Abstract
The Current River is a cold-water, thermally constant Missouri Ozark stream and is one of the few remaining free-flowing rivers in the U.S. The Current River’s baseflow is largely fed by hundreds of springs, which include five first-magnitude springs. Little attention has been given to the influence of spring inflows on river temperature and spring influence on biodiversity. The objectives of this study were to demonstrate how large springs affect river macroinvertebrate communities, and to explore the relationships among macroinvertebrate diversity and habitat variables to estimate spring influences on community structure and diversity. Aquatic macroinvertebrates were collected from 42 riffle/run habitats of the Current River main-stem, tributaries, and springs during the winter season. Samples at each site were collected using a Slack-Surber sampler for macroinvertebrates with additional habitat variables collected: substrate size, embeddedness, periphyton, filamentous green algae, vegetation, depth, current velocity, temperature, dissolved oxygen, specific conductance, and pH. Beta diversity analysis was performed on consecutive pairs of site taxa richness values using the Wilson–Shmida calculation to determine the impact of main-stem confluences with either tributaries or springs, and invertebrate community relationships were explored using nonmetric multidimensional scaling (NMDS). Water temperature and taxa richness exhibited similar patterns, with higher temperatures being associated with lower taxa richness. Downstream of each large-magnitude spring, taxa richness sharply decreased, while taxa richness increased downstream of tributaries. Beta diversity usually declined downstream of the confluences with springs, but increased downstream of the tributaries. Data from large springs were closely grouped in NMDS, while tributaries and main-stem sites were more widely scattered. These data indicate spring inputs produce more homogenous conditions in the main-stem river compared to more heterogenous conditions produced by tributary inputs. Macroinvertebrate diversity along the Current River also does not follow predictions from the river continuum concept, but rather diversity peaks are downstream of springs. Our data clearly demonstrate the strong influence of large springs on macroinvertebrate communities in the Current River.
1. Introduction
Biodiversity varies across space according to both physical and chemical gradients [1,2,3]. Therefore, understanding gradual and abrupt shifts in biodiversity can help us better determine ecological drivers to such change. Measuring only species or taxa richness often does not provide sufficient insight to explain differences among sites [4]. Instead, Heino et al. [5] suggest that studies need to focus more on comparisons of diversity at multiple scales, including beta diversity, in order to understand what shapes community diversity. Beta diversity captures the change in the community composition in relation to environmental gradients. Therefore, analyzing beta diversity should interpret the degree of differentiation among biological communities within a region by capturing the dissimilarity between sample pairs to essentially measure taxonomic turnover. Beta diversity is therefore the link between the local and regional species pools [6,7,8].
The influence of temperature on aquatic life has been the focus of numerous studies on streams and rivers [9,10,11,12,13]. For example, temperature-related cues are crucial for the sustained presence of insects in the benthic macroinvertebrate community because they strongly influence growth and development [9]. Lower diversity has been associated with decreasing water temperature due in large part to the loss of insect taxa [10,12]. However, less attention has been given to the influence of cold spring inflows on river temperature and its influence on biodiversity [14,15,16,17]. As reported by Smith and Wood [18] and Smith et al. [19], species composition within and outside of springs often differs, thus enhancing beta diversity within those systems. Springs often have a lower diversity of aquatic macroinvertebrates compared to surface-fed streams [12,20] due to stable water flows and physical-chemical measures, consistent water temperatures, and lower nutrient loadings. Moreover, Ebersole et al. [13] reported that cold-water inflows can provide thermal refugia to cold-adapted species when they flow into rivers that have higher water temperatures. In contrast to springs, Caissie [21] found water temperature in headwater streams is generally close to groundwater sources, but it then generally increases in a downstream direction. Accordingly, the river continuum concept (RCC) [22] predicts that taxonomic richness should generally resemble a bell curve from headwaters to river mouths with the highest diversity in the mid-reaches.
The Current River in southeastern Missouri is one of the few remaining large, free-flowing streams in the Ozarks Physiographic Province. The karst topography of the Ozarks allows for the formation of many springs, of which there are more than 400 in the Current River basin [23,24]. In the Current River watershed (Figure 1), there are five first magnitude springs (≥2.83 m3/s), and six second magnitude springs (0.28–2.83 m3/s), in addition to the many other springs of lesser magnitude (<0.28 m3/s) [24,25]. Three of the first magnitude and three second magnitude springs are located upstream of the Jacks Fork confluence, which is the major tributary in the basin. Additionally, there are two first magnitude and two second magnitude springs located downstream of that confluence. The springs provide the bulk of the baseflow for the Current River and Jacks Fork, and approximately 92% of the water in the Current River at Van Buren, Missouri, originates from springs in the watershed [26], resulting in largely thermally homogeneous flows downstream of that location. To our knowledge, no other river in North America has a similar arrangement of large springs within such a small spatial area.
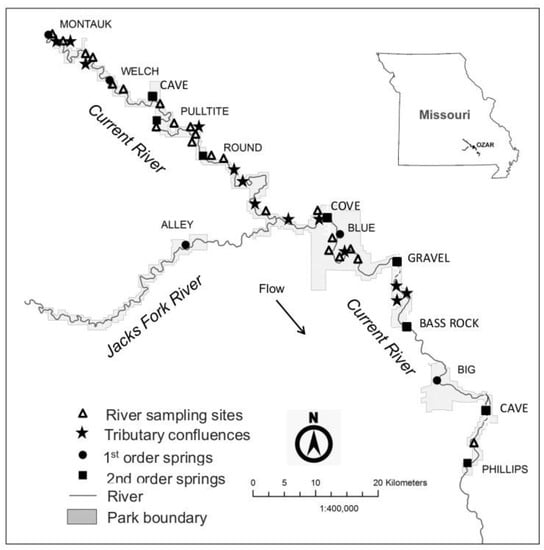
Figure 1.
Map of the Current River Watershed, Missouri, showing approximate locations of high magnitude springs, tributaries, and river sampling sites. River sampling sites are in sequential order from headwaters to downstream (see Appendix A). Montauk Spring is designated as the first river sampling site (C1). Cave (upper and lower), Cove, Gravel and Bass Rock springs were not sampled. Springs are longitudinally oriented on the Current River with their magnitude in parenthesis: Montauk (1), Welch (1), Cave (upper) (2), Pulltite (2), Round (2), Alley (1), Blue (1), Gravel (2), Bass Rock (2), and Big (1), Cave (lower) (2). No samples were collected from Cave (upper and lower), Gravel. Bass Rock or Phillips springs. Major tributaries that are longitudinally oriented on the Current River include Ashley Creek, Shafer Spring Creek, Big Creek West, Sinking Creek, Big Creek East, Sutton Creek, Thompson Creek, Blair Creek, Powder Mill Creek, Rocky Creek, Carr Creek, Rogers Creek, and Mill Creek. No samples were collected from Ashley Creek. The major tributary of the Current River, the Jacks Fork, is here treated as a spring because the majority of the flow in that stream comes from Alley Spring. Although sampling data upstream of Alley Spring are available, they are not included in this study.
Given the unique density and arrangement of large springs along the basin of the Current River, we are interested in the influence of those springs on river beta diversity. We anticipated that areas in the Current River located downstream of large-spring, confluences would have altered water temperatures that would, in turn, result in lower macroinvertebrate diversity compared to areas upstream of these confluences, or to areas located downstream of surface-fed tributaries. Therefore, the aims of our study were to (1) investigate diversity relationships among aquatic macroinvertebrate communities in this unique river system and (2) determine whether they fit within the framework of the RCC and other established stream models. We also assess the relationships among macroinvertebrate diversity and habitat variables among sampling sites to estimate their respective influences on community structure and diversity.
2. Materials and Methods
2.1. Study Area and Site Selection
We sampled the Current River, its tributaries, and springs located in Ozark National Scenic Riverways (OZAR), located in southeastern Missouri. Sampling was conducted at a total of 42 sampling sites: 23 permanent mainstem river sites on the Current River, 6 large-magnitude springs, and 13 surface-fed tributaries (Figure 1, Appendix A). Montauk Spring, the source of the Current River, is an additional large-magnitude spring, but it was treated as a river sampling site for analysis purposes. The springs are cold-water and thermally consistent, having water temperatures of about 13.5–14.5 °C [24]. Surface-fed tributaries contribute much less to the base flow, although upstream of the Jacks Fork they generally have greater flows and larger watershed areas. The surface-fed streams have cold-water temperatures during winter months, but these streams become warmer (>19 °C) during summer. The thermally cold spring flows in this basin support numerous cold-adapted species, including rainbow trout Oncorhynchus mykiss (Walbaum), which are stocked by the Missouri Department of Conservation.
All samples were collected from riffle/run habitats [27] during November through early January during 2005–2009, 2012, 2014, and 2016. Mainstem sampling sites were located at varying distances downstream of the confluences of large springs and surface-fed tributaries.
2.2. Macroinvertebrate Sampling
Benthic macroinvertebrate samples were collected using a Slack-Surber sampler (500 µm mesh, 0.25 m2, n = 9; [28]. Specific field and lab procedures are those described in Bowles et al. [27] and are not repeated here. All identifications of taxa were completed by using a standardized instruction [27]. Data are from the publicly available database of the National Park Service, Heartland Inventory and Monitoring Network, Wilson’s Creek National Battlefield, Missouri, and additional data are from Heth [29] and Heth et al. [30].
From these data we calculated taxa richness (alpha diversity) and number of EPT (Ephemeroptera, Plecoptera, Trichoptera) taxa for analyses in this study. Although raw taxa richness estimates based on our subsampling routine (≥200 organisms, plus large and rare search) possibly could be biased, it has been previously shown that taxa richness increases rapidly in samples up to 200 individuals, but it increases at a much slower rate thereafter [31]. We therefore feel our data adequately represent true richness in our samples without using rarefaction procedures.
2.3. Habitat and Water Quality
Several qualitative habitat variables were measured in association with benthic samples [27]. They include substrate, periphyton, filamentous green algae, and aquatic vegetation. Variables were estimated within the sampling net frame and recorded as percentage categories (0, <10, 10–40, 40–75, >75) and analyzed as midpoints of each category across years for each site. This approach allowed us to estimate the average condition of the variables for those sampling sites. We also visually assessed dominant substrate size using the Wentworth scale [32]. Additionally, we measured depth (cm) and current velocity (m/s) immediately in front of the sampling net frame. Discharge was determined from appropriate USGS gages or measured in situ immediately above the sampling site using a top-setting wading rod fitted with a calibrated Marsh–McBirney Flow-Mate 2000 flow meter [33]. Discrete readings of water-quality parameters (temperature, dissolved oxygen, specific conductance, and pH) were recorded at each riffle/run sampled with calibrated, hand-held instruments (YSI models 55, 63, ProPlus). In addition to the discrete measurements, we recorded continuous, hourly readings of temperature, dissolved oxygen, specific conductance, pH, and turbidity for at least one week prior to sampling using calibrated data loggers (YSI models 6600, 6920) at two fixed sites on the Current River (Figure 1). Water quality data were averaged across years for each site to estimate their general condition and are not intended to represent the broader range of possible conditions over seasons and years.
2.4. Statistical Analysis
Habitat data for sampling sites (Table 1) were checked for normality (Shapiro–Wilks test), log transformed when necessary, and analyzed with a Kruskal–Wallis (alpha = 0.05) and Dunn’s post-hoc test for significant tests. For the Dunn’s post-hoc test comparisons, S = springs, T = tributaries, C = river.

Table 1.
Habitat and diversity variables for springs, tributary and river sampling sites at Ozark National Scenic Riverways, Missouri. Values are means with standard error in parentheses. Kruskal–Wallis test (alpha = 0.05) with Dunn’s post hoc test. For Dunn’s Test, S = springs, T = tributaries C = river.
We evaluated the relationship of taxa and EPT richness and associated environmental variables among collection sites using nonmetric multidimensional scaling (NMDS) with a Bray–Curtis distance measure (PAST statistical software, version 4.06b, [34]. Only significantly different variables were included in the model (Table 1, Kruskal–Wallis test, alpha = 0.05). In order to reduce skew and increase interpretability, we transformed variables prior to analysis using Log10 for water quality data while proportional data were transformed using arcsin square root. Depth and current velocity were not included in this analysis due to their relative uniformity among samples over time. NMDS was selected for analysis because it is suitable for handling non-normal data and non-continuous data with null values [35]. We tested two-dimensional and three-dimensional NMDS models based on the rationale that stress values can be reduced in additional dimensions [34]. The corresponding Shepard’s plot and coefficient of determination (R2) were evaluated to estimate the strength of the model. Each run for each dimension consisted of a sequence of 11 trials, from which the one with smallest stress value was chosen as the best fit [34].
Beta diversity analysis was conducted on taxa richness values among sites using the Wilson–Shmida calculation [36] by analyzing consecutive pairs along the river longitude in relation to the location of large-volume springs and surface-fed tributaries. The Wilson–Shmida test was used because it is considered to be the most robust measure of beta diversity for presence–absence data collected along environmental gradients [36]. Index scores range from 0, meaning complete similarity, and 1, meaning complete dissimilarity. For the purpose of graphical comparison, we multiplied the Wilson–Shmida values by 50 so they could be represented on the same scale as water temperature and taxa richness.
3. Results
All habitat variables were significantly different among the three types of sampling sites (i.e., river, tributaries, springs) with the exception of percent embeddedness (Table 1, Kruskal–Wallis test). Dunn’s post hoc test results showed many of the comparisons among the three habitat types were significant. Generally, water temperature increased at Current River sampling sites located downstream of large springs, while water temperature decreased at sites downstream of surface-fed tributary confluences.
Water temperature and taxa richness exhibited a general pattern with higher temperatures being associated with decreased taxa richness (Figure 2). Downstream of each major spring, taxa richness generally decreased, while conversely taxa richness increased downstream of tributaries. It was also evident that beta diversity declined downstream of the confluence for each spring, but similarly increased downstream of the tributaries. Notable exceptions were for site 16, which has three substantial tributaries located upstream of the site, and two located downstream appear to override the effects associated with the flows of Alley Spring. Interestingly, taxa richness for site 21 peaked sharply compared to other sites located upstream of it. The tributary located upstream of this site, Rocky Creek, had the highest taxa richness among those included in this study.
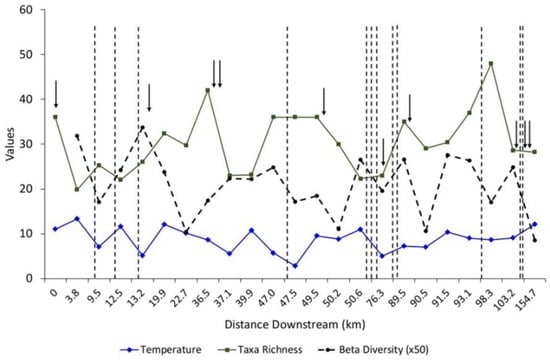
Figure 2.
Mean water temperature (°C), taxa richness (alpha diversity) and beta diversity taken along the Current River, Missouri. Arrows indicate approximate locations where first and second magnitude springs flow into the river, and dashed vertical lines indicate approximate locations where surface-fed tributaries flow into the river. See the Appendix A and Figure 1 for specific locations of the sampling sites.
Wilson–Shmida beta diversity values generally decreased downstream of the large springs but increased downstream of tributaries (Figure 2). In cases where tributaries bracketed either side of a large-spring confluence (i.e., sites 15–17), the dissimilarity associated with the tributaries appears to override the contribution of the springs. In contrast, when large springs bracketed tributaries in the lower river (i.e., site 23), the springs appear to have had the greater influence on the similarity of macroinvertebrate communities.
The NMDS generally corroborated the findings related to taxa richness and temperature (Figure 3). The three-dimensional NMDS model for the diversity and environmental data was found to be stable, with the lowest stress value and a fair fit (Shepard plot stress value = 0.16; Axis 1–0.50, Axis 2 = 0.25). Running the NMDS model in three dimensions did not improve the stress scores or fit. With the exception of Montauk Springs, the large springs were generally closely grouped in the ordination space compared to the widely scattered tributaries and mainstem sites. The tributaries also ordinated as a group, but with more scatter compared to the springs. Consecutive mainstem river sites did not always ordinate closely together as expected and were widely scattered in the ordination space. Some of the river sites were grouped with tributaries. For example, sites C10, C15, C16, and C19 were located further downstream of springs than the previous sampling site, which were located immediately downstream of the springs. Site C4 was located downstream of a spring-fed tributary, and site C23 was located nearly 14 km downstream of Big Spring.
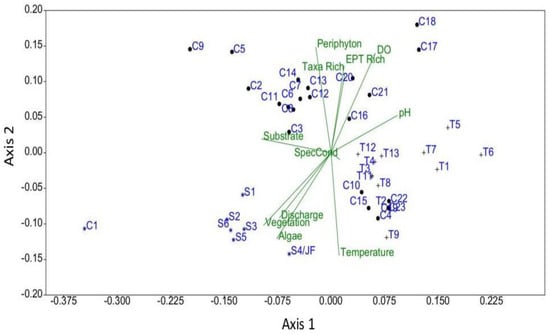
Figure 3.
NMDS biplot of taxa diversity, water quality and habitat data for springs (S), tributaries (T) and river (C) sampling sites located at Ozark National Scenic Riverways, Missouri. Sites for each habitat type are numbered consecutively from upstream to downstream. Refer to the Appendix A for additional interpretation of site codes. Site C1 is Montauk Spring.
4. Discussion
Water temperatures in the Current River downstream of high-magnitude springs were warmer in winter, while sites downstream of tributaries were colder, with the opposite occurring during warmer months. Bowles et al. [37] and Heth et al. [30] reported high diversity of aquatic invertebrates at sampling sites along the length of the Current River, while the diversity of aquatic macroinvertebrates in the large springs is generally much lower compared to the river and tributaries, although densities are typically greater in springs [38,39]. This study further elucidated the dynamics of macroinvertebrate diversity in the river by showing that taxa richness in river samples was lower at sites located downstream of springs, while it increased downstream of tributaries. Furthermore, beta diversity values generally decreased downstream of the large springs but increased downstream of tributaries, which likely relates to the more homogeneous conditions at the former sites compared to more heterogeneous conditions of the latter. This suggests that the contribution of springs to the river resulted in more similar communities by producing more homogeneous conditions in those areas compared to more dissimilar communities downstream of the surface-fed tributaries where conditions were more heterogeneous. We also showed that the influence of surface-fed tributaries on macroinvertebrate communities lessens in a downstream direction due in large part to the overwhelming influence of spring flows.
Ordination showed that springs and tributaries generally clustered within their respective groups, but river sampling sites exhibited a broader ordination with some of those sites being grouped more closely with tributaries. Consecutive mainstem river sites did not always ordinate close together as expected and were widely scattered in the ordination space, and some of the river sites were grouped with tributaries. This suggests that the influences of the springs were being mitigated by the river at those locations relative to those sites closer to the springs. The lack of a strong ordination of Montauk Spring (as the first river site) with the other large springs was not expected. However, Montauk State Fish Hatchery diverts water from the spring to support their operations with the effluent returned to the spring channel approximately 0.8 km downstream of the source. The Montauk hatchery produces 300,000 to 400,000 rainbow trout and stocks about 200,000 trout in the park annually, with concomitant fishing activity. Thus, that portion of the upper river likely has altered ecological functioning both in terms of changes in water chemistry and nutrient additions, and highly augmented predation by trout on the macroinvertebrate populations in the spring and spring-run [40].
The diversity of aquatic macroinvertebrates along the length of the Current River are not well described by previously published stream ecology models, most notably the river continuum concept (RCC). In the RCC, the expected response curves of taxa richness and beta diversity along the river continuum are bell shaped curves with maximum values expected in the mid-reaches, while lower values are expected in the headwaters and downstream reaches [22,41]. Our findings showed much more irregular responses. Similar to the RCC, the serial discontinuity concept (SDC) describes the longitudinal resource gradient from headwater to mouth, but with a response curve related to the longitudinal arrangement of dams along the river [42,43]. Notably, the SDC describes the disruptive impacts that modified thermal and flow regimes have on river functioning along its continuum. Our findings are described better by the SDC than they are by the RCC, but the former model relates to impoundment effects and not springs and is therefore not directly comparable.
The effects of surface tributary inflows on the diversity of receiving streams are generally well described [41]. For example, tributary influences on stream species richness depends on where it enters the stream. If the tributary enters upstream of the point of the system richness maximum, it drives the stream to that maximum. If the tributary enters downstream of the point of system richness maximum, it can partially reset that maximum [41]. In the Current River, such responses were not evident from the data we collected. Taxa richness and beta diversity in this river oscillate broadly from headwaters to its downstream reaches with some of the highest diversity in the headwaters and downstream areas, as well as the mid-reaches. This unexpected pattern may be due in part to the Current River not following a typical pattern of increasing stream order as well as the disruptive influences of numerous large, thermally consistent springs. Although the volume of the river increases greatly along its 296 km length before its confluence with the Black River, the majority of inflows in the Current River come from high-magnitude springs, which technically are first order, rather than the higher order seen in many of the tributaries. Our data shows that these large springs serve to reset taxa richness along the length of the river to which we attribute thermal disruption.
Thermal alterations to streams can have profound impacts on macroinvertebrate diversity. For example, previous studies have reported the importance of cold-water patches related to stream inflows of tributaries, which can serve as seasonal ‘hotspots’ of productivity and diversity for benthic macroinvertebrates and other aquatic life, as well as major nodes of geomorphic adjustments [13,44]. However, the tributaries in those studies were not spring-fed and the inflows demonstrated broad seasonal and temporal variability unlike the spring-fed tributaries in this study. In contrast, the thermally consistent springs flowing into the Current River provide consistent warm-water patches in the winter and cold-water patches in the summer, with the tributaries doing the opposite and in a much more variable fashion [30,37,38,39,45]. Additionally, the springs we studied maintain continuous flows and have never been known to stop flowing, while the tributaries occasionally go through periods of low and partially hyporheic flows, especially during summer. We contend greater thermal variability helps to explain the greater macroinvertebrate diversity we found in the river downstream of tributaries during winter.
Although the influence of tributary inflows on rivers, including beta diversity of aquatic macroinvertebrates, is fairly well documented [41,44,46], springs generally have received much less attention in this regard [12]. Although springs likewise have the capacity to alter receiving streams, they do so quite differently than a surface-fed stream [12,47], and they are capable of strongly influencing physiochemical and biological patterns [26]. Springs establish physicochemical longitudinal patterns that can be predicted based on distance from the spring source. Springs add large quantities of organic matter, stabilize temperature fluctuations, and increase stream velocities [10,12]. Accordingly, those factors can influence diversity of aquatic macroinvertebrate communities [48,49]. As noted by Gonzales-Yrujillo and Alonso-Moreno [50], beta-diversity patterns are often linked to differences in habitat heterogeneity and spatial distance among headwater streams. Their findings suggest that habitat simplification induces functional homogenization of the community and diminishes the extent of change in community composition among sites. Similarly, our data showing beta diversity decreased downstream of springs but increased downstream of tributaries, supports the findings of Gonzales-Yrujillo and Alonso-Moreno [50]. In this study, spring inflows resulted in more homogeneous habitat conditions in the Current River compared to the more heterogeneous tributaries, which was reflected in macroinvertebrate community compositions at those river sites where the latter were most dissimilar. Our finding was the opposite of Gray and Harding [47], who found that spring creeks flowing into braided New Zealand rivers had higher diversity compared to main channel habitats. However, the discharges of the springs studied by Gray and Harding [47] were of far lesser magnitude than those in this study so the relative influences of those springs may not have been as strong.
As reported by Astorga et al. [8], higher disturbance frequency, and perhaps higher stream variability in recovery from disturbance, may contribute to higher species turnover. Therefore, general stability and uniformity of large-volume springs that confluence with the river may serve to mitigate such disturbance and therefore reduce heterogeneity and turnover. The large springs flowing into the Current River are highly homogeneous with respect to temperature, water quality and physical attributes in addition to biological communities [24,38,39,51], and disturbance of those springs due to floods and other factors is not common [52]. We recognize this assessment may be an oversimplification of our sampling sites and the river itself given that streams have complex hierarchical structures that make it difficult to ascertain specific contributions of the many physical and chemical contributions to those sites [48]. However, our data clearly show the large springs have a strong influence on macroinvertebrate communities in the Current River. In other words, the Current River is fed primarily by an irregular pattern of stable spring flows in addition to highly variable tributary flows.
Benda et al. [15] argued that the RCC’s predictions of gradual downstream change in river attributes and associated biological processes are valid over orders of magnitude in river size, but the complexity of riverine ecology must be studied in terms of patchiness or heterogeneity, stochastic disturbance, and hierarchical scaling. Collectively this complexity points to studying streams based on the principles of landscape ecology emphasizing the importance of studying riverine habitats and their patchiness over multi-kilometer scales. While linear approaches to stream ecology such as the RCC predict gradual and continuous downstream changes, especially in biological processes, non-linear models such as stream networks and branching influenced by stochastic influences, interrupts the downstream continua, and such disruptions have been termed ‘river discontinuum’ perspectives [16]. Such interruptions often produce heterogeneous distributions of habitats [16]. Tributaries certainly have the potential to disrupt linear functioning and produce discontinuum, but large volume springs appear to have even more potential to do so.
Because we measured temperatures during the colder months of the year (November–January), when the spring water was warmer than that of the tributaries and river, the opposite response would be expected during the warmer months of the year, with possible neutral responses during early fall and spring. That notion was validated by Westhoff and Paukert [45] who reported that springs influenced river temperatures in the Current River during winter and summer, but the primary influence was from the large springs. Moreover, smaller springs along the Current River that contributed less than 5% of the mainstem discharge did not affect river water temperatures beyond a few hundred meters downstream, but as spring magnitude increased, the influence of groundwater on river water temperature increased [45]. To our knowledge, the observed response of aquatic macroinvertebrate communities to the inflows of multiple large springs is a unique occurrence in North America. While many other streams in North America have spring inflows and even their sources from springs, none have multiple large springs along their entire length serving to periodically alter the ecological functioning of the river at those junctures. However, our findings may be partially applicable to streams with some major spring inflows.
5. Conclusions
The strong influence the springs exert on the ecological functioning of the Current River may provide this system with a unique resilience to physical disturbance. Spring-fed rivers will become increasingly important as refugia for aquatic life due to the impacts of climate change. Such resilience may be crucial as climate change causes thermal and other alterations to regional streams. Rivers worldwide are threatened by a burgeoning human population and its associated stressors [53], and it is imperative that conservation efforts take into account the full array of these impacts to protect them. As noted by Lusardi et al. [54], spring-fed rivers will become increasingly important for aquatic life due to the impacts of climate change. For example, although the Current River is considered a high quality resource and is designated a National Scenic River, the jurisdictional boundary of OZAR encompasses only 4% of the watershed, leaving much of it unprotected from human activities (e.g., agriculture, urbanization, and logging), which could result in the alteration of water quantity and quality. The watersheds for the large springs in the region are especially vulnerable to disturbance. Protecting and maintaining the integrity of those natural resources is therefore a high priority because it also serves as a major economic contributor to the region [55,56,57]. Presently, the strong influence the springs exert on the ecological functioning of the river may provide this system with a unique resilience to physical disturbance. Such resilience may be crucial as climate change causes thermal and other alterations to regional streams.
Author Contributions
R.L.S.H. and D.E.B. were responsible for all aspects related to this paper, including data collection, analyses, and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Data collection was funded by the United States National Park Service.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this study are available in Heth [29] or deposited in the official databases of the Heartland Inventory and Monitoring Network, United States National Park Service, Wilson’s Creek National Battlefield, 6424 West Farm Road 182. They are available upon request.
Acknowledgments
We thank the staff of the Heartland Inventory and Monitoring Network for their assistance in the field and laboratory. We also thank John Havel (Missouri State University) and Robert Sites (University of Missouri) for their respective guidance to RLSH during her graduate studies. Thanks to those who assisted RLSH in the field including Jack Grant, Robert Heth, James Pflug, and Kris Simpson. The constructive comments of two anonymous reviewers improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
List of sampling sites for aquatic invertebrates. Those springs and tributaries marked with an asterisk were not included in this study. Site codes are used only for those sites included in the analysis.
Table A1.
List of sampling sites for aquatic invertebrates. Those springs and tributaries marked with an asterisk were not included in this study. Site codes are used only for those sites included in the analysis.
| Site Name | Site Number | Distance between Sites (km) | Springs | Tributaries | River |
|---|---|---|---|---|---|
| Montauk Spring/Current River | C1 | 0 | X | ||
| Current River at Tan Vat | C2 | 3.80 | X | ||
| Ashley Creek * | -- | 3.85 | X | ||
| Current River at Parker | C3 | 5.15 | X | ||
| Schafer Spring Creek | T2 | 0.11 | X | ||
| Current River at Cedar Grove | C4 | 2.92 | X | ||
| Big Creek (West) | T3 | 0.72 | X | ||
| Current River downstream of Big Creek | C5 | 0.30 | X | ||
| Welch Spring | S1 | 6.00 | X | ||
| Current River downstream of Welch Spring | C6 | 0.66 | X | ||
| Current River at Howell | C7 | 2.78 | X | ||
| Cave Spring * | -- | 6.50 | |||
| Current River upstream of Pulltite Spring | C8 | 7.30 | X | ||
| Pulltite Spring | S2 | 0.30 | X | ||
| Current River downstream of Pulltite Spring | C9 | 0.30 | X | ||
| Current River further downstream Pulltite Spring | C10 | 2.79 | X | ||
| Current River Upstream of Sinking Creek | C11 | 7.09 | X | ||
| Sinking Creek | T4 | 0.23 | X | ||
| Current River downstream of Sinking Creek | C12 | 0.28 | X | ||
| Current River upstream of Round Spring | C13 | 2.00 | X | ||
| Round Spring | S3 | 0.54 | X | ||
| Current River downstream of Round Spring | C14 | 0.20 | X | ||
| Current River further downstream of Round Spring | C15 | 0.43 | X | ||
| Big Creek (East) Creek | T5 | 11.25 | X | ||
| Sutton Creek | T6 | 9.90 | X | ||
| Thompson Creek | T7 | 3.90 | |||
| Current River downstream of Thompson Creek | C16 | 0.57 | X | X | |
| Alley Spring/Jacks Fork | S4/JF | 0,38 | X | ||
| Blair Creek | T8 | 7.44 | X | ||
| Powder Mill Creek | T9 | 3.54 | X | ||
| Current River upstream of Blue Spring | C17 | 1.92 | X | ||
| Blue Spring | S5 | 0.14 | X | ||
| Current River downstream of Blue Spring | C18 | 0.79 | X | ||
| Current River further downstream of Blue Spring | C19 | 1.00 | X | ||
| Current River upstream of Rocky Creek | C20 | 1.66 | X | ||
| Rocky Creek | T10 | 2.07 | X | ||
| Current River Downstream of Rocky Creek | C21 | 3.08 | X | ||
| Current River near Log Yard | C22 | 4.91 | X | ||
| Carr Creek | T11 | 1.20 | X | ||
| Gravel Spring * | -- | 10.19 | X | ||
| Rogers Creek | T12 | 9.50 | X | ||
| Mill Creek | T13 | 5.80 | X | ||
| Bass Rock Spring * | -- | 1.50 | |||
| Big Spring | S6 | 9.40 | X | ||
| Current River downstream of Big Spring | C23 | 13.94 | X |
References
- Poff, N.L. Landscape filters and species traits: Towards mechanistic understanding and prediction in stream ecology. J. N. Am. Benthol. Soc. 1997, 16, 391–409. [Google Scholar] [CrossRef]
- Heino, J.; Parviainen, J.; Paavola, R.; Jehle, M.; Louhi, P.; TMuotka, T. Characterizing macroinvertebrate assemblage structure in relation to stream size and tributary position. Hydrobiologia 2005, 539, 121–130. [Google Scholar] [CrossRef]
- Hellmann, J.K.; Erikson, J.S.; Queenborough, S.A. Evaluating macroinvertebrate community shifts in the confluence of freestone and limestone streams. J. Limnol. 2015, 74, 64–74. [Google Scholar] [CrossRef]
- Fleishman, E.; Noss, R.F.; Noon, B.R. Utility and limitations of species richness metrics for conservation planning. Ecol. Indic. 2006, 6, 543–553. [Google Scholar] [CrossRef]
- Heino, J.; Muotka, T.; Paavola, R. Determinants of macroinvertebrate diversity in headwater streams: Regional and local influences. J. Anim. Ecol. 2003, 72, 425–434. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 280–338. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Astorga, A.; Death, R.; Paavola, R.; Chakraborty, M.; Muotka, T. Habitat heterogeneity drives the geographical distribution of beta diversity: The case of New Zealand stream invertebrates. Ecol. Evol. 2014, 4, 2693–2702. [Google Scholar] [CrossRef]
- Ward, J.V.; Stanford, J.A. Thermal responses in the evolutionary ecology of aquatic insects. Annu. Rev. Entomol. 1982, 27, 97–117. [Google Scholar] [CrossRef]
- Barquín, J.; Death, R.G. Spatial patterns of macroinvertebrate diversity in New Zealand springbrooks and rhithral streams. J. N. Am. Benthol. Soc. 2006, 25, 768–786. [Google Scholar] [CrossRef]
- Webb, B.W.; Hannah, D.M.; Moore, R.D.; Brown, L.E.; Nobilis, F. Recent advances in stream and river temperature research. Hydrol. Process. 2008, 22, 902–918. [Google Scholar] [CrossRef]
- Barquín, J.; Death, R.G. Downstream changes in spring-fed stream invertebrate communities: The effect of increased temperature range? J. Limnol. 2011, 70, 134–146. [Google Scholar] [CrossRef][Green Version]
- Ebersole, J.L.; Wigington, P.L., Jr.; Leibowitz, S.G.; Comeleo, R.L.; Van Sickle, J. Predicting the occurrence of cold-water patches at intermittent and ephemeral tributary confluences with warm rivers. Freshw. Sci. 2015, 34, 111–124. [Google Scholar] [CrossRef]
- Mattson, R.A.; Epler, J.H.; Hein, M.K. Description of benthic communities in karst, spring-fed streams of north central Florida. J. Kans. Entomol. Soc. 1995, 68, 18–41. [Google Scholar]
- Benda, L.; Andras, K.; Miller, D.; Bigelow, P. Confluence effects in rivers: Interactions of basin scale, network geometry, and disturbance regimes. Water Resour. Bull. 2004, 40, 1–15. [Google Scholar] [CrossRef]
- Benda, L.; Poff, N.L.; Miller, D.; Dunne, T.; Reeves, G.; Pess, G.; Pollock, M. The network dynamics hypothesis: How channel networks structure riverine habitats. BioScience 2004, 54, 413–427. [Google Scholar] [CrossRef]
- Scarsbrook, M.; Barquin, J.; Gray, D. New Zealand Coldwater Springs and Their Biodiversity. Science for Conservation 278; Science & Technology Publishing, Department of Conservation: Wellington, New Zealand, 2007; p. 72. [Google Scholar]
- Smith, H.; Wood, P.J.; Gunn, J. The influence of habitat structure and flow permanence on invertebrate communities in karst spring systems. Hydrobiologia 2003, 510, 53–66. [Google Scholar] [CrossRef]
- Smith, H.; Wood, P.J. Flow permanence and macroinvertebrate community variability in limestone spring systems. Hydrobiologia 2002, 487, 45–58. [Google Scholar] [CrossRef]
- Lusardi, R.A.; Bogan, M.T.; Moyle, P.B.; Dahlgren, R.A. Environment shapes invertebrate assemblage structure differences between volcanic spring-fed and runoff rivers in northern California. Freshw. Sci. 2016, 35, 1010–1022. [Google Scholar] [CrossRef]
- Caissie, D. The thermal regime of rivers: A review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Vineyard, J.D.; Feder, G.L.; Pflieger, W.L.; Lipscomb, R.G. Springs of Missouri with Sections on Fauna and Flora; Water Resources Report No. 29; Missouri Geological Survey and Water Resources: Rolla, MO, USA, 1974; p. 212. [Google Scholar]
- Bowles, D.E.; Dodd, H.R. Floristics and community ecology of aquatic vegetation occurring in seven large springs at Ozark National Scenic Riverways, Missouri (U.S.A.), 2007–2012. J. Bot. Res. Inst. Tex. 2015, 9, 235–249. [Google Scholar]
- Meinzer, O.E. Large Springs in the United States; U.S. Geological Survey Water-Supply Paper 557; United States Geological Survey: Washington, DC, USA, 1927; p. 94. [Google Scholar]
- Mugel, D.N.; Richards, J.M.; Schumacher, J.G. Geohydrologic Investigations and Landscape Characteristics of Areas Contributing Water to Springs, the Current River, and Jacks Fork, Ozark National Scenic Riverways, Missouri; U.S. Geological Survey Scientific Investigations Report 2009–5138; United States Geological Survey: Washington, DC, USA, 2009; p. 80. [Google Scholar]
- Bowles, D.E.; Luraas, J.A.; Morrison, L.W.; Dodd, H.R.; Williams, M.H.; Rowell, G.A.; DeBacker, M.D.; Hinsey, J.A.; Usrey, F.D.; Haack, J.L. Protocol for Monitoring Aquatic Invertebrates at Ozark National Scenic Riverways, Missouri, and Buffalo National River, Arkansas; Natural Resource Report NPS/HTLN/NRR—2007/00; US National Park Service: Fort Collins, CO, USA, 2007; p. 138. [Google Scholar]
- Moulton, S.R., II; Kennen, J.G.; Goldstein, R.M.; Hambrook, J.A. Revised Protocols for Sampling Algal, Invertebrate, and Fish Communities as Part of the National Water-Quality Assessment Program. Open-File Report 02-150; US Geological Survey: Reston, VT, USA, 2022; p. 75. [Google Scholar]
- Heth, R.L. Diversity of Macroinvertebrates in Tributaries of the Jacks Fork and Current Rivers, Ozark National Scenic Riverways, Missouri and Efficacy of Spring-Fed Tributaries as Refugia. Ph.D. Dissertation, University of Missouri-Columbia, Columbia, MO, USA, 2015; p. 223. [Google Scholar]
- Heth, R.L.; Bowles, D.E.; Havel, J.E. Potential impacts of stream crossing traffic on macroinvertebrate communities in a Missouri Ozark River. River Res. Appl. 2016, 32, 925–934. [Google Scholar] [CrossRef]
- Vinson, M.R.; Hawkins, C.P. Effects of sampling area and subsampling procedure on comparisons of taxa richness among streams. J. N. Am. Benthol. Soc. 1996, 15, 392–399. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Carter, R.W.; Davidian, J. General Procedure for Gaging Streams. Book 3, Chapter A6 of Techniques of Water-Resources Investigations of the United States Geological Survey; United States Government Printing Office: Washington, DC, USA, 1966; p. 13. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Kruskal, J.B. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Wilson, M.V.; Shmida, A. Measuring beta diversity with presence-absence data. J. Ecol. 1984, 72, 1055–1064. [Google Scholar] [CrossRef]
- Bowles, D.E.; Morrison, L.W.; Cribbs, J.T. Aquatic invertebrate community structure, biological condition, habitat, and water-quality at Ozark National Scenic Riverways, Missouri, 2005–2014, Missouri, 2005–2014. J. Ark. Acad. Sci. 2018, 72, 67–80. [Google Scholar]
- Bowles, D.E.; Dodd, H.R.; Hinsey, J.A.; Cribbs, J.T.; Luraas, J.A. Spring Communities Monitoring at Ozark National Scenic Riverways, Missouri: 2007–2009 Status Report; Natural Resource Technical Report NPS/OZAR/NRTR—2011/511; National Park Service: Fort Collins, CO, USA, 2011; p. 41. [Google Scholar]
- Bowles, D.E.; Dodd, H.R.; Hinsey, J.A.; Cribbs, J.T.; Williams, J.M. Aquatic Invertebrate Community Structure in Springs at Ozark National Scenic Riverways, Missouri, 2007–2016; Natural Resource Data Series NPS/HTLN/NRDS—2018/1161; National Park Service: Fort Collins, CO, USA, 2018; p. 37. [Google Scholar]
- Buria, L.; Albarino, R.; Villanueva, V.D.; Modenutti, B.; Balseiro, E. Impact of exotic rainbow trout on the benthic macroinvertebrate community from Andean-Patagonian headwater streams. Fundam. Appl. Limnol. Arch. Fur Hydrobiol. 2007, 168, 145–154. [Google Scholar] [CrossRef]
- Minshall, G.W.; Cummins, K.W.; Peterson, R.C.; Cushing, C.E.; Bruns, D.A.; Sedell, J.R.; Vannote, R.L. Developments in stream ecosystem theory. Can. J. Fish. Aquat. Sci. 1985, 42, 1045–1055. [Google Scholar] [CrossRef]
- Ward, J.V.; Stanford, J.A. The serial discontinuity concept of lotic ecosystems. In Dynamics of Lotic Ecosystems; Fontaine, T.D., Bartell, S.M., Eds.; Ann Arbor Scientific Publishers: Ann Arbor, MI, USA, 1983; pp. 29–42. [Google Scholar]
- Stanford, J.A.; Ward, J.V. Revisiting the serial discontinuity concept. River Res. Appl. 2001, 17, 303–310. [Google Scholar] [CrossRef]
- Svendsen, K.M.; Renshaw, C.E.; Magilligan, F.J.; Nislow, K.H.; Kaste, J.M. Flow and sediment regimes at tributary junctions on a regulated river: Impact on sediment residence time and benthic macroinvertebrate communities. Hydrol. Process. 2009, 23, 284–296. [Google Scholar] [CrossRef]
- Westhoff, J.T.; Paukert, C.P. Climate change simulations predict altered biotic response in a thermally heterogeneous stream system. PLoS ONE 2014, 9, e111438. [Google Scholar] [CrossRef]
- Dong, R.; Wang, Y.; Lu, C.; Lei, G.; Wen, L. The seasonality of macroinvertebrate β diversity along the gradient of hydrological connectivity in a dynamic river-floodplain system. Ecol. Indic. 2021, 121, 107112. [Google Scholar] [CrossRef]
- Gray, D.; Harding, J. Braided river benthic diversity at multiple spatial scales: A hierarchical analysis of ß diversity in complex floodplain systems. J. N. Am. Benthol. Soc. 2009, 28, 537–551. [Google Scholar] [CrossRef]
- Melo, A.S.; Schneck, F.; Hepp, L.U.; Simões, N.R.; Siqueira, T.; Bini, L.M. Focusing on variation: Methods and applications of the concept of beta diversity in aquatic ecosystems. Acta Limnol. Bras. 2011, 23, 318–331. [Google Scholar] [CrossRef][Green Version]
- Castro, D.M.P.; Callisto, M.; Solar, R.R.C.; Macedo, D.R.; Fernandes, G.W. Beta diversity of aquatic invertebrates increases along an altitudinal gradient in a Neotropical mountain. Biotropica 2019, 51, 399–411. [Google Scholar] [CrossRef]
- Gonzales-Yrujillo, J.D.; Alonso-Moreno, Y.L. Habitat simplification changes temporal patterns of invertebrate beta diversity in a high-Andean stream. Neotrop. Biodivers. 2020, 6, 206–216. [Google Scholar] [CrossRef]
- Bowles, D.E.; Dodd, H.R. Aquatic Vegetation Monitoring in Springs at Ozark National Scenic Riverways, 2007–2015; Natural Resource Data Series NPS/OZAR/NRDS—2016/1044; National Park Service: Fort Collins, CO, USA, 2016; p. 21. [Google Scholar]
- Bowles, D.E. Resiliency and recovery of aquatic vegetation following scouring floods in two first-magnitude springs, Missouri, USA. Hydrobiology 2022, 1, 164–182. [Google Scholar] [CrossRef]
- Wang, H.; He, G. Rivers: Linking nature, life, and civilization. River 2022, 1, 25–3612. [Google Scholar] [CrossRef]
- Lusardi, R.A.; Nichols, A.L.; Willis, A.D.; Jeffries, C.A.; Kiers, A.H.; Van Nieuwenhuyse, E.E.; Dahlgren, R.A. Not all rivers are created equal: The importance of spring-fed rivers under a changing climate. Water 2021, 13, 1652. [Google Scholar] [CrossRef]
- Cui, Y.; Mahoney, E.; Herbowicz, T. Economic Benefits to Local Communities from National Park Visitation, 2011; Report No. 48824–6446; Michigan State University, Department of Community, Agriculture, Recreation, and Resource Studies: East Lansing, MI, USA, 2013; p. 36. [Google Scholar]
- Cullinane, T.C.; Huber, C.; Koontz, L. 2012 National Park Visitor Spending Effects: Economic Contributions to Local Communities, States, and the Nation; Natural Resource Report NPS/NRSS/EQD/NRR 2014/765; National Park Service: Fort Collins, CO, USA, 2014; p. 42. [Google Scholar]
- National Park Service (NPS). Ozark National Scenic Riverways, Final General Management Plan/Environmental Impact Statement; Ozark National Scenic Riverways: Van Buren, MO, USA, 2014; p. 616. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).