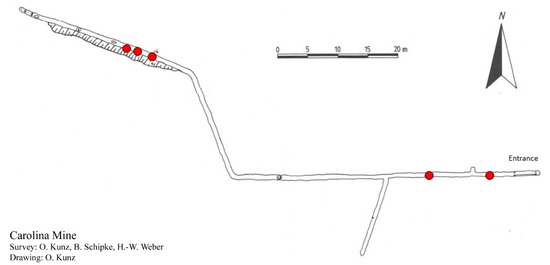
Figure 2.
Plan of the Carolina Mine from 1992, sourced from Arbeitsgemeischaft Höhle und Karst Sauerland Hemer e.V. Red dots represent the sites where specimens of Niphargus carolinensis sp. nov. were found.
During 11–12 May 2018, baits were placed at different distances from the entrance in the Carolina Mine. Holes of 5 mm were drilled in the sides of plastic cups. The cups were placed upright in gently flowing water, so that the upper part of the cup was above the water surface. Chicken liver (approximately 10 g) was placed into the cups. The contents were emptied the following day and analyzed using the binocular equipment. Two spe-cimens of Niphargus carolinensis sp. nov. were found at a distance of 10 m from the adit mouth eaves, 3 specimens were found 20 m from the eaves and 4 specimens were found 80–85 m from the eaves (Figure 2, Table 1).

Table 1.
Specimen list. * = holotype; Date = date of collecting; pres. = preservation (ET96 = pure ethanol 96%, minus 20 °C; KGG = Kaiser’s glycerol gelatin according to 2.4; PG = propylene glycol 100% = propylene diol-1,2, minus 20 °C); Spec. = storage of the specimen (Weber = private collection Dieter Weber, ISER = Institutul de Speologie “Emil Racoviță”, -** = completely used for DNA isolation); isolate = storage of the isolate (ULB = Evolutionary Biology and Ecology research unit of the Université libre de Bruxelles; SDEI = Senckenberg German Entomological Institute); COI = Genbank number of the COI sequence; 28S = Genbank number of the 28S rRNA sequence; ITS2 = Genbank number of the ITS sequence.
Collecting was repeated on 5 February 2022, as first sequences indicated that a new species was found. In particular, all gravel banks were examined according to the Karaman–Chappuis method [27]. No niphargids were found. At a distance of 90 m from the eaves, one specimen was captured by hand, and 95 were captured from the eaves. In the area of the wooden shoring, another three specimens were captured (Table 1).
After it was obvious that Niphargus carolinensis sp. nov. was mainly found under collapsed wooden crossbeams, a further eight specimens were captured on 6/7 September 2022 using three baits of chicken liver (Table 1). Another bait placed 90 m from the eaves into a larger lake did not yield any niphargids.
The methods used for storing the samples are shown in Table 1.
2.3. Laboratory Work
The DNA of three individuals was extracted from one pereopod using the NucleoSpin Tissue Kit (Macherey-Nagel, Düren, Germany), according to the instructions provided by the manufacturer. DNA isolates were stored at −20 °C in the collections of the Evolutionary Biology and Ecology research unit of the Université Libre de Bruxelles and the Senckenberg German Entomological Institute (Table 1).
Polymerase chain reaction (PCR) was performed to amplify the standard barcoding fragment of the cytochrome c oxidase subunit 1 gene (COI) [28], using the primer pair HCO2198-JJ and LCO1490-JJ [29] (10 pmol/μL), premixed at a ratio of 1:1. A list of primers, together with their nucleotide sequence, for both PCR and sequencing is provided in Table 2. For COI, the PCR mix contained 1.5 μL DNA extract (with varying concentrations, not measured), 1 μL of primer mix, 5 µL of “DreamTaq DNA Polymerase” (Thermo Scientific, Macquarie Park, Australia) and 5 µL of ultrapure water. PCR cycling conditions were an initial 5 min denaturation step at 95 °C, 38 cycles of 30 s of denaturation at 95 °C, 90 s of annealing at 49 °C, 60 s of extension at 72 °C and a final elongation step of 30 min at 68 °C.

Table 2.
List of primers used for PCR and sequencing (Seq). Ref. = Reference.
A fragment of the nuclear 28S ribosomal RNA gene (28S rRNA) was analyzed using the PCR primer Niph15 and Niph16 [30]. The PCR mixture for the 28S rRNA marker contained 2 μL of DNA extract (with varying concentration), 1 μL of each primer (10 pmol/μL), 0.2 μL of REDTaq polymerase (Sigma-Aldrich, St. Louis, MI, USA), 5 µL of REDTaq reaction buffer and 15.8 µL of ultrapure water. PCR cycling conditions for 28S rRNA were an initial 3-minute denaturation at 95 °C, 56 cycles of 30 s of denaturation at 94 °C, 60 s of annealing at 45 °C and 90 s of extension at 72 °C.
The nuclear internal transcribed spacer 2 (ITS2) marker was analyzed using the PCR primer pair shown in [31], which was premixed at a ratio of 1:1. The PCR mix for the ITS2 marker contained 1 μL of DNA extract (with varying concentration), 1 μL of primer mix (10 pmol/μL), 7.5 μL of Qiagen mix and 5.5 µL of ultrapure water. PCR cycling conditions were an initial 5 min denaturation step at 95 °C, 35 cycles of 30 s of denaturation at 95 °C, 90 s of annealing at 52 °C, 60 s of extension at 72 °C and a final elongation step of 30 min at 68 °C.
Bidirectional Sanger sequencing was performed at Genoscreen (Lille, France) or Macrogen (Amsterdam, The Netherlands). For COI and ITS2, the same primers as for PCR amplification were used. Moreover, 28S rRNA was sequenced with three primers: Niph15, Niph20 and Niph21.
To compare new findings with other findings, we sequenced 137 specimens of the genus Niphargus from North Rhine–Westphalia, mainly using those from one collecting site. From the Carolina Mine and, therefore, from the new species, three specimens were sequenced (Table 1).
The sequences were deposited in GenBank (Table 1).
2.4. Sequence Analysis
Chromatograms were assembled into contigs using Sequencher version 4.1.4 (Gene Codes Corporation, Ann Arbor, MI, USA) and inspected visually.
We downloaded all COI sequences from relevant species from North Rhine–Westphalia present in GenBank (www.ncbi.nlm.nih.gov/Genbank) in January 2023. We removed incomplete and not fully resolved sequences, obtained 316 sequences, deleted overlong sequence parts, and added to this dataset our newly achieved COI sequences of N. carolinensis. Sequences were aligned using MUSCLE [32], as implemented in Mega X [33]. This dataset was used for species delimitation.
Similarly, all 28S rRNA sequences of Niphargus were downloaded from GenBank (www.ncbi.nlm.nih.gov/Genbank) in March 2023. We removed short sequences, as well as all sequences that did not belong to the 28S rRNA fragment. We added the sequences of Microniphargus leruthi as an outgroup, as well as unpublished sequences of Niphargus irlandicus and Niphargus boulangei, as both were indicated in a preliminary test as being close to N. carolinensis. We obtained 258 sequences, trimmed the dataset to 602 columns, corresponding to fewer base pairs in one specimen, and used DNAcollapser [34] to remove duplicates. We aligned the dataset using MAFFT [35] version 7 with iterative refinement method E-INS-I in the updated version of 2015. Phylogenetic trees were performed in MEGA X [33] using p-distances with 1000 bootstrap replicates according to the Bayesian Information Criterion [36].
Internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2 complete sequences (ITS) were downloaded from Genbank (www.ncbi.nlm.nih.gov/Genbank, accessed 9 October 2022) for all available species known to exist in Germany: Niphargus hrabei, Niphargus schellenbergi, Niphargus thienemanni and Niphargus tonywhitteni, with a final set of 167 sequences. Sequences were aligned using MUSCLE [32], as implemented in Mega X [33].
The sequences of the new species were compared to published sequences using Nucleotide Blast [37] to make sure the species is not known to exist outside Germany. Species delimitation was carried out using Automatic Barcode Gap Discovery (ABGD) [38] and Assemble Species via Automatic Partitioning (ASAP) [39].
2.5. Morphological Analysis
At the laboratory, the specimens (DW220205-01 male and DW 220907-01 female) were immersed in glycerol, and the dissected body appendages were mounted on slides using Kaiser’s glycerol gelatin (Merck KGaA, Darmstadt, Germany). The slides were inspected with an Olympus SZX16 stereomicroscope and provided with an Olympus SC180 camera and a microscope Olympus BX51, as well as the Olympus SC50 camera. The drawings were performed upon printed photographs via manual inking and the continuous inspection of the slides under the microscope. The terminology used for body parts, as well as the choice of appendages taken into consideration for measurements, are those previously described [40].
3. Results
3.1. Molecular Identification
All three sequenced specimens have identical COI. Comparing the sequences in Nucleotide Blast did not yield any closely related species.
Comparing the 28S rRNA sequence in Nucleotide Blast did not yield any closely related sequences. The closest relatives are Niphargus aff. fontanus (up to 90%), Niphargus aff. aquilex (up to 90%), Niphargus enslini (90%) and Niphargus puteanus (up to 90%).
ITS2 did not reveal any closely related species: Niphargus racovitzai (87%), Niphargus pontoruffoi (87%) and Niphargus dobrogicus (87%) were the most closely related species.
The Neighbor Joining (NJ) tree of the 28S rRNA placed N. irlandicus/glenniei clade as the sister clade to all other Niphargus species, and within those, Niphargus carolinensis was the sister clade to all remaining Niphargus species (Figure 3). The split of N. carolinensis to the main clade of Niphargus is of high support. The Maximum Likelihood (ML) tree yielded low support.
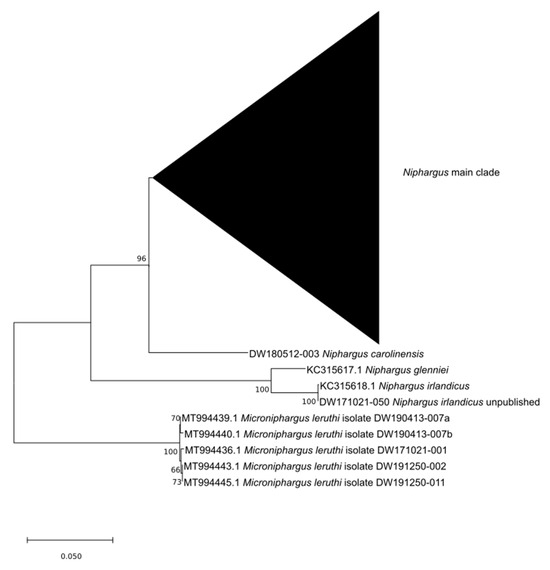
Figure 3.
NJ tree of the 28S rRNA gene fragments. The triangle shows the collapsed Niphargus main clade. Microniphargus leruthi (family Pseudoniphargidae) is chosen as an outgroup.
ABGD based on COI of the species from North Rhine–Westphalia in standard settings and all distance models resulted in 19 species (2 putative species of the N. kochianus species complex, 4 putative species of the N. puteanus species complex, 1 putative species of the N. fontanus complex, 2 putative species of the N. caspary species complex, 8 putative species of the N. aquilex species complex and 1 putative species of the N. schellenbergi complex), with N. carolinensis defined as a separate species. ASAP in standard settings results in 22 species (3 putative species of the N. kochianus species complex, 5 putative species of the N. puteanus species complex, 2 putative species of the N. fontanus species complex, 2 putative species of the N. caspary species complex, 8 putative species of the N. aquilex species complex and 1 putative species of the N. schellenbergi complex), with N. carolinensis defined as a separate species.
3.2. Morphology-Based Description
Order Amphipoda (Latreille, 1816);
Family Niphargidae (Bousfield, 1977);
Genus Niphargus (Schiödte, 1849);
Niphargus carolinensis sp. nov. (Weber and Brad, 2023).
3.2.1. Diagnosis
Small-to-medium-sized Niphargus, with little setose appendages. No outstanding diagnosis features were noticed, which would be particularly specific for the newly species herein described. The molecular evidence best supports the reason for the present species description.
3.2.2. Etymology
The species name derives from the name of the Carolina Mine in western Germany (North Rhine–Westphalia), where it was discovered.
3.2.3. Type Locality
Germany, North Rhine–Westphalia, 2 km NE Lüdenscheid, 51.25 °N and 7.64 °E of the Carolina Mine, leg. D. Weber.
3.2.4. Description
Material Examined
Holotype. Germany. Male, the Carolina Mine, North Rhine–Westphalia, Germany (Figure 1A). The holotype specimen was dissected and used here for species description.
Paratypes. Female, inspected for sexual variability. Two additional paratypes exa-mined for potential intraspecific variability.
The dissected body appendages were transferred to permanent slides and stored in the collection of Emil Racoviță Institute of Speleology, Cluj, Romania, with the inventory number DW220205-001 (male holotype) and DW220907-001 (female paratype).
Description (Male)
The total body length of the male is 8.93 mm (Figure 4A). A chart containing detailed measurements of all diagnosis-relevant body appendages is presented in Table 3.
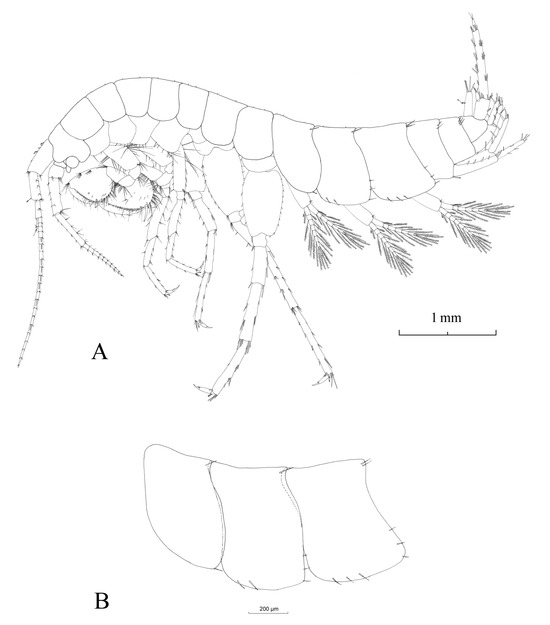
Figure 4.
Habitus of male Niphargus carolinensis sp. nov. (A); epimeral plates I to III, from left to right (B).

Table 3.
Measurements in mm (according to landmarks in Fišer et al., 2009 [40]) of the various appendages of male (inventory number DW220205-001) Niphargus carolinensis sp. nov. sampled from the Carolina Mine (North Rhine–Westphalia, Germany).
Head: the head (Figure 4A) with no rostrum represents 7.41% of the total body length.
Antenna I: Antenna I (Figure 5A), with the main flagellum formed of 20 articles, represents approximately one third of the total body length (Table 3). The length of the antenna I peduncle is one third of the total length of antenna I. The accessory flagellum (Figure 5B) is biarticulated; the proximal article is as long as the first two articles of the main flagellum. The distal article bears apically three setae of different lengths and one aestethasc and represents one third of the total length of the accessory flagellum. Aestethascs is three quarters of the lengths of the respective flagellum articles (Figure 5C).
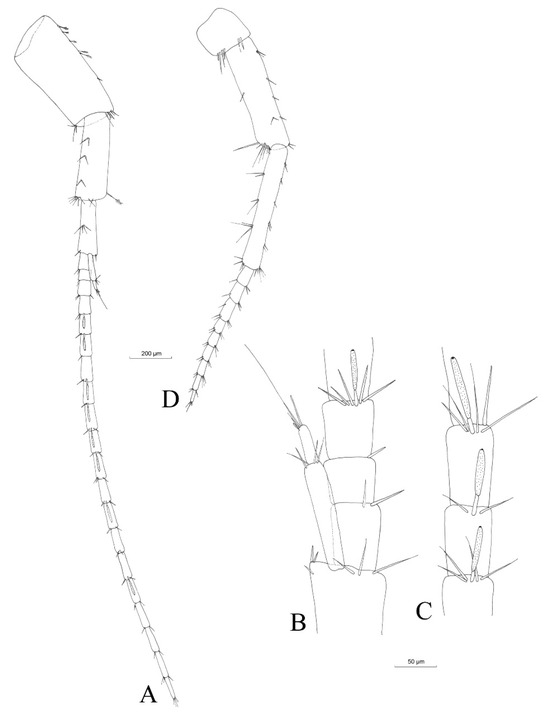
Figure 5.
Niphargus carolinensis sp. nov. male antenna I (A), details of accessory flagellum (B) and aestethascs (C) and antenna II (D).
Antenna II: the flagellum (Figure 5D) is formed of nine articles and represents one third of the total length of antenna II.
Mouthparts
Labium (Figure 6D). Short inner lobes with no setae. Outer lobes with one row of fine setae subapically on inner sides.
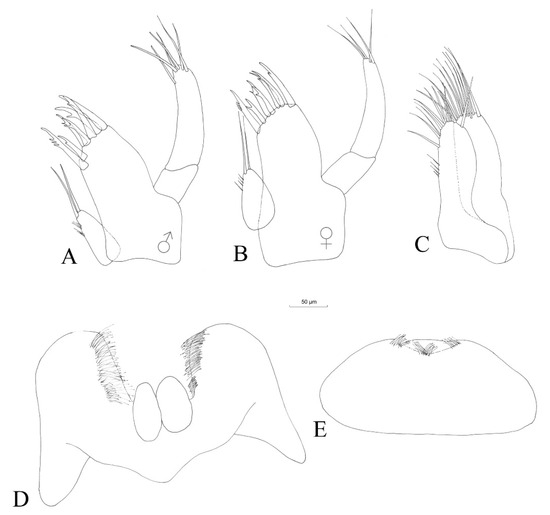
Figure 6.
Niphargus carolinensis sp. nov. maxilla I male (A) and female (B), male maxilla II (C), labium (D) and labrum (E).
Labrum (Figure 6E) is of typical, subovoid shape.
Maxilla I (Figure 6A) with six apical setae on the distal article of the palp. Six spines of the outer lobe with one tooth each and one spine with three smaller teeth. Inner lobe with two apical setae.
Maxilla II (Figure 6C) with the inner lobe slightly shorter than the outer lobe. One apical row of setae on each lobe. Four fine setae along the outer margin of inner lobe.
Left mandible (Figure 7A) with four teeth on incisor process and two teeth on lacinia mobilis. Five serrate setae and two groups with four and seven smaller setae between lacinia mobilis and the molar process, respectively.
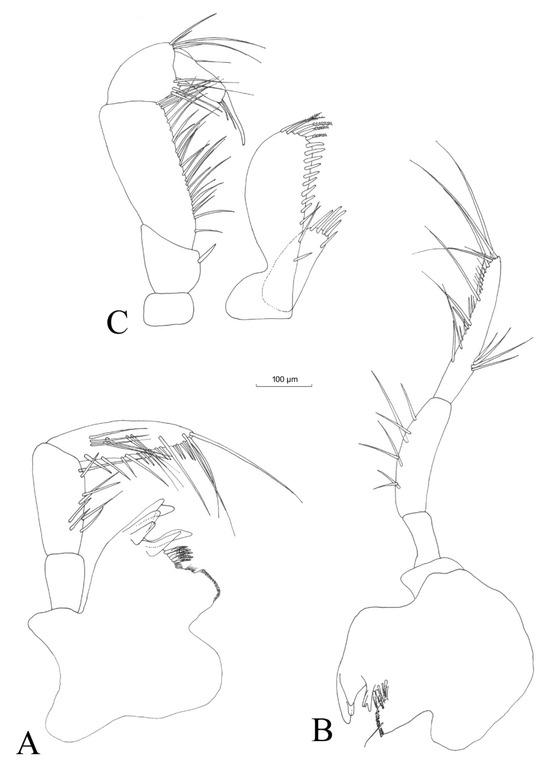
Figure 7.
Niphargus carolinensis sp. nov. male mandibles left (A) and right (B), and maxilliped (C).
Right mandible (Figure 7B) with three teeth on incisor process and three teeth on the lacinia mobilis, three serrate and two small setae between lacinia mobilis and the molar process and a long seta on the molar process.
The mandibular palps (Figure 7A,B) are highly similar and of the same length. The three articles of mandibular palps account for 17.9% (article 1), 35.7% (article 2) and 46.4% (article 3) of the total length of the palp (Table 3). Proximal article without setae, median article with 7–8 ventral setae and distal article of the palp with one group of 3–4 A setae, three groups with 1–2 B setae, 9–11 D setae and 4–5 E setae (for the nomenclature of mandibular palps setae, see [40].
Maxilliped (Figure 7C) with the palp formed of four articles. Article 1 with one strong seta on the inner margin, and article 2 with numerous setae aligned along the inner margin. Article 3 with one group of four setae located on the inner margin and one group of five apical setae. Article 4 with one seta located on the outer margin. The outer lobe of maxilliped with three setae, nine flattened spines and five serrated setae located apically. Inner lobe provided apically with four flattened spines and subapically with one seta.
Gnathopod I (Figure 8A) with a coxal plate in the form of a rectangular trapezoid, with a depth larger than its width (ratio depth:width 1.0:0.7). The basis length:width ratio is 1.0:0.4 (Table 3). Ischiopodite with one posteroventral group of three setae. The basis length:carpus length ratio is 1.0:0.5. The carpus has a row of nine setae of various lengths along the ventral margin and a group of five setae located anterodorsally. The propodite is slightly longer than wide and has six groups of 1–6 setae on its ventral margin, as well as two antero-dorsal groups of 4 and 7 setae and one antero-apical group of 6 setae. Two small setae are on the lateral surface of the propodite close to its ventral side, three mesial setae are on the lateral surface and one group of four setae is close to the dorsal margin. One group of three long setae is present in the vicinity of palmar corner. One strong palmar spine, one smaller spine and one outer denticulate spine is present in the palmar corner. Dactylopodite has a claw one third of the total dactylopodite length with four small setae along the outer margin.
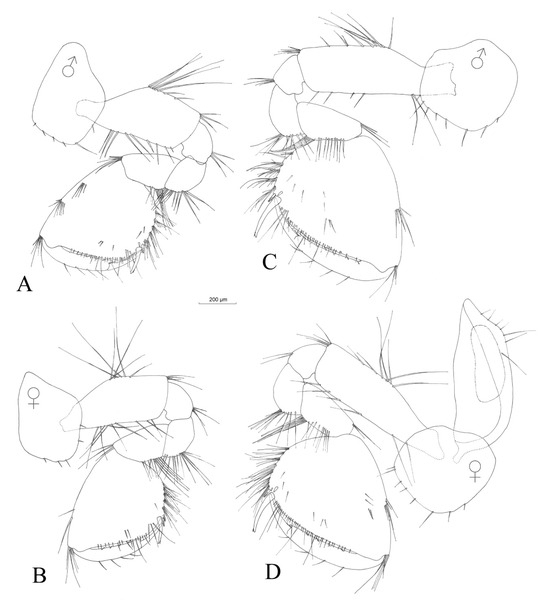
Figure 8.
Niphargus carolinensis sp. nov. gnathopod I male (A) and female (B). Gnathopod II male (C) and female (D).
Gnathopod II (Figure 8C) is larger than gnathopod I. The coxal plate is nearly as wide as it is deep (Table 3). The basis length:width ratio is 1.0:0.3. Ischiopodite with one posteroventral group of four setae. The basis length:carpus length ratio is 1.0:0.5. Carpus with six setae along its ventral margin, nine setae on its surface close to ventral margin and three setae located anterodorsally. The propodite has approximately the same length and width; the propodite presents in six groups of 1–9 setae on its ventral margin, one antero-dorsal group of 5 setae and one antero-apical group of 4 setae. On its surface, the propodite has three mesial groups of 1–3 small setae and three more setae closer to its ventral margin. One group of three long setae close to the palmar spine. Strong palmar spine, one smaller spine and one outer denticulate spine in palmar corner. Dactylopodite with the claw one third of the total dactylopodite length and four small setae along the outer margin.
Pereopod III (Figure 9A) with rectangular coxal plate and a ratio of depth:width 1.0:0.8 (Table 3). Dactylus (Figure 9B) with the nail measuring half of the total length of the dactylus, with one dorsal seta with plumose tip and one seta at nail base. The propodus length:dactylus length ratio is 1.0:0.5. Pereopod III is nearly equal in length to pereopod IV (the pereopod III length:pereopod IV length ratio is 1.0:0.91).
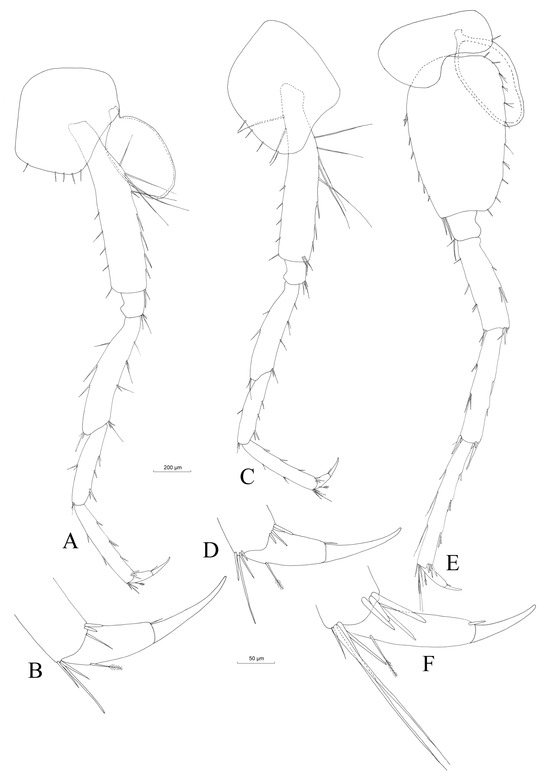
Figure 9.
Niphargus carolinensis sp. nov. male pereopods III (A), IV (C) and V (E), with details on dactylopodites ((B), (D) and (F), respectively).
Pereopod IV (Figure 9C) with a relatively rectangular coxal plate, with a concavity on the posterior margin. The depth:maximum width ratio is 1.0:1.0. Robust dactylus (Figure 9D) with the nail measuring almost half of total dactylus length, with one dorsal seta with plumose tip and one seta at nail base. The propodus length:dactylus length ratio is 1.0:0.4.
Pereopod V (Figure 9E) with the coxal plate of irregular shape, with a deep concavity on the ventral side and one anterior seta. The basis has an ovoid–rectangular shape with an length:width ratio of 1.0:0.6, 11 groups of 1–2 short and thick setae on the posterior margin and six similar setae on the anterior margin. Two postero-apical setae. Dactylus (Figure 9F) with on seta with plumose end on the outer margin and one seta at the base of the nail, which represents 34% of the total dactylus length. The propodus length:dactylus length ratio is 1.0:0.3.
Pereopod VI (Figure 10A). With more than 4 mm, pereopod VI is the longest leg of the inspected male Niphargus carolinensis sp. nov. (Table 3). Coxal plate similar in shape to that of pereopod V, but slightly smaller, with two posterior setae. Basis with ovoid–trapezoidal shape, seven groups of 1–2 small setae on the anterior margin and 11 small setae on the posterior margin; the length:width ratio is 1.0:0.6. Dactylus (Figure 10B) with one plumose seta on the outer margin and two setae at the base of nail. Nail length is one third of the total dactylus length. The ratio of propodite length:dactylus length is 1.0:0.3.
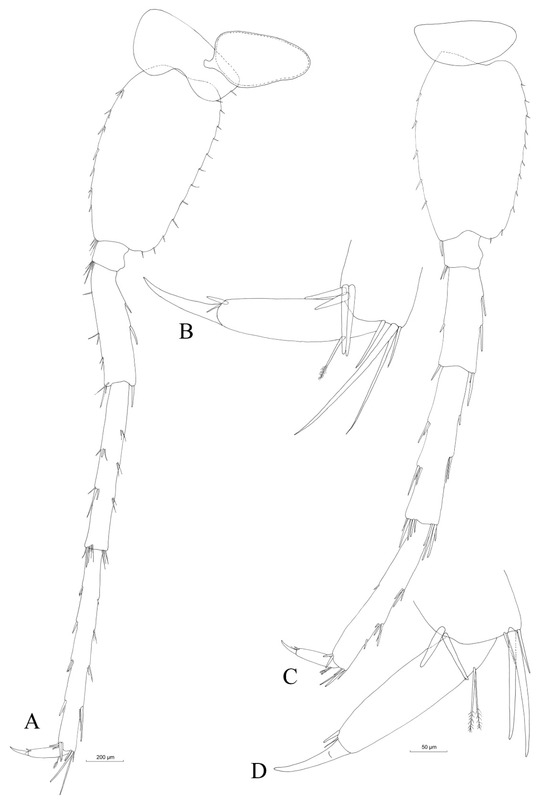
Figure 10.
Niphargus carolinensis sp. nov. male pereopods VI (A) and VII (C). Details on dactylopodites ((B) and (D), respectively).
Pereopod VII (Figure 10C). The coxal plate of pereopod VII is trapezoidal, with no setae. The base has an ovoid–trapezoidal shape, with a ratio of length:width of 1.0:0.65. The basis presents 5 thick setae on the anterior margin and 11 setae on the posterior margin. Dactylus (Figure 10D) with two plumose setae on the outer margin and two setae at the base of the nail. Nail length is one third of the total dactylus length. The ratio of propodite length:dactylus length is 1.0:0.35.
Pereopods’ V:VI:VII ratio is 1.0:1.33:1.22 (Table 3).
Pleopods are highly similar (pleopod II is depicted in Figure 11A), with equal rami and two hooks on the retinaculum.
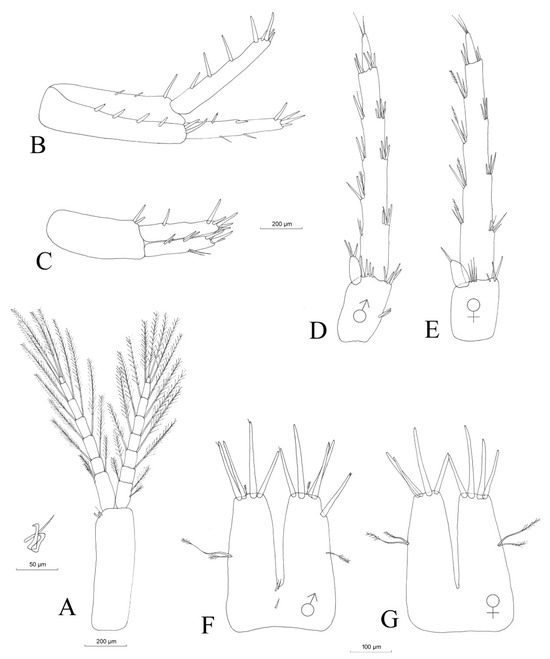
Figure 11.
Niphargus carolinensis sp. nov. male pleopod II (A) with a detail of the retinaculum. Male uropods I (B), II (C) and III (D). Female uropod III (E). Male (F) and female (G) telson.
Uropod I (Figure 11B) with two rows of 2–4 dorsal and 3 apical spines onto peduncle. The endopodite is slightly longer than the exopodite, and the ratio of endopodite length:exopodite length is 1.0:0.81. One strong spine at the base of the uropod I.
Uropod II (Figure 11C): Peduncle with two apical and no dorsal spines. The pendopodite is longer than the exopodite, and the endopodite length:exopodite length ratio is 1.0:0.87; both rami have a low number of spines.
Uropod III (Figure 11D) is short, being approximately 15% of the total body length (Table 3). Sexually not differentiated. Basipodite with two groups of four apical spine-like setae. Short endopodite, slightly less than half of basipodite, with three apical setae. The proximal segment of the exopodite is five times longer than that of the distal segment (ratio 1.0:0.18). The anterior margin of the proximal segment of exopodite has four groups of two setae, with one of them containing one plumose seta. The posterior margin of the proximal segment has three groups of 4–5 setae. The proximal segment of the exopodite is provided apically with five setae, while the distal segment has two setae.
Epimeral plates (Figure 4B): Epimeral plate I with acute postero-ventral angle. Relatively rounded ventral margin; convex posterior margin with one postero-ventral and one postero-dorsal seta. Epimeral plate II with right postero-ventral angle, convex ventral margin with two spines and a relatively straight posterior margin with three setae and one postero-dorsal seta. Epimeral plate III is slightly different compared to epimeral plates I and II, as the postero-ventral angle is rather acute, the ventral margin is straight with three spines and the posterior margin is concave to straight, with three setae and two postero-dorsal setae.
Urosomite I (Figure 4A) with two dorso-lateral spines, and urosomite II with three dorso-lateral spines of various lengths. Dorsal margin of urosomite III with no spines (Figure 4A).
Telson (Figure 11F) longer than wide (length:width ratio of 1.0:0.8). Four apical spines of different lengths, and one plumose seta on each lobe; the longest spine is slightly more than half of the total telson length (Table 3). Two fragile setae with plumose ends along each side. Three small dorsal setae at the cleft base.
3.2.5. Sexual Dimorphism
No obvious sexual dimorphism was observed. Males and females seem identical, with small differences in maxilla I, where the distal article of the palp has only four setae in the case of females (Figure 6B), compared to six setae in the case of males (Figure 6A). Gnathopods I and II are identical in shape and slightly smaller in females (Figure 8B,D). An oostegite is present next to gnathopod II in females (Figure 8D). The endopodite of the female uropod III is provided apically with only one seta (Figure 11E), compared to that of the males, which has three setae (Figure 11D).
4. Discussion
4.1. New Species
The specimens from the Carolina Mine have strong genetic variation to all other known Niphargus species. Molecular species delimitation models fail when no sequences of comparative species are available. In this case, however, all species in question have been sufficiently sequenced.
4.1.1. Position of N. carolinensis sp. nov. in the Niphargid Tree of Life
As the 28S rRNA data already indicated a low relationship between N. carolinensis and other Niphargus species, it is not a surprise that phylogenetic trees based on the COI always yielded low support. The ITS2 marker can only be used for reconstruction when dealing with sufficiently similar species, which is not the case for the species in question. In contrast, the NJ tree of the 28S rRNA gene shows the position of N. carolinensis outside the main Niphargus clade with high support and, thus, indicates a very early split of N. carolinensis from the other Niphargus species. The close relationships between N. boulangei and N. irlandicus suggested in initial tests have not been confirmed.
It is noticeable that the species delimitation according to ABGD splits numerous species deposited in Genbank into several species, which may give the impression that the method is flawed. However, we assume that, rather erroneously, several species are stored under the same name in Genbank, as we proved in the case of Niphargus aquilex [41].
4.1.2. Morphological Difference between Niphargus carolinensis sp. nov. and Species in Nearby Vicinity
Morphologically, Niphargus carolinensis sp. nov. is different from the species present in nearby locations. The first varying characteristic is the constitution of uropod III. In the case of all three species present in locations neighboring the Carolina Mine, the distal article of the exopodite of uropod III is elongated in males. This aspect is not present in the newly described species herein. This distal article of uropod III exopodite is short and identical in males and females. Adult individuals of N. schellenbergi and N. fontanus are sexually dimorphic and a lot larger compared to specimens of N. carolinensis sp. nov. Compared to Niphargus carolinensis sp. nov., N. fontanus is approximately twice as large [42].
Other similarly sized species with gnathopod II being visibly larger than gnathopod I and broad basipodites display a few morphological differences compared to N. carolinensis sp. nov., as described herein. Hence, N. laisi described from Austria has the spines on maxilla I outer lobe with 4–6 teeth and the ventral margin of gnathopods dactylopodites with only 1 seta [43]. N. renei from France has two spines on the maxilla I outer lobe with more than one tooth and only three setae on the ventral margin on gnathopods dactylopodites [44]. An outstanding character of N. arolaensis discovered in drinking water wells in Switzerland is the presence of two spiniform setae on the posteroventral angle of the first urosomite near the insertion of uropod I [45]. Most Niphargus species, including N. carolinensis sp. nov., bear only one seta in this region. Also, maxilla I of N. arolaensis is provided on its outer lobe with seven comb-like spiniform setae, and only two setae are present on the ventral margins of gnathopod dactylopodites. Compared to N. carolinensis sp. nov., N. tonywhitteni is a lot larger, and it has uropod III with an elongated second article of the exopodite and a rather different telson with respect to shape and chaetotaxy [46].
Niphargus aquilex s.str. and several species within the N. aquilex complex usually have only one seta along the outer margin of the gnathopod dactylopodites, while four setae are present in the case of N. carolinensis sp. nov. The species in N. aquilex complex that we refer to are currently under description and were collected from Luxembourg; the regions Normandy, Alsace and Lorraine (France); and the German federal states of Rhenish–Pala-tinate and Saarland.
4.2. Ecological Data
Niphargus carolinensis sp. nov. specimens were found mainly on and below the wooden crossbeams that were installed around the year 1764, or in their immediate vici-nity. N. carolinensis sp. nov. specimens probably feed on this 250-year-old wood, the only organic material visible in the Carolina mine. It may graze on bacteria and fungi feeding on this wood. This is a sign of how low the food requirements of subterranean niphargids may be. We found fewer specimens in chicken liver baits than expected, if compared to other Niphargus species.
Niphargus carolinensis sp. nov. is absent in running water and lakes, as well as in interstitial spaces in the mine. It is also absent in neighboring springs, meaning that it is classified as strictly stygobiontic.
4.3. Endemic Species in the North
Niphargus carolinensis sp. nov. was so far identified in one location only, i.e., the Carolina Mine, as we searched thoroughly in groundwater sources in the nearby vicinity and only encountered other species (Figure 1C). In a karst spring located approximately 100 m from the Carolina Mine, we sampled specimens of Niphargus fontanus (blue dot in Figure 1C). Niphargus schellenbergi and a species of the Niphargus aquilex complex were sampled from locations at distances less than 1 km away from the Carolina Mine (Figure 1C).
The presence of Niphargus carolinensis sp. nov. is a case of extreme endemicity. Northern areas are usually characterized by smaller biodiversity compared to more southern parts of the Northern Hemisphere [47]. Likewise, the number of Niphargus species in Germany is far smaller compared to that in Slovenia, for example [48], and their distribution range is far higher (Table 4), confirming the so-called Rapoport rule [4,49]. Strongly endemic Niphargus species are known. However, they all originate from the southern distribution areas of niphargids [50,51,52,53].
In the northern areas, at best, Niphargus boulangei [54] could have been considered narrow endemic, with only one published collecting site; however, other localities of this species, far from the type locality, have since become known (Weber, unpublished). Thus, N. carolinensis sp. nov. is the only known narrow endemic Niphargus species in the northern distribution area (roughly North of 47° N) of the genus Niphargus.

Table 4.
Distribution ranges of Niphargus species from northern half of the distribution of this genus. Only identification results that have been molecularly confirmed are considered. Niphargus virei names follow [55], and Niphargus virei A, with two figures as species, is not finally defined.
Table 4.
Distribution ranges of Niphargus species from northern half of the distribution of this genus. Only identification results that have been molecularly confirmed are considered. Niphargus virei names follow [55], and Niphargus virei A, with two figures as species, is not finally defined.
| Species | Distribution | Literature |
|---|---|---|
| Niphargus carolinensis | 0.06 | This study |
| Niphargus enslini | 82 | [19] |
| Niphargus fontanus | 1150 | Weber and Schmitt in prep. |
| Niphargus puteanus | 756 | [20] |
| Niphargus schellenbergi | 551 | [15] |
| Niphargus tonywhitteni | 400 | [56] |
| Niphargus virei A | 420, 640 | [15,57] |
| Niphargus virei C | 93 | [15,57] |
We are convinced that N. carolinensis sp. nov. mainly lives in a water body outside the mine and has been living there for significantly longer time than the age of the mine ((300 years). Here, as in many other cases, the mine is not the main habitat, but it is a window to the subterranean environment. Even under the unlikely assumption that N. carolinensis exclusively lives deep in the interior of the mountain, while other niphargid species are more likely to survive in springs, the species should most probably have been found in at least one of the neighboring natural caves. Finally, the question remains open as to why N. carolinensis only occurs in this narrowly defined area.
4.4. Low Intraspecific Variability
The comparison of the COI sequences of N. carolinensis sp. nov. with other Niphargus species of the northern range of the genus, of which several specimens were sequenced from the same site, yielded the following results: Niphargus enslini shows one base pair difference at the same site [19]; Niphargus puteanus shows three sites with one base pair difference at the same site and one site with 18 base pair differences at this site [20]; Niphargus schellenbergi shows 10 sites with only identical COI, three sites with one base pair difference and one site with 2, 6, 12 and 23 base pair differences; Niphargus aquilex EF sensu McInerney [55] with four sites and N. aquilex A sensu McInerney [55] with two sites show no differences in COI at the same site [22]. Although the various Niphargus species show strong differences in COI across their ranges, strong variation within a site tends to be the exception, so the identical COI sequences of N. carolinensis seem not to be exceptional.
5. Conclusions
With N. carolinensis, we succeeded in discovering the only strictly endemic species in the north of the distribution range, which separated very early from the main clade of niphargids.
Due to this high degree of endemicity in the newly described species, we suggest that the Carolina Mine should be strictly protected. Visits to the Carolina Mine should be only performed for scientific matters, and the visiting scientists should avoid stepping in the water pools and substrates.
Author Contributions
Conceptualization, D.W.; methodology, D.W. and T.B.; software, D.W.; validation, D.W. and T.B.; formal analysis, D.W.; investigation, D.W. and T.B.; resources, D.W. and T.B.; data curation, D.W. and T.B.; writing—original draft preparation, D.W. (except for 2.4, 3.2 and 4 (partly)) and T.B. (except for 2.4, 3.2 and 4 (partly)); writing—review and editing, D.W. and T.B.; visualization, T.B.; supervision, D.W. and T.B.; project administration, D.W.; funding acquisition, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by Biodiversa+, the European Biodiversity Partnership under the 2021–2022 BiodivProtect joint call for research proposals, co-funded by the European Commission (GA N°101052342) and with funding from organizations such as the Ministry of Universities and Research (Italy); Agencia Estatal de Investigación—Fundación Biodiversidad (Spain); Fundo Regional para a Ciência e Tecnologia (Portugal); Suomen Akatemia—Ministry of the Environment (Finland); the Belgian Science Policy Office (Belgium); Agence Nationale de la Recherche (France); Deutsche Forschungsgemeinschaft e.V. (Germany); Schweizerischer Nationalfonds (Grant N° 31BD30_209583, Switzerland); Fonds zur Förderung der Wissenschaftlichen Forschung (Austria); the Ministry of Higher Education, Science and Innovation (Slovenia); and the Executive Agency for Higher Education, Research, Development and Innovation Funding (Romania).
Data Availability Statement
The DNA sequences of the various phylogenetic markers used within the present article are deposited in GenBank and presented in Table 1, herein.
Acknowledgments
Heinz Werner Weber supplied the literature on the Carolina Mine. Udo Tauchert helped with sampling Niphargus carolinensis in the Carolina Mine. We thank Serban Sarbu for revising the English in this paper. The authors also thank Cene Fišer and two additional reviewers for their suggestions on improving the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Horton, T.; Lowry, J.; De Broyer, C.; Bellan-Santini, D.; Copilaș-Ciocianu, D.; Corbari, L.; Costello, M.J.; Daneliya, M.; Dauvin, J.-C.; Fišer, C.; et al. World Amphipoda Database. 2023. Available online: https://www.marinespecies.org/amphipoda (accessed on 7 September 2023).
- Karaman, G.S. New Data on Two Subterranean Species of the Family Niphargidae from Spain, Niphargus gallicus Schell., 1935 and N. delamarei Ruffo, 1954 (Contribution to the Knowledge of the Amphipoda 282. Contrib. Sect. Nat. Math. Biotech. Sci. 2017, 36, 105–120. [Google Scholar] [CrossRef]
- Esmaeili-Rineh, S.; Sari, A.; Fišer, C.; Bargrizaneh, Z. Completion of Molecular Taxonomy: Description of Four Amphipod Species (Crustacea: Amphipoda: Niphargidae) from Iran and Release of Database for Morphological Taxonomy. Zool. Anz. 2017, 271, 57–79. [Google Scholar] [CrossRef]
- Stevens, G.C. The Latitudinal Gradient in Geographical Range: How so Many Species Coexist in the Tropics. Am. Nat. 1989, 133, 240–256. [Google Scholar] [CrossRef]
- Hartke, T.R.; Fišer, C.; Hohagen, J.; Kleber, S.; Hartmann, R.; Koenemann, S. Morphological and Molecular Analyses of Closely Related Species in the Stygobiontic Genus Niphargus (Amphipoda). J. Crustac. Biol. 2011, 31, 701–709. [Google Scholar] [CrossRef][Green Version]
- Karaman, S. Beitrag Zur Kenntnis der Süsswasser-Amphipoden. Prirodosl. Razpr. 1932, 2, 179–232. [Google Scholar]
- Schellenberg, A. Krebstiere Oder Crustacea. In IV: Flohkrebse oder Amphipoda; die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebenweise; Gustav Fischer: Jena, Germany, 1942. [Google Scholar]
- Schellenberg, A. Bemerkungen zu meinem Niphargus-Schlüssel und zur Verbreitung und Variabilität der Arten, Nebst Beschreibung Neuer Niphargus-Formen. Mitteilungen Aus Dem Zool. Mus. Berl. 1937, 22, 1–30. [Google Scholar] [CrossRef]
- Schellenberg, A. Die Höhere Krebsfauna im Süsswasser Deutschlands, ihre Zusammensetzung und ihr Artenzuwachs. Arch. Für Hydrobiol. 1937, 31, 229–241. [Google Scholar]
- Schellenberg, A. Schlüssel der Amphipodengattung Niphargus mit Fundortangaben und Mehreren Neuen Formen. Zool. Anz. 1935, 111, 204–211. [Google Scholar]
- Schellenberg, A. Der Niphargus Des Thüringer Waldes und Die Glazialreliktenfrage. Arch. Für Hydrobiol. 1935, 29, 274–281. [Google Scholar]
- Schellenberg, A. Weitere Deutsche und Ausländische Niphargiden. Zool. Anz. 1933, 102, 22–33. [Google Scholar]
- Schellenberg, A. Vier Blinde Amphipodenarten in einem Brunnen Oberbayerns. Zool. Anz. 1932, 98, 131–139. [Google Scholar]
- Weber, D. Grundwasserkrebse Niphargus und Microniphargus Im Harz an ihrer Nördlichen Verbreitungsgrenze—Ergebnisse Phylogenetischer Untersuchungen. Abh. Zur Karst-Und Höhlenkunde 2022, 40, 213–224. [Google Scholar]
- Weber, D. Molecular Phylogeny and Systematics of Central and Western European Niphargids (Crustacea: Amphipoda); Université liebre de Bruxelles: Bruxelles, Belgium, 2022. [Google Scholar]
- Weber, D. Die Niphargen (Malacostraca: Amphipoda: Niphargidae) der Schwäbischen Alb—Ergebnisse Phylogenetischer Studien. Laichinger Höhlenfreund 2022, 57, 25–35. [Google Scholar]
- Weber, D. Die Höhlenfauna und -Flora des Höhlenkatastergebietes Rheinland-Pfalz/Saarland, 4. Teil. Abhandlungen zur Karst- und Höhlenkunde; Verband der deutschen Höhlen- und Karstforscher e.V.: München, Germany, 2001; Volume 33, p. 1088. [Google Scholar]
- Weber, D. Die Evertebartenfauna der Höhlen und Künstlichen Hohlräume des Katastergebietes Westfalen Einschließlich der Quellen- und Grundwasserfauna. Abhandlungen zur Karst- und Höhlenkunde; Verband der deutschen Höhlen- und Karstforscher e.V.: München, Germany, 1991; Volume 25, p. 701. [Google Scholar]
- Weber, D.; Brad, T.; Stoch, F.; Flot, J.-F. Rediscovery and Redescription of Niphargus enslini Karaman, 1932 (Amphipoda, Niphargidae) in Southern Germany. Subterr. Biol. 2021, 40, 65–89. [Google Scholar] [CrossRef]
- Weber, D.; Flot, J.-F.; Weigand, H.; Weigand, A.M. Demographic History, Range Size and Habitat Preferences of the Groundwater Amphipod Niphargus puteanus (C.L. Koch in Panzer, 1836). Limnologica 2020, 82, 125765. [Google Scholar] [CrossRef]
- Weber, D.; Flot, J.-F. Rote Liste und Gesamtartenliste der Grundwasserkrebse (Niphargidae) des Saarlandes. In Rote Liste Gefährdeter Tiere und Pflanzen des Saarlandes, pdf-Ausgabe; Saarbrücken und Landsweiler-Reden: Schiffweiler, Germany, 2020; pp. 3–9. [Google Scholar]
- Weber, D.; Weigand, A.M. Groundwater Amphipods of the Hyporheic Interstitial: A Case Study from Luxembourg and The Greater Region. Diversity 2023, 15, 411. [Google Scholar] [CrossRef]
- Schminke, H.K. Amphipoda (Flohkrebse). In Grundwasserfauna Deutschlands. Ein Bestimmungswerk; DWA Deutsche Vereinigung für Wasserwirtschaft, Abwasser und Abfall e.V.: Hennef, Germany, 2007; pp. 239–272. [Google Scholar]
- Pust, J. Untersuchungen Zur Systematik, Morphologie und Ökologie der in Westfälischen Höhlen Vorkommenden Aquatischen Höhlentiere; Abhandlungen aus dem Westfälischen Museum für Naturkunde; Westfälisches Museum für Naturkunde: Münster, Germany, 1990; Volume 4. [Google Scholar]
- Ginet, R. Bilan Systematique Du Genre Niphargus En France; Université Claude-Bernard Lyon I: Lyon, France, 1995. [Google Scholar]
- Binczyk, K. Kupfererzbergbau Im Rahmedetal. De Rammuthe 2008, 2008, 16–20. [Google Scholar]
- Chappuis, P.A. Eine Neue Methode zur Untersuchung der Grundwasserfauna. Acta Sci. Math. Et Nat./Univ. Fr. Joseephina 1942, 6, 3–7. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Astrin, J.J.; Stüben, P.E. Phylogeny in Cryptic Weevils: Molecules, Morphology and New Genera of Western Palaearctic Cryptorhynchinae (Coleoptera: Curculionidae). Invertebr. Syst. 2008, 22, 503–522. [Google Scholar] [CrossRef]
- Verovnik, R.; Sket, B.; Trontelj, P. The Colonization of Europe by the Freshwater Crustacean Asellus aquaticus (Crustacea: Iso-poda) Proceeded from Ancient Refugia and Was Directed by Habitat Connectivity. Mol. Ecol. 2005, 14, 4355–4369. [Google Scholar] [CrossRef] [PubMed]
- Flot, J.-F.; Wörheide, G.; Dattagupta, S. Unsuspected Diversity of Niphargus Amphipods in the Chemoautotrophic Cave Ecosystem of Frasassi, Central Italy. BMC Evol. Biol. 2010, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Villesen, P. FaBox: An Online Toolbox for Fasta Sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Statist. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for Primary Species Delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble Species by Automatic Partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Fišer, C.; Trontelj, P.; Luštrik, R.; Sket, B. Towards a Unified Taxonomy of Niphargus (Crustacea: Amphipoda): A Review of Morphological Variability. Zootaxa 2009, 2061, 1–22. [Google Scholar] [CrossRef]
- Weber, D.; Brad, T.; Weigand, A.; Flot, J.-F. Water Diviners Multiplied: Cryptic Diversity in the Niphargus aquilex Species Complex in Northern Europe. Biorxiv Prepr. Zool. 2023. [Google Scholar] [CrossRef]
- Spence Bate, C. On the Genus Niphargus (Schiödte). Nat. Hist. Rev. Q. J. Sci. 1859, 6, 163–166. [Google Scholar]
- Schellenberg, A. Subterrane Amphipoden Badens, nebst einem Neuen Niphargus Aus Polen. Zool. Anz. 1936, 113, 67–73. [Google Scholar]
- Karaman, G.S. Contribution to the Knowledge of the Amphipoda 150. One New Species of Genus Niphargus (Gammaridea, Niphargidae) from France, Niphargus renei n.sp. Ann. De Limnol. 1986, 22, 17–25. [Google Scholar] [CrossRef]
- Alther, R.; Bongni, N.; Borko, Š.; Fišer, C.; Altermatt, F. Citizen Science Approach Reveals Groundwater Fauna in Switzerland and a New Species of Niphargus (Amphipoda, Niphargidae). Subterr. Biol. 2021, 39, 1–31. [Google Scholar] [CrossRef]
- Fišer, C.; Alther, R.; Zakšek, V.; Borko, Š.; Fuchs, A.; Altermatt, F. Translating Niphargus Barcodes from Switzerland into Taxonomy with a Description of Two New Species (Amphipoda, Niphargidae). ZooKeys 2018, 760, 113–141. [Google Scholar] [CrossRef]
- Zagmajster, M.; Eme, D.; Fišer, C.; Galassi, D.; Marmonier, P.; Stoch, F.; Cornu, J.-F.; Malard, F. Geographic Variation in Range Size and Beta Diversity of Groundwater Crustaceans: Insights from Habitats with Low Thermal Seasonality: Range Size and Beta Diversity in Non-Seasonal Habitats. Glob. Ecol. Biogeogr. 2014, 23, 1135–1145. [Google Scholar] [CrossRef]
- Fišer, C.; Mavrič, B.; Pekolj, A.; Zagmajster, M. Checklist of Amphipod Crustaceans (Crustacea: Amphipoda) in Slovenia. Nat. Slov. 2021, 23, 5–24. [Google Scholar]
- Rapoport, E.H. Areografía. In Estrategias Geográficas de Las Especies; Fondo de Cultura Económica: Mexico City, Mexico, 1975. [Google Scholar]
- Fišer, C.; Sket, B.; Stoch, F. Distribution of Four Narrowly Endemic Niphargus Species (Crustacea: Amphipoda) in the Western Dinaric Region with Description of a New Species. Zool. Anz. 2006, 245, 77–94. [Google Scholar] [CrossRef]
- Fišer, C.; Konec, M.; Alther, R.; Švara, V.; Altermatt, F. Taxonomic, Phylogenetic and Ecological Diversity of Niphargus (Amphipoda: Crustacea) in the Hölloch Cave System (Switzerland). Syst. Biodivers. 2017, 15, 218–237. [Google Scholar] [CrossRef]
- Borowsky, B. Responses to Light in Two Eyeless Cave Dwelling Amphipods (Niphargus ictus and Niphargus frasassianus). J. Crustac. Biol. 2011, 31, 613–616. [Google Scholar] [CrossRef]
- Angyal, D.; Balázs, G.; Zakšek, V.; Krízsik, V.; Fišer, C. Redescription of Two Subterranean Amphipods Niphargus molnari Méhely, 1927 and Niphargus gebhardti Schellenberg, 1934 (Amphipoda, Niphargidae) and their Phylogenetic Position. ZooKeys 2015, 509, 53–85. [Google Scholar] [CrossRef] [PubMed]
- Wichers, H.J. Sur Une Nouvelle Espèce d’Amphipode, Intermédiaire Entre Niphargus et Niphargellus, Du Boulonnais. Beaufortia 1964, 147, 185–198. [Google Scholar]
- McInerney, C.E.; Maurice, L.; Robertson, A.L.; Knight, L.R.F.D.; Arnscheidt, J.; Venditti, C.; Dooley, J.S.G.; Mathers, T.; Matthijs, S.; Erikkson, K.; et al. The Ancient Britons: Groundwater Fauna Survived Extreme Climate Changes over Tens of Millions of Years across NW Europe. Mol. Ecol. 2014, 23, 1153–1166. [Google Scholar] [CrossRef]
- Weber, D. Niphargus Tonywhitteni Fišer et al. 2018 Recorded for the First Time in Bavaria (Malacostraca, Amphipoda, Niphargidae); Spixiana: München, Germany, 2023. [Google Scholar]
- Weber, D.; Stoch, F.; Flot, J.-F. New Insight into the Phylogeny and Biogeography of the Subterranean Niphargus virei Clade (Amphipoda: Niphargidae) in Western Europe. In 3rd Central European Symposium for Aquatic Macroinvertebrate Research, Lodz; University of Lodz: Lodz, Poland, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).