Insights into Diatom Substrate Preferences in the Inter-Tidal Zone of a Subarctic Coast
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling and Substrates
2.3. Environmental Data
2.4. Diatom Analysis
2.5. Data Analysis
3. Results
3.1. Dominant Species
3.2. Diversity
3.3. Substrate Preferences
4. Discussion
4.1. Identified Diatom Assemblages
4.2. Significance of Substrates
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crossland, C.J.; Baird, D.; Ducrotoy, J.-P.; Lindeboom, H. The coastal zone-a domain of global interactions. In Costal Fluxes in the Anthropocene; Crossland, C.J., Kremer, H.H., Lindebomm, H.J., Marshall Crossland, J.I., Le Tissier, M.D.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–38. [Google Scholar]
- Masselink, G.; Gehrels, R. Introduction to coastal environments and global change. In Coastal Environments & Global Change; Masselink, G., Gehrels, R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–27. [Google Scholar]
- Adams, S.M. Assessing cause and effect of multiple stressors on marine systems. Mar. Pollut. Bull. 2005, 51, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Popper, A.N.; Hawkins, A.D.; Fay, R.R.; Mann, D.A.; Bartol, S.; Carlson, T.J.; Coombs, S.; Ellison, W.T.; Gentry, R.L.; Halvorsen, M.B.; et al. The nature of man-made sound. In ASA S3/SC1. 4 TR-2014 Sound Exposure Guidelines for Fishes and Sea Turtles: A Technical Report Prepared by ANSI-Accredited Standards Committee S3/SC1 and Registered with ANSI; Springer: New York, NY, USA, 2014; pp. 23–32. [Google Scholar]
- Beiras, R. Plastics and other solid wastes. In Marine Pollution: Sources, Fate and Effects of Pollutants in Marine Ecosystems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 69–88. [Google Scholar] [CrossRef]
- Oppenheimer, M.; Glavovic, B.C.; Hinkel, J.; van de Wal, R.; Magnan, A.K.; Abd-Elgawad, A.; Cai, R.; Cifuentes-Jara, M.; DeConto, R.M.; Ghosh, T.; et al. Sea Level Rise and Implications for Low-Lying Islands, Coasts and Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; pp. 321–445. [Google Scholar] [CrossRef]
- Milne, G.A. Sea level. In Coastal Environments & Global Change; Masselink, G., Gehrels, R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 28–51. [Google Scholar]
- Switzer, A.D. Coastal hazards: Storms and Tsunamis. In Coastal Environments & Global Change; Masselink, G., Gehrels, R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 104–127. [Google Scholar]
- Orr, J.C. Recent and future changes in ocean carbonate chemistry. In Ocean Acidification; Gattuso, J.-P., Hansson, L., Eds.; Oxford University Press: New York, NY, USA, 2017; pp. 41–66. [Google Scholar]
- Cheng, L.; Abraham, J.; Trenberth, K.E.; Fasullo, J.; Boyer, T.; Locarnini, R.; Zhang, B.; Yu, F.; Wan, L.; Chen, X.; et al. Upper ocean temperature hit record high in 2020. Adv. Atmos. Sci. 2021, 38, 523–530. [Google Scholar] [CrossRef]
- Chen, J.; Kang, W.; Du, W.; Guo, J.; Xu, M.; Zhang, Y.; Zhong, X.; Zhang, W.; Chen, J. Perspectives on Future Sea Ice and Navigability in the Arctic. Cryosphere 2021, 15, 5473–5482. [Google Scholar] [CrossRef]
- Afenyo, M.; Lin, Y.; Ng, A.K.Y.; Jiang, C. The opportunities and challenges of developing the Arctic area and shipping in Canada. In Artic Shipping: Climate Change Commercial Traffic and Port Development; Lasserre, F., Faury., O., Eds.; Routledge: London, UK, 2019; pp. 216–226. [Google Scholar]
- Ferrario, F.; Araújo, C.A.S.; Bélanger, S.; Bourgault, D.; Carrière, J.; Carrier-Belleau, C.; Dreujou, E.; Johnson, L.E.; Juniper, S.K.; Mabit, R.; et al. Holistic Environmental Monitoring in Ports as an Opportunity to Advance Sustainable Development, Marine Science, and Social Inclusiveness. Elem. Sci. Anthr. 2022, 10, 00061. [Google Scholar] [CrossRef]
- Desrosiers, C.; Leflaive, J.; Eulin, A.; Ten-Hage, L. Bioindicators in marine waters: Benthic diatoms as a tool to assess water quality from eutrophic to oligotrophic coastal ecosystems. Ecol. Indic. 2013, 32, 25–34. [Google Scholar] [CrossRef]
- Lavoie, I.; Hamilton, P.B.; Campeau, S.; Grenier, M.; Dillon, P.J. Guide D’identification des Diatomées des Rivières de l’Est du Canada; Presse de l’Université du Québec: Québec City, QC, Canada, 2008. [Google Scholar]
- Battarbee, R.W.; Charles, D.F.; Bigler, C.; Cumming, B.F.; Renberg, I. Diatoms as indicators of surface-water acidity. In The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Smol, J.P., Stoermer, E., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 98–121. [Google Scholar]
- Spaulding, S.A.; Potapova, M.G.; Bishop, I.W.; Lee, S.S.; Gasperak, T.S.; Jovanoska, E.; Furey, P.C.; Edlund, M.B. Diatoms.org: Supporting taxonomists, connecting communities. Diatom Res. 2021, 36, 291–304. [Google Scholar] [CrossRef]
- Lavoie, I.; Saulnier-Talbot, E. Les diatomées: Petits chef d’œuvre de la nature et microtechnologie 100% naturelle au service du biosuivi des écosystèmes aquatiques. In Vivo 2016, 36, 10–12. [Google Scholar]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar]
- Julius, M.L.; Theriot, E.C. The diatoms: A primer. In The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Smol, J.P., Stoermer, E., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 8–22. [Google Scholar]
- Battarbee, R.W.; Jones, V.J.; Flower, R.J.; Cameron, N.G.; Bennion, H.; Carvalho, L.; Juggins, S. Diatoms. In Tracking Environmental Change Using Lake Sediments, Volume 3: Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 155–202. [Google Scholar]
- Kriwy, P.; Sven, U. Microbial Diversity in Marine Biofilms along a Water Quality Gradient on the Great Barrier Reef. Syst. Appl. Microbiol. 2011, 34, 116–126. [Google Scholar] [CrossRef]
- Gremmen, N.; Van de Vijver, B.; Frenot, Y.; Lebouvier, M. Distribution of moss-inhabiting diatoms along an altitudinal gradient at Sub-Antarctic Îles Kerguelen. Antarct. Sci. 2007, 19, 17–24. [Google Scholar] [CrossRef]
- Jewson, D.H.; Lowry, S.F.; Bowen, R. Co-existence and survival of diatoms on sand grains. Eur. J. Phycol. 2006, 41, 131–146. [Google Scholar] [CrossRef]
- Vos, P.C.; de Wolf, H. Geologie en diatomeeën. Grondboor En Hamer 1988, 42, 57–68. [Google Scholar]
- Bere, T.; Tundisi, J.G. The Effects of Substrate Type on Diatom-Based Multivariate Water Quality Assessment in a Tropical River (Monjolinho), São Carlos, SP, Brazil. Water Air Soil Pollut. 2011, 216, 391–409. [Google Scholar] [CrossRef]
- Potapova, M.; Charles, D.F. Choice of Substrate in Algae-Based Water-Quality Assessment. J. N. Am. Benthol. Soc. 2005, 24, 415–427. [Google Scholar] [CrossRef]
- Lapointe, M. Modern diatom assemblages in surface sediments from the maritime estuary and the Gulf of St. Lawrence, Québec (Canada). Mar. Micropaleontol. 2000, 40, 43–65. [Google Scholar] [CrossRef]
- Carrière, J.; Le Hénaff, A.; Demers, K.A. Description du site d’étude. In Observatoire Environnemental de la Baie de Sept-Îles; Carrière., J., Ed.; INREST: Sept-Îles, QC, Canada, 2018; Volume 1, pp. 47–79. [Google Scholar]
- Demers, K.A.; Le Hénaff, A.; Carrière, J. État des glaces. In Observatoire Environnemental de la Baie de Sept-Îles; Carrière, J., Ed.; INREST: Sept-Îles, QC, Canada, 2018; Volume 1, pp. 593–612. [Google Scholar]
- Bourque, M.; Malouin, J. Guide D’intervention en Matière de Conservation et de Mise en Valeur Des habitats Littoraux de la MRC de Sept-Rivières; Comité ZIP Côte-Nord du Golfe: Sept-Îles, QC, Canada, 2009; pp. 1–1555. [Google Scholar]
- Bernatchez, P.; Fraser, C.; Friesinger, S.; Jolivet, Y.; Dugas, S.; Drejza, S.; Morissette, A. Sensibilité des côtes et Vulnérabilité des Communautés du Golfe du Saint-Laurent aux Impacts des Changements Climatiques; Rapport de recherche remis au Consortium OURANOS et au FACC; Laboratoire de Dynamique et de Gestion Intégrée des Zones Côtières, UQAR: Rimouski, QC, Canada, 2008. [Google Scholar]
- Dreujou, E.; McKindsey, C.W.; Grant, C.; Tréau de Coeli, L.; St-Louis, R.; Archambault, P. Biodiversity and habitat assessment of coastal benthic communities in a sub-arctic industrial harbor area. Water 2020, 12, 2424. [Google Scholar] [CrossRef]
- Shaw, J.-L.; Bourgault, D.; Dumont, D.; Lefaivre, D. Hydrodynamics of the Bay of Sept-Îles. Atmos.-Ocean. 2023, 61, 105–121. [Google Scholar] [CrossRef]
- Shaw, J.-L.; Bourgault, D.; Dumont, D. Hydrodynamique de la Baie de Sept-Îles. Master’s Thesis, Université du Québec à Rimouski, Rimouski, QC, Canada, 2019. [Google Scholar]
- Fisheries and Ocean Canada. 2023. Sept-Îles-02780. Available online: https://www.marees.gc.ca/fr/stations/02780 (accessed on 25 April 2023).
- Carrière, J.; Le Hénaff, A. Mise en contexte. In Observatoire Environnemental de la Baie de Sept-Îles; Carrière, J., Ed.; INREST: Sept-Îles, QC, Canada, 2018; Volume 1, pp. 35–42. [Google Scholar]
- Purmer. (s.d.). Available online: http://ferme-purmer.com/ (accessed on 2 June 2022).
- Port of Sept-Îles. Available online: https://www.portsi.com/port/ (accessed on 25 March 2023).
- Beauchesnes, D.; Daigle, R.M.; Vissault, S.; Gravel, D.; Bastien, A.; Bélanger, S.; Bernatchez, P.; Blais, M.; Bourdages, H.; Chion, C.; et al. Characterizing exposure to and sharing knowledge of drivers of environmental change in the St. Lawrence System in Canada. Front. Mar. Sci. 2020, 7, 383. [Google Scholar] [CrossRef]
- Wu, X.; de Vernal, A.; Fréchette, B.; Moros, M.; Perner, K. The signal of climate changes over the last two millennia in the Gulf of St. Lawrence, eastern Canada. Quat. Res. 2021, 106, 28–43. [Google Scholar] [CrossRef]
- Carrière, J.; Le Hénaff, A.; Demers, K.A. Qualité de l’eau. In Observatoire Environnemental de la Baie de Sept-Îles; Carrière, J., Ed.; INREST: Sept-Îles, QC, Canada, 2018; Volume 1, pp. 83–211. [Google Scholar]
- Saint-Louis, R.; Montero-Serrano, J.-C. Qualité des sédiments. In Observatoire Environnemental de la Baie de Sept-Îles; Carrière, J., Ed.; INREST: Sept-Îles, QC, Canada, 2018; Volume 1, pp. 213–374. [Google Scholar]
- Saulnier-Talbot, É.; Pienitz, R. Isolation au postglaciaire d’un bassin côtier près de Kuujjuaraapik-Whapmagoostui, en Hudsonie (Québec): Une analyse biostratigraphique diatomifère. Géographie Phys. Et Quat. 2001, 55, 63–74. [Google Scholar] [CrossRef]
- Campeau, S.; Pienitz, R.; Héquette, A. Diatoms as quantitative paleodepth indicators in coastal areas of the southeastern Beaufort Sea, Arctic Ocean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 146, 67–97. [Google Scholar] [CrossRef]
- Nevrova, E.L.; Petrov, A. Benthic diatoms species richness at Dvuyakornaya Bay and other coastal sites of Crimea (the Black Sea) under various environments. Mediterr. Mar. Sci. 2019, 20, 506–520. [Google Scholar] [CrossRef]
- Poulin, M.; Bérard-Therriault, L.; Cardinal, A. Les diatomées benthiques de substrats durs des eaux marines et saumâtres du Québec. 1.Cocconeioideae (Achnanthales, Achnanthaceae). Nat. Can. 1984, 111, 45–61. [Google Scholar]
- Cardinal, A.; Poulin, M.; Bérard-Therriault, L. Les diatomées benthiques de substrats durs des eaux marines et saumâtres du Québec. 4.Naviculales, Naviculaceae (à l’exclusion des genres Donkinia, Gyrosygma et Pleurosigma. Nat. Can. 1984, 111, 369–394. [Google Scholar]
- Poulin, M.; Bérard-Therriault, L.; Cardinal, A.; Hamilton, P.B. Les diatomées (Bacillariophyta) benthiques de substrats durs des eaux marines et saumâtres du Québec. 9. Bacillariaceae. Nat. Can. 1990, 117, 73–101. [Google Scholar]
- Snoeijs, P. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1993; Volume 1. [Google Scholar]
- Snoeijs, P.; Vilbaste, S. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1994; Volume 2. [Google Scholar]
- Snoeijs, P.; Potapova, M. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1995; Volume 3. [Google Scholar]
- Snoeijs, P.; Kasperovičienė, J. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1996; Volume 4. [Google Scholar]
- Snoeijs, P.; Balashova, N. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1998; Volume 5. [Google Scholar]
- Campeau, S.; Pienitz, R.; Héquette, A. Diatoms from the Beaufort Sea Coast, Southern Arctic Ocean (Canada); Bibliotheca Diatomologica: Stuttgart, Germany, 1999. [Google Scholar]
- Pienitz, R.; Fedje, D.; Poulin, M. Marine and Non-Marine Diatoms from the Haida Gwaii Archipelago and Surroundings Coasts, Northeastern Pacific, Canada; Bibliotheca Diatomologica: Stuttgart, Germany, 2003. [Google Scholar]
- Park, J.S.; Lee, S.D.; Kang, S.E.; Lee, J.H. New records of the marine pennate diatoms in Korea. J. Ecol. Environ. 2014, 37, 231–244. [Google Scholar] [CrossRef][Green Version]
- Lee, S.D.; Yun, S.M.; Park, J.S.; Lee, J.H. Floristic survey of diatom in the Three Islands (Baeknyeong, Daecheong, Socheong) from Yellow Sea of Korea. J. Ecol. Environ. 2015, 38, 563–598. [Google Scholar] [CrossRef]
- Fallu, M.-A.; Pienitz, R. Diatomées lacustres de Jamésie-Hudsonie (Québec) et modèle de reconstitution des concentrations de carbone organique dissous. Écoscience 1999, 6, 603–620. [Google Scholar] [CrossRef]
- Lavoie, I.; Dillon, P.J.; Campeau, S. The effect of excluding diatom taxa and reducing taxonomic resolution on multivariate analyses and stream bioassessment. Ecol. Indic. 2009, 9, 213–225. [Google Scholar] [CrossRef]
- Wachnicka, A.; Gaiser, E.; Collins, L.; Frankovich, T.; Boyer, J. Distribution of diatoms and development of diatom-based models for inferring salinity and nutrient concentrations in Florida Bay and adjacent coastal wetlands of South Florida (USA). Estuaries Coasts 2010, 33, 1080–1098. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package, Version 2.6-4; R: Vienna, Austria, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 3 March 2023).
- Bray, J.R.; Curtis, J.T. An ordination for the upland forest of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Juggins, S.; Telford, R.J. Exploratory data analysis and data display. In Tracking Environmental Change Using Lake Sediments, Volume 5: Data Handling and Numerical Techniques; Birks, H.J.B., Lotter, A.F., Juggins, S., Smol, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 123–142. [Google Scholar]
- Hassan, G.S.; Marcela, A.E.; Federico, I.I. Diatom-based inference model for paleosalinity reconstructions in estuaries along the northeastern coast of Argentina. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 275, 77–91. [Google Scholar] [CrossRef]
- Birks, H.J.B. Numerical methods for the analysis of diatom assemblage data. In The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Smol, J.P., Stoermer, E., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 23–54. [Google Scholar]
- Weckström, K.; Juggins, S. Coastal diatom-environment relationship from the Gulf of Finland, Baltic Sea. J. Phycol. 2006, 42, 21–35. [Google Scholar] [CrossRef]
- ter Braak, C.J.; Verdonschot, P.F. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Weckström, K.; Korhola, A.; Shemeikka, P. Physical and chemical characteristics of shallow embayments on the southern coast of Finland. Hydrobiologia. 2002, 477, 115–127. [Google Scholar] [CrossRef]
- Teittinen, A.; Virta, L.; Li, M.; Wang, J. Factors influencing the biodiversity of three microbial groups within and among islands of the Baltic Sea. FEMS Microbiol. Ecol. 2021, 97, fiab049. [Google Scholar] [CrossRef]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom Flora of Marine Coasts I. Iconographia Diatomologica; Lange-Bertalot, H., Ed.; Koeltz Scientific Books: Königstein, Germany, 2000; Volume 7. [Google Scholar]
- Vilbaste, S.; Sundbäck, K.; Nilsson, C.; Truu, J. Distribution of benthic diatoms in the littoral zone of the Gulf of Riga, the Baltic Sea. Eur. J. Phycol. 2000, 35, 373–385. [Google Scholar] [CrossRef]
- Van der Werff, A.; Huls, H. Diatomeeënflora van Nederland (Diatom Flora of the Netherlands); Otto Koeltz Science Publishers: Koenigstein, Germany, 1976. [Google Scholar]
- Vos, P.C.; de Wolf, H. Methodological aspects of paleo-ecological diatom research in coastal areas of the Netherlands. Geol. En Mijnb. 1988, 67, 31–40. [Google Scholar]
- Ribeiro, L. Intertidal Benthic Diatoms of the Tagus Estuary: Taxonomic Composition and Spatial-Temporal Variation. Ph.D. Thesis, Lisbon University, Lisbon, Portugal, 2010. [Google Scholar]
- Al-Yamani, F.Y.; Saburova, M.A. Illustrated Guide on the Benthic Diatoms of Kuwait’s Marine Environment; Kuwait Institute for Scientific Research: Safat, Kuwait, 2011. [Google Scholar]
- Chung, M.H.; Lee, K.-S. Species composition of the epiphytic diatoms on the leaf tissues of three zostera species distributed on the Southern Coast of Korea. Algae 2008, 23, 75–81. [Google Scholar] [CrossRef]
- Hall, R.I.; Smol, J.P. Diatoms as indicators of lake eutrophication. In The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Smol, J.P., Stoermer, E., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 122–151. [Google Scholar]
- Busse, S.; Snoeijs, P. Gradient responses of diatom communities in the Bothnian Sea (northern Baltic Sea), with emphasis on responses to water movement. Phycologia 2003, 42, 451–464. [Google Scholar] [CrossRef]
- Cetin, A.K. Epilithic, Epipelic, and Epiphytic Diatoms in the Göksu Stream: Community Relationships and Habitat Preferences. J. Freshw. Ecol. 2008, 23, 143–149. [Google Scholar] [CrossRef]
- Antoniades, D.; Douglas, M.S.V.; Smol, J.P. Biogeographic distributions and environmental controls of stream diatoms in the Canadian Arctic Archipelago. Botany 2008, 87, 443–454. [Google Scholar] [CrossRef]
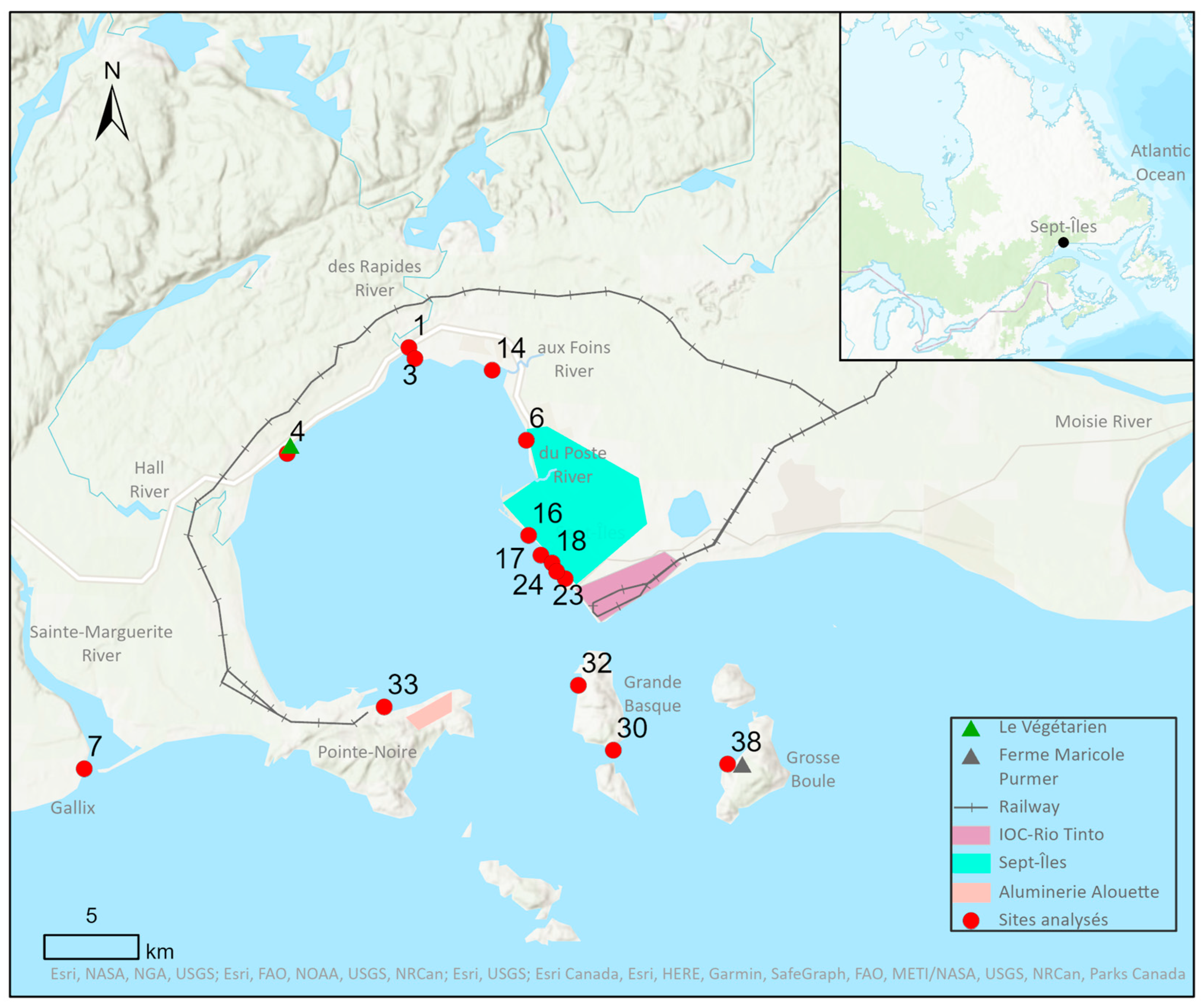

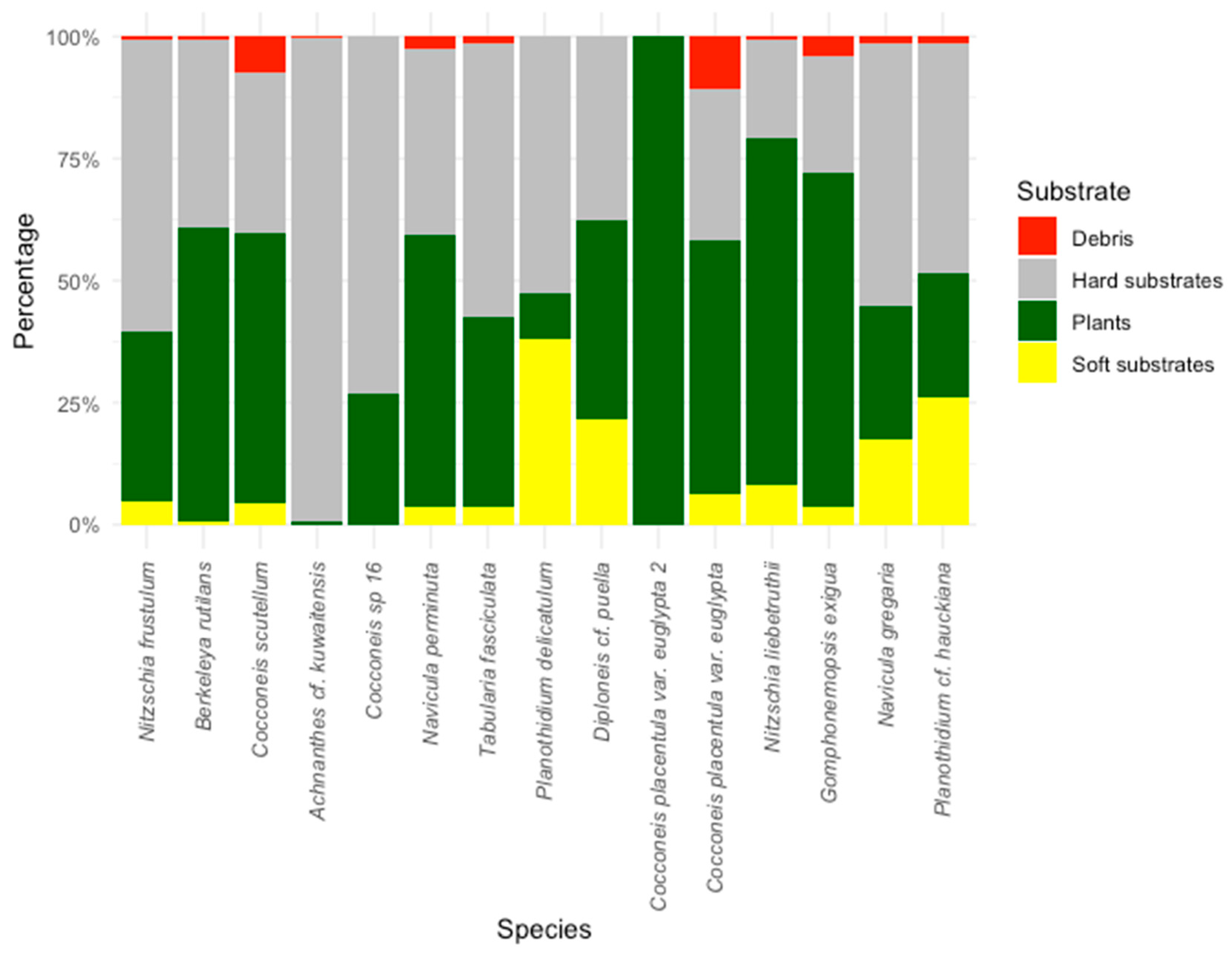
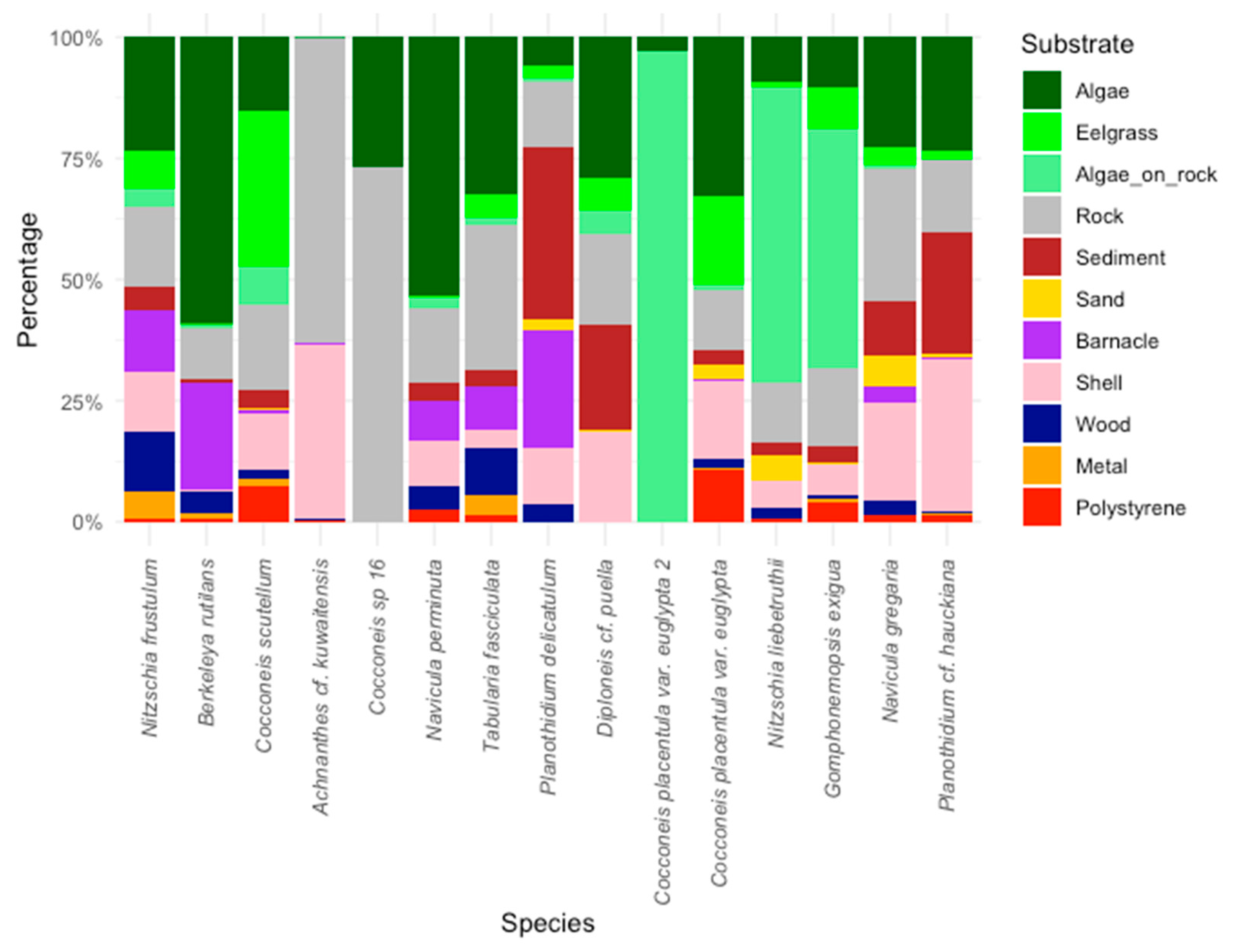
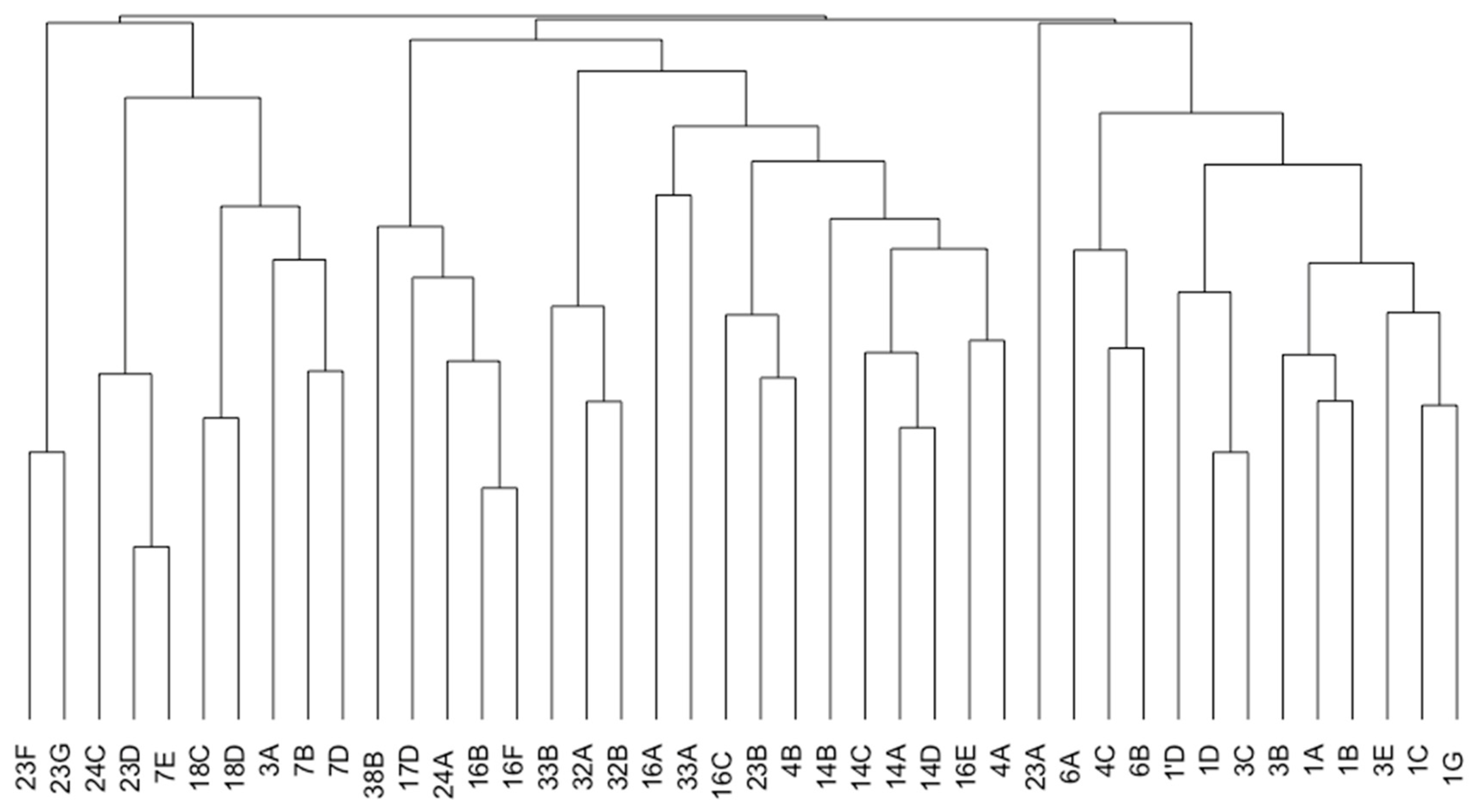
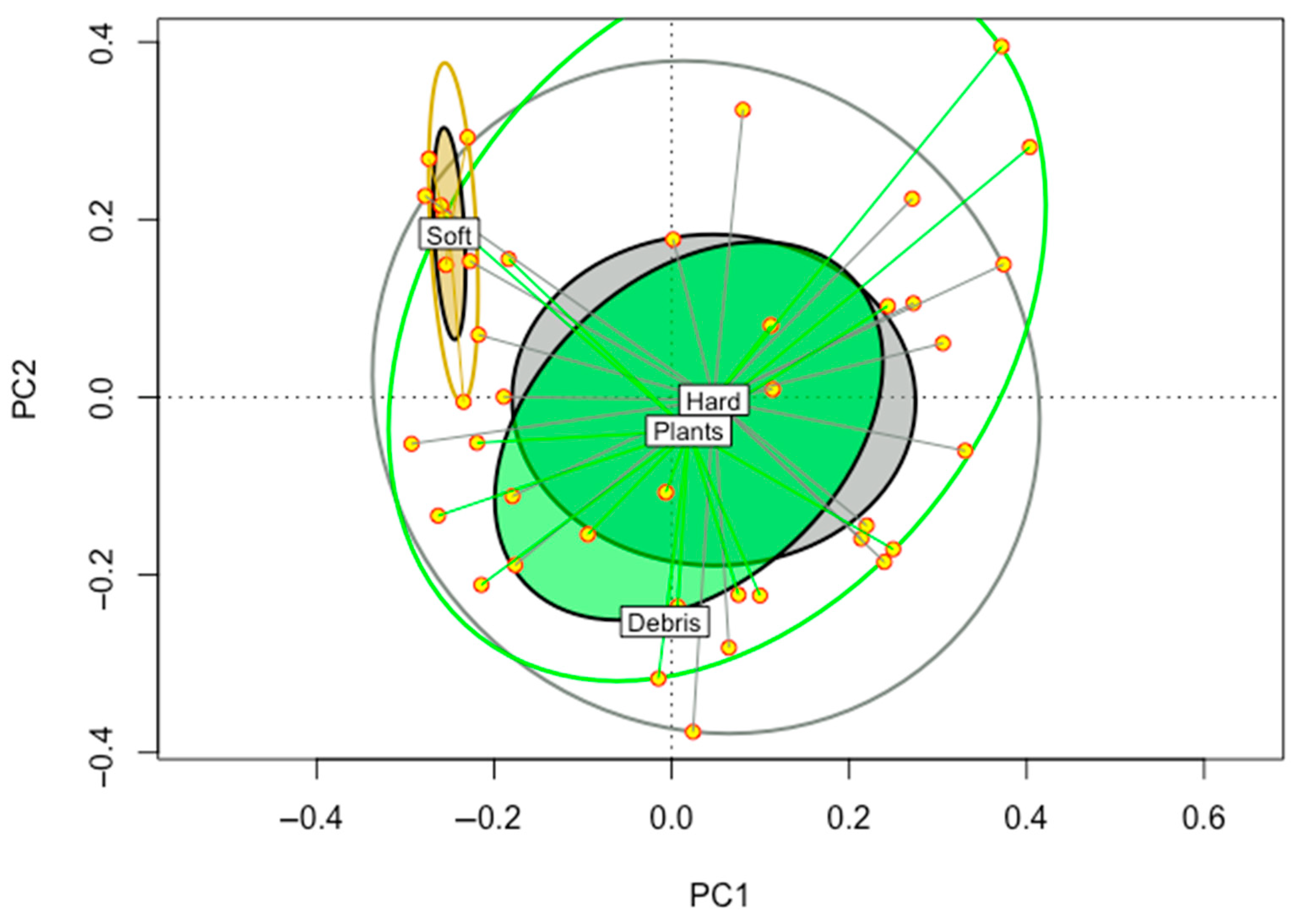
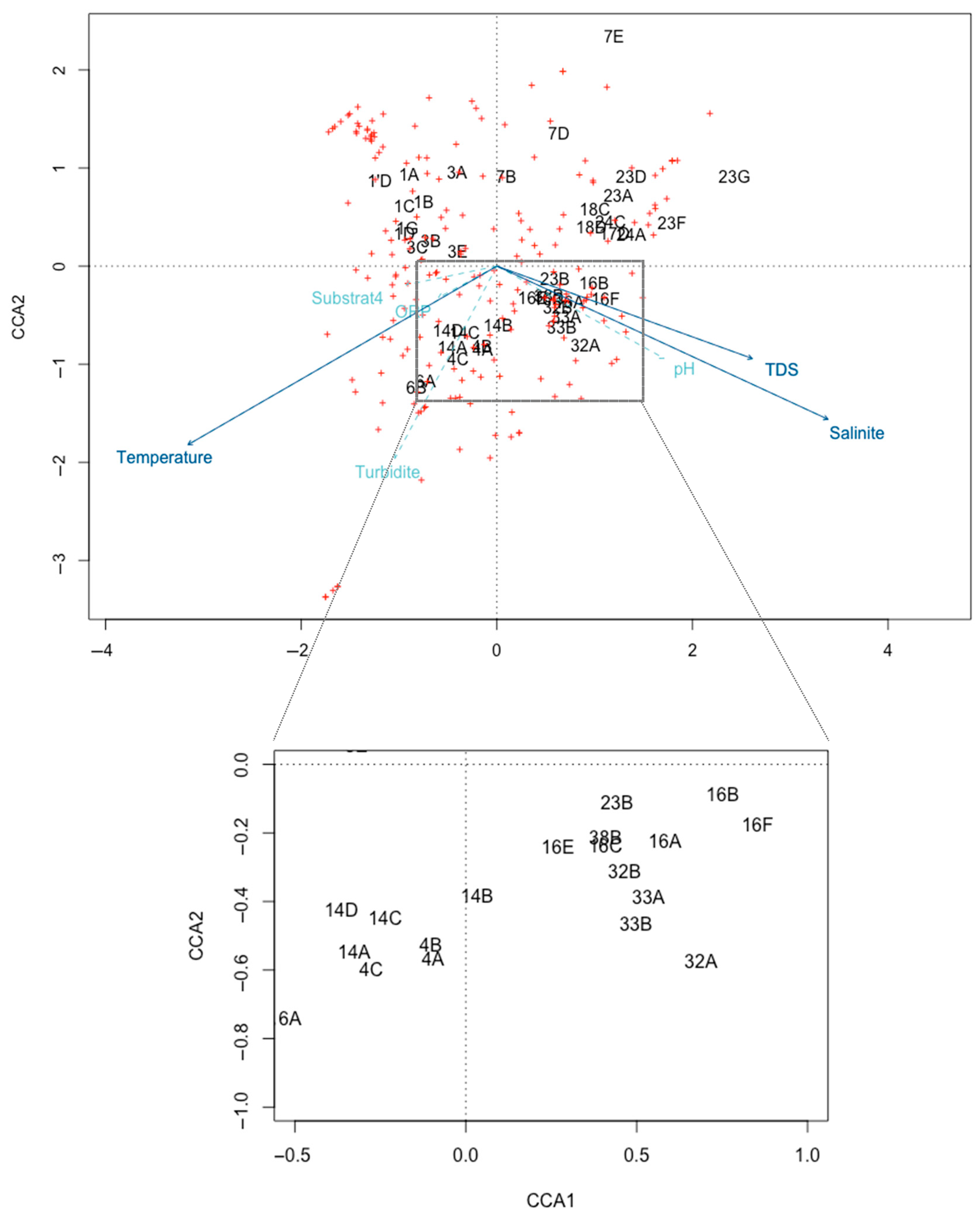
| Sites | 1 | 3 | 4 | 6 | 7 | 14 | 16 | 17 | 18 | 23 | 24 | 32 | 33 | 38 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Types of collected substrates | Algae, eelgr. 1, rock, shell, sed. 2, sand | Wood, rock, sed., algae | Shell, eelgr., sed. | Shell, sed. | Rock, barn. 3, algae | Algae, shell, rock, algae-r. 4 | Algae-r., wood, eelgr., rock | Metal | Algae, rock | Algae-r., styrof. 5, algae, shell, rock | Algae, barn. | Rock, algae | Algae-r., rock | Barn. |
| pH | 8.0 | 6.4 | 8.1 | 7.7 | 7.4 | 7.6 | 8.1 | 8.3 | 8.1 | 8.1 | 8.3 | 8.6 | 8.4 | 8.4 |
| ORP (mV) | 208.4 | 231.9 | 184.1 | 219.93 | 184.6 | 187.5 | 216.57 | 175.9 | 201.3 | 179.0 | 190.9 | 208.5 | 304.1 | 204.1 |
| TDS (g/L) | 12.5 | 1.9 | 17.4 | 15.34 | 7.8 | 5.8 | 21.38 | 21.7 | 19.7 | 21.2 | 21.5 | 20.0 | 20.1 | 22.0 |
| Salinity (PSU) | 2.6 | 2.0 | 21.90 | 17.04 | 8.3 | 6.4 | 27.63 | 28.0 | 25.2 | 27.3 | 27.7 | 25.7 | 26.0 | 28.4 |
| Turbidity (FNU) | 5.3 | 10.5 | 14.0 | 31.13 | 5.5 | 60.3 | 42.50 | 1.9 | 1.7 | 7.7 | 1.7 | 0.0 | 1.10 | 10.00 |
| Water temp. 6 (°C) | 20.5 | 17.5 | 21.4 | 25.46 | 15.8 | 21.5 | 14.60 | 14.6 | 15.5 | 15.5 | 14.7 | 19.2 | 17.5 | 17.7 |
| Taxa | Proportion (%) |
|---|---|
| Nitzschia frustulum | 9.62 |
| Berkeleya rutilans | 7.27 |
| Achnanthes cf. kuwaitensis | 3.75 |
| Cocconeis sp. 16 | 3.52 |
| Navicula perminuta | 3.30 |
| Tabularia fasciculata | 3.00 |
| Planothidium delicatulum | 2.45 |
| Diploneis cf. puella | 2.35 |
| Cocconeis var. euglypta 2 | 2.12 |
| Cocconeis placentula var. euglypta | 2.12 |
| Nitzschia liebetruthii | 2.07 |
| Gomphonemopsis exigua | 2.03 |
| Navicula gregaria | 1.94 |
| Planothidium cf. hauckiana | 1.83 |
| Taxa | Frequency (%) |
|---|---|
| Nitzschia frustulum | 90.48 |
| Tabularia fasciculata | 90.48 |
| Cocconeis scutellum | 83.33 |
| Cocconeis costata | 76.19 |
| Navicula perminuta | 76.19 |
| Berkeleya rutilans | 73.81 |
| Gomphonemopsis exigua | 69.05 |
| Navicula sp. 102 | 69.05 |
| Thalassionema nitzschioides | 69.05 |
| Achnanthidium delicatulum | 66.67 |
| Cocconeis placentula var. euglypta | 64.29 |
| Nitzschia palea | 64.28 |
| Planothidium cf. hauckiana | 61.90 |
| Navicula gregaria | 61.90 |
| Nitzschia dissipata | 59.24 |
| Navicula group 1 (gidle view) | 57.14 |
| Grammatophora oceanica | 57.14 |
| Navicula phyllepta shape 1 | 52.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arseneault, E.; Pienitz, R.; Carrière, J.; Saulnier-Talbot, É. Insights into Diatom Substrate Preferences in the Inter-Tidal Zone of a Subarctic Coast. Hydrobiology 2023, 2, 537-553. https://doi.org/10.3390/hydrobiology2040036
Arseneault E, Pienitz R, Carrière J, Saulnier-Talbot É. Insights into Diatom Substrate Preferences in the Inter-Tidal Zone of a Subarctic Coast. Hydrobiology. 2023; 2(4):537-553. https://doi.org/10.3390/hydrobiology2040036
Chicago/Turabian StyleArseneault, Emilie, Reinhard Pienitz, Julie Carrière, and Émilie Saulnier-Talbot. 2023. "Insights into Diatom Substrate Preferences in the Inter-Tidal Zone of a Subarctic Coast" Hydrobiology 2, no. 4: 537-553. https://doi.org/10.3390/hydrobiology2040036
APA StyleArseneault, E., Pienitz, R., Carrière, J., & Saulnier-Talbot, É. (2023). Insights into Diatom Substrate Preferences in the Inter-Tidal Zone of a Subarctic Coast. Hydrobiology, 2(4), 537-553. https://doi.org/10.3390/hydrobiology2040036






