Total Mass Flux in the Northern Humboldt Current System: Rates and Contribution Sources from Central Peru (12° S)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Automatic Sediment Trap Deployment and Recovery
2.3. Samples Splitting
2.3.1. Aliquot “A”
Zooplankton Picking
Filtering and Weighing of Particulate Matter
2.3.2. Aliquot “B”
Collection, Measurement, and Characterization of Fecal Pellets
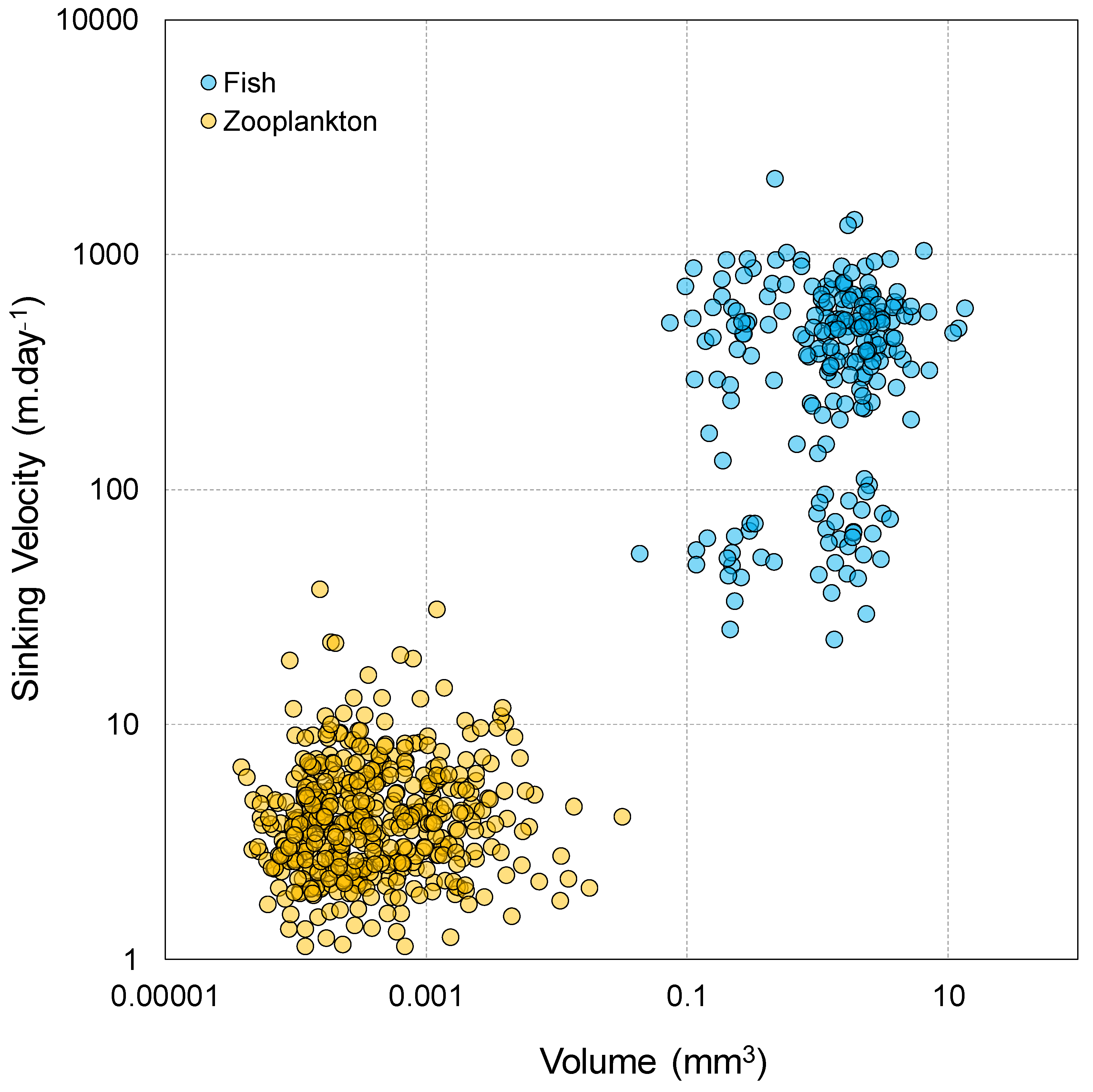
2.4. Sinking Velocity
3. Results
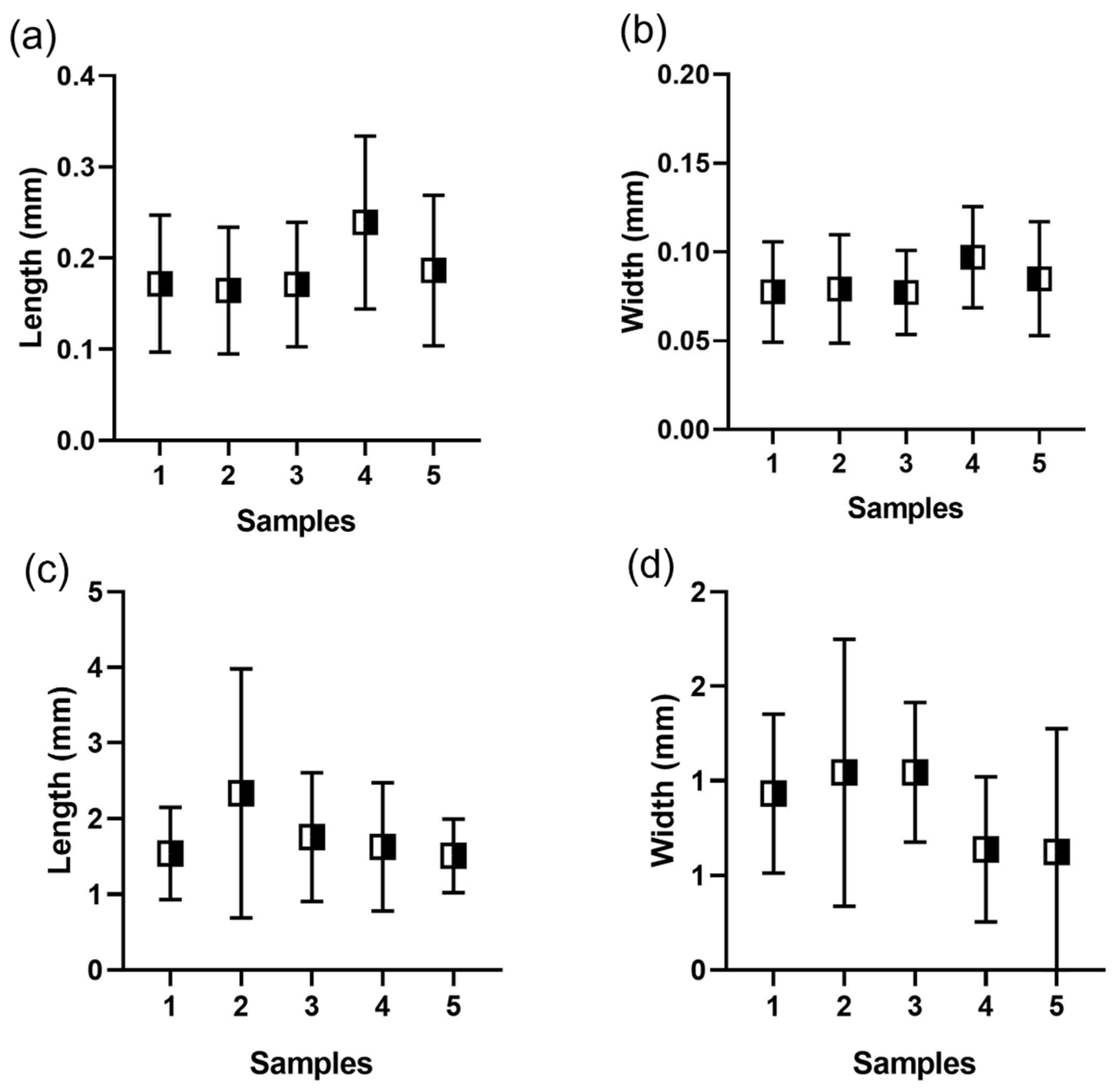
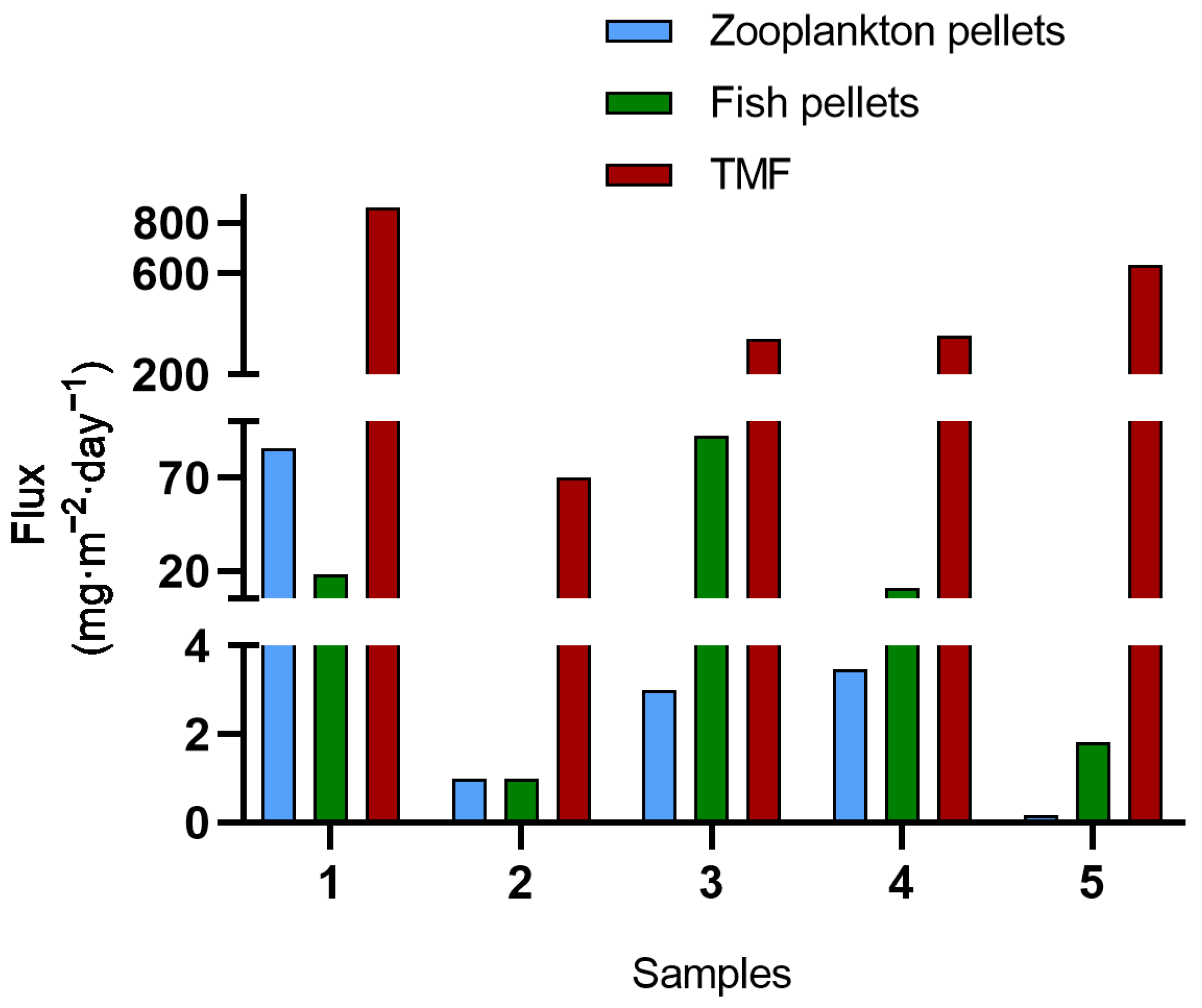
3.1. Fecal Pellets Count, Sources, and Contribution to TMF
3.2. TMFs, Fecal Pellets Flux, and Sinking Velocities
4. Discussion
4.1. Contrasting TMF Estimates
4.2. TMF Rates and Fecal Pellets Contribution
4.3. Fecal Pellets Quantification and Attributes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | Length Range (mm) | Length Mean (mm) | Width Range (mm) | Width Mean (mm) |
|---|---|---|---|---|
| Zooplankton fecal pellets | ||||
| 1 | 0.068–0.530 | 0.17 ± 0.08 | 0.038–0.207 | 0.08 ± 0.03 |
| 2 | 0.080–0.366 | 0.16 ± 0.07 | 0.039–0.143 | 0.08 ± 0.03 |
| 3 | 0.088–0.440 | 0.17 ± 0.07 | 0.045–0.150 | 0.08 ± 0.02 |
| 4 | 0.112–0.611 | 0.24 ± 0.09 | 0.045–0.189 | 0.09 ± 0.03 |
| 5 | 0.090–0.569 | 0.19 ± 0.08 | 0.042–0.279 | 0.09 ± 0.03 |
| Fish fecal pellets | ||||
| 1 | 0.628–2.799 | 1.54 ± 0.61 | 0.43–1.795 | 0.93 ± 0.42 |
| 2 | 1.170–3.498 | 2.33 ± 1.65 | 0.544–1.543 | 1.04 ± 0.71 |
| 3 | 0.738–6.070 | 1.76 ± 0.85 | 0.345–1.836 | 1.04 ± 0.37 |
| 4 | 0.814–5.227 | 1.63 ± 0.85 | 0.260–1.601 | 0.64 ± 0.38 |
| 5 | 0.808–4.099 | 1.51 ± 0.49 | 0.643–2.796 | 1.32 ± 0.33 |
| Sample | Producer | Weight (Aliquot) (mg) | Weight (Sample) (mg) |
|---|---|---|---|
| 1 | Zooplankton | 68.8 | 83.2 |
| Fish | 14.4 | ||
| 2 | Zooplankton | 0.8 | 1.6 |
| Fish | 0.8 | ||
| 3 | Zooplankton | 2.4 | 76.8 |
| Fish | 74.4 | ||
| 4 | Zooplankton | 2.8 | 11.6 |
| Fish | 8.8 | ||
| 5 | Zooplankton | 3.2 | 38.4 |
| Fish | 35.2 |
| Samples | Total Mass Flux (mg·m−2·day−1) |
|---|---|
| 1 | 860.9 |
| 2 | 70.2 |
| 3 | 343.4 |
| 4 | 354.8 |
| 5 | 634.5 |
| Mean flux | 601.9 |
| Sample | Source | Pellets Mass Flux (mg·m−2·day−1) | Contribution to TMF (%) |
|---|---|---|---|
| 1 | Zooplankton | 85.59 | 9.94 |
| 1 | Fish | 17.91 | 2.08 |
| 2 | Zooplankton | 1 | 1.42 |
| 2 | Fish | 1 | 1.42 |
| 3 | Zooplankton | 2.99 | 0.87 |
| 3 | Fish | 92.56 | 26.95 |
| 4 | Zooplankton | 3.48 | 0.98 |
| 4 | Fish | 10.95 | 3.09 |
| 5 | Zooplankton | 0.17 | 0.03 |
| 5 | Fish | 1.83 | 0.29 |
References
- Karl, D.M.; Knauer, G.A.; Martin, J.H. Downward flux of particulate organic matter in the ocean: A particle decomposition paradox. Nature 1988, 332, 438–441. [Google Scholar] [CrossRef]
- Dale, A.W.; Graco, M.; Wallmann, K. Strong and Dynamic Benthic-Pelagic Coupling and Feedbacks in a Coastal Upwelling System (Peruvian Shelf). Front. Mar. Sci. 2017, 4, 29. [Google Scholar] [CrossRef]
- Giordani, P.; Helder, W.; Koning, E.; Miserocchi, S.; Danovaro, R.; Malaguti, A. Gradients of benthic–pelagic coupling and carbon budgets in the Adriatic and Northern Ionian Sea. J. Mar. Syst. 2002, 33–34, 365–387. [Google Scholar] [CrossRef]
- Griffiths, J.R.; Kadin, M.; Nascimento, F.J.A.; Tamelander, T.; Törnroos, A.; Bonaglia, S.; Bonsdorff, E.; Brüchert, V.; Gårdmark, A.; Järnström, M.; et al. The importance of benthic-pelagic coupling for marine ecosystem functioning in a changing world. Glob. Change Biol. 2017, 23, 2179–2196. [Google Scholar] [CrossRef]
- Echevin, V.; Aumont, O.; Ledesma, J.; Flores, G. The seasonal cycle of surface chlorophyll in the Peruvian upwelling system: A modelling study. Prog. Oceanogr. 2008, 79, 167–176. [Google Scholar] [CrossRef]
- Górska, B.; Soltwedel, T.; Schewe, I.; Włodarska-Kowalczuk, M. Bathymetric trends in biomass size spectra, carbon demand, and production of Arctic benthos (76-5561 m, Fram Strait). Prog. Oceanogr. 2020, 186, 102370. [Google Scholar] [CrossRef]
- Levin, L.A. Oxygen minimum zone benthos: Adaptation and community response to hypoxia. In Oceanography and Marine Biology; Gibson, R.N., Atkinson, R.J.A., Eds.; CRC Press: London, UK, 2003; Volume 41, pp. 1–45. [Google Scholar]
- Kalvelage, T.; Lavik, G.; Lam, P.; Contreras, S.; Arteaga, L.; Löscher, C.R.; Oschlies, A.; Paulmier, A.; Stramma, L.; Kuypers, M.M. Nitrogen cycling driven by organic matter export in the South Pacific oxygen minimum zone. Nat. Geosci. 2013, 6, 228–234. [Google Scholar] [CrossRef]
- van der Heijden, L.H.; Niquil, N.; Haraldsson, M.; Asmus, R.M.; Pacella, S.R.; Graeve, M.; Rzeznik-Orignac, J.; Asmus, H.; Saint-Béat, B.; Lebreton, B. Quantitative food web modeling unravels the importance of the microphytobenthos-meiofauna pathway for a high trophic transfer by meiofauna in soft-bottom intertidal food webs. Ecol. Model. 2020, 430, 109129. [Google Scholar] [CrossRef]
- Schukat, A.; Hagen, W.; Dorschner, S.; Correa, J.; Pinedo, E.L.; Ayón, P.; Auel, H. Zooplankton ecological traits maximize the trophic transfer efficiency of the Humboldt Current upwelling system. Prog. Oceanogr. 2021, 193, 102551. [Google Scholar] [CrossRef]
- Karstensen, J.; Ulloa, O. Peru-Chile current system. Earth Syst. Environ. Sci. 2009, 3, 437–444. [Google Scholar] [CrossRef]
- Chaigneau, A.; Dominguez, N.; Eldin, G.; Vasquez, L.; Flores, R.; Grados, C.; Echevin, V. Near-coastal circulation in the Northern Humboldt Current System from shipboard ADCP data. J. Geophys. Res. 2013, 118, 5251–5266. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Swartzman, G.; Bertrand, A.; Bertrand, S. Anchovy (Engraulis ringens) and sardine (Sardinops sagax) spatial dynamics and aggregation patterns in the Humboldt Current ecosystem, Peru, from 1983–2003. Fish. Oceanogr. 2007, 16, 155–168. [Google Scholar] [CrossRef]
- Carr, M.E.; Kearns, E.J. Production regimes in four Eastern Boundary Current systems. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 3199–3221. [Google Scholar] [CrossRef]
- Spinrad, R.W.; Glover, H.; Ward, B.B.; Codispoti, L.A.; Kullenberg, G. Suspended particle and bacterial maxima in Peruvian coastal waters during a cold water anomaly. Deep-Sea Res. Part Oceanogr. Res. Pap. 1989, 36, 715–733. [Google Scholar] [CrossRef]
- Ochoa, N.; Taylor, M.H.; Purca, S.; Ramos, E. Intra- and interannual variability of nearshore phytoplankton biovolume and community changes in the northern Humboldt Current system. J. Plankton Res. 2010, 32, 843–855. [Google Scholar] [CrossRef]
- Ledesma, J.; Tam, J.; Graco, M.; León, V.; Gonzáles, G.F.; Morón, O. Caracterización de la Zona de Mínimo de Oxígeno (ZMO) frente a la costa peruana entre 3° N y 14° S, 1999–2009. Bol. Inst. Mar Perú 2011, 26, 49–57. [Google Scholar]
- Velazco, F.; Mendoza, U.; Solís, J.; Fernandez, E.; Caquineau, S.; Sifeddine, A.; Graco, M.; Bouloubassi, I.; Turcq, B.; Leigh, B.; et al. Flujos de material particulado y formación de una lámina de sedimentos en la plataforma continental interna frente al Callao durante El Niño Costero 2017. Bol. Inst. Mar Perú. 2021, 36, 441. [Google Scholar] [CrossRef]
- Aramayo, V.; Romero, D.; Quipúzcoa, L.; Graco, M.; Marquina, R.; Solís, J.; and Velazco, F. Respuestas del bentos marino frente a El Niño costero 2017 en la plataforma continental de Perú central (Callao, 12° S). Bol. Inst. Mar. Perú 2021, 36, 476–509. [Google Scholar] [CrossRef]
- Tarazona, J.; Arntz, W. The Peruvian Coastal Upwelling System. In Coastal Marine Ecosystems of Latin America; Seeliger, U., Kjerfve, B., Eds.; Springer: Berlin, Germany, 2001; Volume 144, pp. 229–244. [Google Scholar] [CrossRef]
- Graco, M.; Anculle, T.; Aramayo, V.; Bernales, A.; Carhuapoma, W.; Correa, D.; Fernández, E.; Díaz, W.; Ledesma, J.; Marquina, R.; et al. Análisis de las condiciones oceanográficas y biológicas del afloramiento costero frente a Callao en períodos contrastantes durante el 2018. Bol. Inst. Mar Perú 2019, 34, 519–543. [Google Scholar]
- Velazco, F. Sedimentos marinos superficiales en la bahía del Callao, Perú. 1997. Bol. Inst. Mar Perú 2011, 26, 75–82. [Google Scholar]
- Velazco, F.; Solís, J.; Delgado, C.; Gomero, R. Sedimentos superficiales y morfología de la plataforma y talud continental superior entre 3°30′ S y 15°30′ S, Perú. Bol. Inst. Mar Perú. 2015, 42, 526–537. [Google Scholar]
- Mendoza, U.; Ayón, P.; Leigh, B.; Oyola, W.; Bach, L.; Caquineau, S.; Bouloubassi, I.; Velazco, F.; Turck, B.; Sifeddine, A.; et al. Procedimientos para división y retirada de nadadorespellets fecales de muestras de trampa de sedimento colectadas en el sistema de afloramiento del norte de la Corriente de Humboldt. Inf. Inst. Mar Perú. 2022, 49, 315–322. [Google Scholar]
- Chiarini, F.; Capotondi, L.; Giglio, F.; Dunbar, R.; Giglio, F.; Mammì, I.; Mucciarone, D.; Ravaioli, M.; Tesi, T.; Langone, L. A revised sediment trap splitting procedure for samples collected in the Antarctic Sea. Methods Ocean. 2013, 8, 14–22. [Google Scholar] [CrossRef]
- Conte, M.H.; Ralph, N.; Ross, E.H. Seasonal and interannual variability in deep ocean particle fluxes at the Oceanic Flux Program (OFP)/Bermuda Atlantic Time Series (BATS) site in the western Sargasso Sea near Bermuda. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 1471–1505. [Google Scholar] [CrossRef]
- Honjo, S.; Manganini, S.J. Annual biogenic particle fluxes to the interior of the North Atlantic Ocean; studied at 34° N 21° W and 48° N 21° W. Deep-Sea Res. Part II Top. Stud. Oceanogr. 1993, 40, 587–607. [Google Scholar] [CrossRef]
- Miquel, J.C.; Fowler, S.W.; La Rosa, J.; Buat-Menard, P. Dynamics of the downward flux of particles and carbon in the open northwestern Mediterranean Sea. Deep-Sea Res. Part I Oceanogr. Res. Pap. 1994, 41, 243–261. [Google Scholar] [CrossRef]
- Karl, D.M.; Christian, J.R.; Dore, J.E.; Hebel, D.V.; Letelier, R.M.; Tupas, L.M.; Winn, C.D. Seasonal and interannual variability in primary production and particle flux at station ALOHA. Deep-Sea Res. Part II Top. Stud. Oceanogr. 1996, 43, 539–568. [Google Scholar] [CrossRef]
- Boxhammer, T.; Bach, L.T.; Czerny, J.; Riebesell, U. Technical note: Sampling and processing of mesocosm sediment trap material for quantitative biogeochemical analysis. Biogeosciences 2016, 13, 2849–2858. [Google Scholar] [CrossRef]
- Ehrhardt, M.; Koeve, W. Determination of particulate organic carbon and nitrogen. In Methods of Seawater Analysis; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Wiley: Weinheim, Germany, 1999; Volume 3, pp. 437–444. [Google Scholar]
- Yoon, W.; Kim, S.; Han, K. Morphology and sinking velocities of fecal pellets of copepod, molluscan, euphausiid, and salp taxa in the northeastern tropical Atlantic. Mar. Biol. 2001, 139, 923–928. [Google Scholar] [CrossRef]
- Komar, P.D.; Morse, A.P.; Small, L.F.; Fowler, S. An analysis of sinking rates of natural copepod and euphausiid fecal pellets. Limnol. Oceanogr. 1981, 26, 172–180. [Google Scholar] [CrossRef]
- Dillon, W.P. Flotation techniques for separating fecal pellets and small organisms from sand. Limnol. Oceanogr. 1964, 4, 467–614. [Google Scholar] [CrossRef]
- Urban, J.L.; Deibel, D.; Schwinghamer, P. Seasonal variations in the densities of fecal pellets produced by Oikopleura vanhoeffeni (C. Larvacea) and Calanus finmarchicus (C. Copepoda). Mari. Biol. 1993, 117, 607–613. [Google Scholar] [CrossRef]
- Janke, N.C. Effect of shape upon the settling velocity of regular convex geometric particles. J. Sediment. Petrol. 1966, 36, 370–376. [Google Scholar] [CrossRef]
- Bretagnon, M.; Paulmier, A.; Garçon, V.; Dewitte, B.; Illig, S.; Leblond, N.; Coppola, L.; Campos, F.; Velazco, F.; Panagiotopoulos, C.; et al. Modulation of the vertical particle transfer efficiency in the oxygen minimum zone off Peru. Biogeosciences 2018, 15, 5093–5111. [Google Scholar] [CrossRef]
- Cerda, M.; Knoppers, B.; Valdés, J.; Fettah Siffedine, A.; Ortlieb, L.; Sabadini-Santos, E. Variación espacial y temporal de las masas de agua, nutrientes y sedimentación de la materia orgánica e inorgánica en la bahía Mejillones del sur (23° S), Chile. Rev. Chilena Hist. Nat. 2010, 83, 402–409. [Google Scholar] [CrossRef]
- Farías, L.; Chuecas, L.; Salamanca, M. Effect of coastal upwelling on nitrogen regeneration from sediments and ammonium supply to the water column in Concepcion Bay, Chile. Estuar. Coast. Shelf Sci. 1996, 43, 137–155. [Google Scholar] [CrossRef]
- Staresinic, N.; Farrington, J.; Gagosian, R.B.; Clifford, C.H.; Hulburt, E.M. Downward Transport of Particulate Matter in the Peru Coastal Upwelling: Role of the Anchoveta, Engraulis ringens. In Coastal Upwelling Its Sediment Record: Part A: Responses of the Sedimentary Regime to Present Coastal Upwelling; Suess, E., Thiede, J., Eds.; Springer: New York, NY, USA, 1983; pp. 225–240. [Google Scholar] [CrossRef]
- Rowe, G.T. Benthic production and processes off Baja California, Northwest Africa and Peru: A classification of benthic subsystems in upwelling ecosystems. Int. Symp. Upw. W Afr. Inst. Inv. Pesq. 1985, 2, 589–612. Available online: https://tamug-ir.tdl.org/handle/1969.3/28322 (accessed on 7 August 2023).
- Rodier, M.; Le Borgne, R. Export flux of particles at the equator in the western and central Pacific ocean. Deep. Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 2085–2113. [Google Scholar] [CrossRef]
- Pilskaln, C.; Neumann, S.; Bane, J. Periplatform flux in the northern Bahamas. Deep-Sea Res. 1989, 36, 1391–1406. [Google Scholar] [CrossRef]
- Estapa, M.; Buesseler, K.; Durkin, C.A.; Omand, M.; Benitez-Nelson, C.R.; Roca-Martí, M.; Breves, E.; Kelly, R.P.; Pike, S. Biogenic sinking particle fluxes and sediment trap collection efficiency at Ocean Station Papa. Elem. Sci. Anthr. 2021, 9, 122. [Google Scholar] [CrossRef]
- Daneri, G.; Dellarossa, V.; Quiñones, R.; Jacob, B.; Montero, P.; Ulloa, O. Primary production and community respiration in the Humboldt Current System off Chile and associated oceanic areas. Mar. Ecol. Prog. Ser. 2000, 197, 41–49. [Google Scholar] [CrossRef]
- Contreras, S.; Pantoja, S.; Neira, C.; Lange, C.B. Biogeochemistry of surface sediments off Concepción (∼36° S), Chile: El Niño vs. non-El Niño conditions. Prog. Oceanogr. 2007, 75, 576–585. [Google Scholar] [CrossRef]
- ENFEN Report. Available online: http://met.igp.gob.pe/elnino/lista_eventos.html (accessed on 10 April 2023).
- Espinoza-Morriberon, D.; Echevin, V.; Colas, F.; Tam, J.; Ledesma, J.; Vasquez, L.; Graco, M. Impacts of El Nino events on the Peruvian upwelling system productivity. J. Geophys. Res. Oceans. 2017, 122, 5423–5444. [Google Scholar] [CrossRef]
- Stukel, M.; Ohman, M.; Benitez-Nelson, C.; Landry, M. Contributions of mesozooplankton to vertical carbon export in a coastal upwelling system. Mar. Ecol. Prog. Ser. 2013, 491, 47–65. [Google Scholar] [CrossRef]
- Steinberg, D.; Lomas, M.; Cope, J. Long-term increase in mesozooplankton biomass in the Sargasso Sea: Linkage to climate and implications for food web dynamics and biogeochemical cycling. Glob. Biogeochem. Cycles 2012, 26, 1004. [Google Scholar] [CrossRef]
- Shatova, O.; Koweek, D.; Conte, M.; Weber, J. Contribution of zooplankton fecal pellets to deep ocean particle flux in the Sargasso Sea assessed using quantitative image analysis. J. Plankton Res. 2012, 34, 905–921. [Google Scholar] [CrossRef]
- Turner, J. Zooplankton Fecal Pellets, Marine Snow, Phytodetritus and the Ocean’s Biological Pump. Prog. Oceanogr. 2014, 13, 205–248. [Google Scholar] [CrossRef]
- Wexels, C.; Wassmann, P.; Olli, K.; Pasternak, A.; Arashkevich, E. Seasonal variation in production, retention, and export of zooplankton faecal pellets in the marginal ice zone and central Barents Sea. J. Mar. Syst. 2002, 38, 175–188. [Google Scholar] [CrossRef]
- Frangoulis, C.; Skliris, N.; Lepoint, G.; Elkalay, K.; Goffart, A.; Pinnegar, J.K.; Hecq, J.-H. Importance of copepod carcasses versus fecal pellets in the upper water column of an oligotrophic area. Estuar. Coast. Shelf Sci. 2011, 92, 456–463. [Google Scholar] [CrossRef]
- Olli, K.; Wassmann, P.; Reigstad, M.; Ratkova, T.N.; Arashkevich, E.; Pasternak, A.; Matrai, P.A.; Knulst, J.; Tranvik, L.; Klais, R.; et al. The fate of production in the central Arctic Ocean—Top-down regulation by zooplankton expatriates? Prog. Oceanogr. 2007, 72, 84–113. [Google Scholar] [CrossRef]
- Wilson, S.; Steinberg, D.; Buesseler, K. Changes in fecal pellet characteristics with depth as indicators of zooplankton repackaging of particles in the mesopelagic zone of the subtropical and subarctic North Pacific Ocean. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 1636–1647. [Google Scholar] [CrossRef]
- Urrére, M.; Knauer, G. Zooplankton fecal pellet flaxes and vertical transport of participate organic material in the pelagic environment. J. Plankton Res. 1981, 3, 369–387. [Google Scholar] [CrossRef]
- Noji, T.; Estep, K.; MacIntyre, F.; Norrbin, F. Image analysis of faecal material grazed upon by three species of copepods: Evidence for coprohexy, coprophagy, and coprochaly. J. Mar. Biol. Assoc. 1991, 71, 465–480. [Google Scholar] [CrossRef]
- Ayón, P.; Pinedo, E.; Schukat, A.; Taucher, J.; Kiko, R.; Hauss, H.; Dorschner, S.; Hagen, W.; Segura-Noguera, M.; Lischka, S. Zooplankton community succession and trophic links during a mesocosm experiment in the coastal upwelling off Callao Bay (Peru). Biogeosciences 2023, 20, 945–969. [Google Scholar] [CrossRef]
- McDonnell, A.M.P.; Buesseler, K.O. Variability in the average sinking velocity of marine particles. Limnol. Oceanogr. 2010, 55, 2085–2096. [Google Scholar] [CrossRef]
- Saba, G.K.; Steinberg, D.K. Abundance, Composition and Sinking Rates of Fish Fecal Pellets in the Santa Barbara Channel. Sci. Rep. 2012, 2, 716. [Google Scholar] [CrossRef]



| Bottles Number | Sampling Start Date | Sampling End Date | Period |
|---|---|---|---|
| 1 | 14 March 2020 | 23 March 2020 | 10 days |
| 2 | 24 March 2020 | 2 April 2020 | 10 days |
| 3 | 3 April 2020 | 12 April 2020 | 10 days |
| 4 | 13 April 2020 | 22 April 2020 | 10 days |
| 5 | 23 April 2020 | 18 December 2020 | 239 days |
| Parameter | Sample | Type of Pellet | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Count | 384 | 112 | 208 | 332 | 3360 | Zooplankton |
| Cylindrical shape (%) | 36.5 | 17.9 | 13.5 | 33.7 | 16.2 | |
| Ovoid shape (%) | 63.5 | 82.1 | 86.5 | 66.3 | 83.8 | |
| Volume (mm3) | 0.3 | 0.1 | 0.1 | 0.4 | 3.2 | |
| Count | 148 | 8 | 216 | 116 | 1472 | Fish |
| Volume (mm3) | 205.6 | 27.2 | 406.2 | 76.1 | 3392.6 | |
| Total count | 532 | 120 | 424 | 448 | 4832 | |
| Total volume (mm3) | 206.1 | 27.4 | 406.4 | 76.9 | 3398.1 | |
| Study Area | Depth (m) | Sampling Date | El Niño Event | TMF (mg·m−2·day−1) | Fecal Pellets Contribution (%) | Study |
|---|---|---|---|---|---|---|
| Callao Bay—Peru | 30 | March–December 2020 | 601.9 | 0.03–26.95% | This study | |
| Callao Bay—Peru | 30 | January–April 2017 | 2017 coastal El Niño | 4502 ± 1892 | [18] | |
| Mejillones Bay—Chile | 30 | January–February 2004 | 1100 ± 400 | [38] | ||
| Concepcion Bay—Chile | 30 | January 1993 | 4600 ± 300 | [39] | ||
| Punta Santa Ana—Peru | 50 | 1978 | 0–17% | [40] | ||
| ~50 km off Lima, Peru | 34 | January–March 2013 | 427 ± 217 | 60–70% | [37] | |
| June–November 2013 | 986 ± 1422 | |||||
| Callao Bay—Peru | 30 | 1976–1978 | 16,800 | [41] | ||
| Central Pacific, Ecuador | 105 | September–October 1994 | El Niño | 973 ± 72 | [42] | |
| 125 | 277 ± 81 | |||||
| 155 | 639 ± 264 | |||||
| 175 | 297 ± 77 | |||||
| Northern Bahamas—USA (Atlantic Ocean) | 500 | February–June 1985 | 59 | 3% | [43] | |
| Ocean Station Papa—USA (Alaska) | 96 | August 2018 | 89 ± 3.8 | [44] | ||
| 97 | 83.5 ± 14.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leigh, B.; Aramayo, V.; Mendoza, U.; Velazco, F.; Kiko, R.; Ayón, P.; Fernández, E.; Graco, M. Total Mass Flux in the Northern Humboldt Current System: Rates and Contribution Sources from Central Peru (12° S). Hydrobiology 2023, 2, 521-536. https://doi.org/10.3390/hydrobiology2040035
Leigh B, Aramayo V, Mendoza U, Velazco F, Kiko R, Ayón P, Fernández E, Graco M. Total Mass Flux in the Northern Humboldt Current System: Rates and Contribution Sources from Central Peru (12° S). Hydrobiology. 2023; 2(4):521-536. https://doi.org/10.3390/hydrobiology2040035
Chicago/Turabian StyleLeigh, Bobby, Víctor Aramayo, Ursula Mendoza, Federico Velazco, Rainer Kiko, Patricia Ayón, Ernesto Fernández, and Michelle Graco. 2023. "Total Mass Flux in the Northern Humboldt Current System: Rates and Contribution Sources from Central Peru (12° S)" Hydrobiology 2, no. 4: 521-536. https://doi.org/10.3390/hydrobiology2040035
APA StyleLeigh, B., Aramayo, V., Mendoza, U., Velazco, F., Kiko, R., Ayón, P., Fernández, E., & Graco, M. (2023). Total Mass Flux in the Northern Humboldt Current System: Rates and Contribution Sources from Central Peru (12° S). Hydrobiology, 2(4), 521-536. https://doi.org/10.3390/hydrobiology2040035






