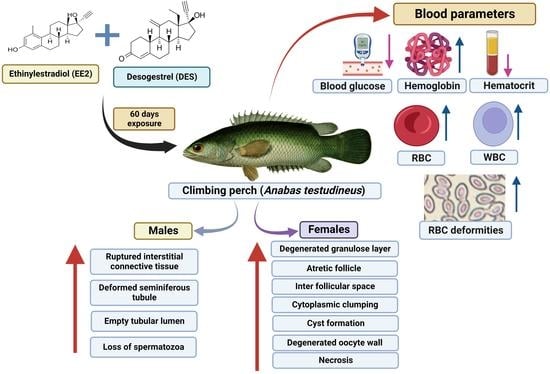
Contraceptive-Pill-Sourced Synthetic Estrogen and Progestogen in Water Causes Decrease in GSI and HSI and Alters Blood Glucose Levels in Climbing Perch (Anabas testudineus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Experimental Setup and Chemical Exposure
2.3. Collection of Gonads and Liver and Estimation of Gonadosomatic Index (GSI) and Hepatosomatic Index (HSI)
2.4. Histopathological Observations of Gonads
2.5. Measurement of Hemato-Biochemical Parameters
2.6. Erythrocytic Cellular Abnormalities (ECA)
2.7. Statistical Analysis
3. Results
3.1. Changes in GSI and HSI
3.2. Changes in Ovarian Histology
3.3. Changes in Histo-Architecture of Testes
3.4. Changes in Hemato-Biochemical Parameters
3.5. Erythrocytic Cellular Abnormalities (ECA)
4. Discussion
4.1. Alterations in GSI and HSI Values
4.2. Histolopathogical Alterations in Ovary
4.3. Histological Alterations in Testes
4.4. Alterations of Blood Cells and Glucose Levels
4.5. Alterations in Erythrocytic Cellular Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, A.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ. Res. 2018, 165, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Nielsen, S.; Lanzky, P.; Ingerslev, F.; Lützhøft, H.H.; Jørgensen, S. Occurrence, fate and effects of pharmaceutical substances in the environment—A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Rabiet, M.; Togola, A.; Brissaud, F.; Seidel, J.-L.; Budzinski, H.; Elbaz-Poulichet, F. Consequences of Treated Water Recycling as Regards Pharmaceuticals and Drugs in Surface and Ground Waters of a Medium-sized Mediterranean Catchment. Environ. Sci. Technol. 2006, 40, 5282–5288. [Google Scholar] [CrossRef] [PubMed]
- Togola, A.; Budzinski, H. Multi-residue analysis of pharmaceutical compounds in aqueous samples. J. Chromatogr. A 2008, 1177, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.D.; Kulis, J.; Thomson, B.; Chapman, T.H.; Mawhinney, D.B. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 2006, 366, 772–783. [Google Scholar] [CrossRef]
- Kümmerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—A review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef]
- Matamoros, V.; Arias, C.; Brix, H.; Bayona, J.M. Preliminary screening of small-scale domestic wastewater treatment systems for removal of pharmaceutical and personal care products. Water Res. 2009, 43, 55–62. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, I.; Callejón, M.; Alonso, E. Occurrence of pharmaceutically active compounds during 1-year period in wastewaters from four wastewater treatment plants in Seville (Spain). J. Hazard. Mater. 2009, 164, 1509–1516. [Google Scholar] [CrossRef]
- Tamtam, F.; Mercier, F.; le Bot, B.; Eurin, J.; Dinh, Q.T.; Clément, M.; Chevreuil, M. Occurrence and fate of antibiotics in the Seine River in various hydrological conditions. Sci. Total Environ. 2008, 393, 84–95. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Mimeault, C.; Woodhouse, A.J.; Miao, X.-S.; Metcalfe, C.D.; Moon, T.W.; Trudeau, V.L. The human lipid regulator, gemfibrozil bioconcentrates and reduces testosterone in the goldfish, Carassius auratus. Aquat. Toxicol. 2005, 73, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; de Biase, A.; Deeva, I.B.; Degan, P.; Doronin, Y.K.; Iaccarino, M.; Oral, R.; Trieff, N.M.; Warnau, M.; Korkina, L.G. The role of oxidative stress in developmental and reproductive toxicity of tamoxifen. Life Sci. 2001, 68, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Laville, N.; Ait-Aissa, S.; Gomez, E.; Casellas, C.; Porcher, J.-M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, J.P.; Kime, D.E.; van der Ven, L.T.M.; Wester, P.W.; Brion, F.; Maack, G.; Stahlschmidt-Allner, P.; Tyler, C.R. Long-Term Exposure to Environmental Concentrations of the Pharmaceutical Ethynylestradiol Causes Reproductive Failure in Fish. Environ. Health Perspect. 2004, 112, 1725–1733. [Google Scholar] [CrossRef] [Green Version]
- Gross-Sorokin, M.Y.; Roast, S.D.; Brighty, G.C. Assessment of Feminization of Male Fish in English Rivers by the Environment Agency of England and Wales. Environ. Health Perspect. 2006, 114, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, D.J.; Mastrocco, F.; Hutchinson, T.H.; Länge, R.; Heijerick, D.; Janssen, C.; Anderson, P.D.; Sumpter, J.P. Derivation of an Aquatic Predicted No-Effect Concentration for the Synthetic Hormone, 17α-Ethinyl Estradiol. Environ. Sci. Technol. 2008, 42, 7046–7054. [Google Scholar] [CrossRef] [Green Version]
- Islam, K.; Haque, R.; Hema, P.S. Regional variations of contraceptive use in Bangladesh: A disaggregate analysis by place of residence. PLoS ONE 2020, 15, e0230143. [Google Scholar] [CrossRef]
- OECD. OECD Studies on Water Pharmaceutical Residues in Freshwater. Hazards And Policy Responses; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Ullah, A.N.Z.; Humble, M.E. Determinants of oral contraceptive pill use and its discontinuation among rural women in Bangladesh. Reprod. Med. Biol. 2006, 5, 111–121. [Google Scholar]
- Thakur, M.; Rachamalla, M.; Niyogi, S.; Datusalia, A.K.; Flora, S.J.S. Molecular Mechanism of Arsenic-Induced Neurotoxicity including Neuronal Dysfunctions. Int. J. Mol. Sci. 2021, 22, 10077. [Google Scholar] [CrossRef]
- Salahinejad, A.; Attaran, A.; Meuthen, D.; Rachamalla, M.; Chivers, D.P.; Niyogi, S. Maternal exposure to bisphenol S induces neuropeptide signaling dysfunction and oxidative stress in the brain, and abnormal social behaviors in zebrafish (Danio rerio) offspring. Sci. Total Environ. 2022, 830, 154794. [Google Scholar] [CrossRef]
- Kumar, A.; Raj, V.; Srivastava, A.; Ali, M.; Ghosh, A.K.; Rachamalla, M.; Kumar, D. Autophagy in arsenic exposed population and cancer patients. In Autophagy and Metabolism; Academic Press: Cambridge, MA, USA, 2022; pp. 141–161. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Bottoni, P.; Caroli, S.; Caracciolo, A. Pharmaceuticals as priority water contaminants. Toxicol. Environ. Chem. 2010, 92, 549–565. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Tirumala, M.G.; Anchi, P.; Raja, S.; Rachamalla, M.; Godugu, C. Novel Methods and Approaches for Safety Evaluation of Nanoparticle Formulations: A Focus Towards In Vitro Models and Adverse Outcome Pathways. Front. Pharmacol. 2021, 12, 2157. [Google Scholar] [CrossRef]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.J. Contamination of Surface, Ground, and Drinking Water from Pharmaceutical Production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Saal, F.S.V.; Tillitt, D.E. Transgenerational effects from early developmental exposures to bisphenol A or 17α-ethinylestradiol in medaka, Oryzias latipes. Sci. Rep. 2015, 5, srep09303. [Google Scholar] [CrossRef] [Green Version]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef] [Green Version]
- Siegenthaler, P.F.; Zhao, Y.; Zhang, K.; Fent, K. Reproductive and transcriptional effects of the antiandrogenic progestin chlormadinone acetate in zebrafish (Danio rerio). Environ. Pollut. 2017, 223, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Crane, M.; Maltby, L. The lethal and sublethal responses ofgammarus pulexto stress: Sensitivity and sources of variation in an in situ bioassay. Environ. Toxicol. Chem. 1991, 10, 1331–1339. [Google Scholar] [CrossRef]
- Cajaraville, M.P.; Bebianno, M.J.; Blasco, J.; Porte, C.; Sarasquete, C.; Viarengo, A. The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: A practical approach. Sci. Total Environ. 2000, 247, 295–311. [Google Scholar] [CrossRef]
- Chiang, M.W.-L.; Au, D.W.-T. Histopathological Approaches in Ecotoxicology. In Encyclopedia of Aquatic Ecotoxicology; Springer: Berlin, Germany, 2013; pp. 597–614. [Google Scholar] [CrossRef]
- Matthiessen, P.; Wheeler, J.; Weltje, L. A review of the evidence for endocrine disrupting effects of current-use chemicals on wildlife populations. Crit. Rev. Toxicol. 2017, 48, 195–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yancheva, V.; Velcheva, I.; Stoyanova, S. Georgieva Histological Biomarkers in Fish as a Tool in Ecological Risk Assessment and Monitoring Programs: A Review. Appl. Ecol. Environ. Res. 2016, 14, 47–75. [Google Scholar] [CrossRef]
- Ruane, N.; Nolan, D.; Rotllant, J.; Costelloe, J.; Bonga, S.W. Experimental exposure of rainbow trout Oncorhynchus mykiss (Walbaum) to the infective stages of the sea louseLepeophtheirus salmonis (Krøyer) influences the physiological response to an acute stressor. Fish Shellfish Immunol. 2000, 10, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Okomoda, V.T.; Ataguba, G.; Ayuba, V. Hematological Response of Clarias Gariepinus Fingerlings Exposed to Acute Concentrations of Sunsate. J. Stress Physiol. Biochem. 2013, 9, 271–278. [Google Scholar]
- Lohner, T.W.; Reash, R.J.; Willetb, V.E.; Rose, L.A. Assessment of Tolerant Sunfish Populations (Lepomis sp.) Inhabiting Selenium-Laden Coal Ash Effluents: 1. Hematological and Population Level Assessment. Ecotoxicol. Environ. Saf. 2001, 50, 203–216. [Google Scholar] [CrossRef]
- Cazenave, J.; Wunderlin, D.A.; Hued, A.C.; Bistoni, M.D.L.A. Haematological parameters in a neotropical fish, Corydoras paleatus (Jenyns, 1842) (Pisces, Callichthyidae), captured from pristine and polluted water. Hydrobiologia 2005, 537, 25–33. [Google Scholar] [CrossRef]
- Elahee, K.; Bhagwant, S. Hematological and gill histopathological parameters of three tropical fish species from a polluted lagoon on the west coast of Mauritius. Ecotoxicol. Environ. Saf. 2007, 68, 361–371. [Google Scholar] [CrossRef]
- Sharmin, S.; Shahjahan, M.; Hossain, M.A.; Haque, M.A.; Rashid, H. Histopathological Changes in Liver and Kidney of Common Carp Exposed to Sub-Lethal Doses of Malathion. Pak. J. Zool. 2015, 47, 1495–1498. [Google Scholar]
- Salam, M.A.; Shahjahan, M.; Sharmin, S.; Haque, F.; Rahman, M.K. Effects of Sub-Lethal Doses of an Organophosphorus Insecticide Sumithion on Some Hematological Parameters in Common Carp, Cyprinus carpio. Pak. J. Zool. 2015, 47, 1487–1491. [Google Scholar]
- Islam, J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World Aquac. Soc. 2021, 53, 314–366. [Google Scholar] [CrossRef]
- Islam, J.; Slater, M.J.; Bögner, M.; Zeytin, S.; Kunzmann, A. Extreme ambient temperature effects in European seabass, Dicentrarchus labrax: Growth performance and hemato-biochemical parameters. Aquaculture 2020, 522, 735093. [Google Scholar] [CrossRef]
- Sharkey, L.C. Veterinary Hematology and Clinical Chemistry. Vet. Clin. Pathol. 2004, 33, 252-252. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2018, 500, 237–242. [Google Scholar] [CrossRef]
- Bhat, R.A.; Saoca, C.; Cravana, C.; Fazio, F.; Guerrera, M.C.; Labh, S.N.; Kesbiç, O.S. Effects of heavy pollution in different water bodies on male rainbow trout (Oncorhynchus mykiss) reproductive health. Environ. Sci. Pollut. Res. 2022, 1–13. [Google Scholar] [CrossRef]
- Munshi, J.S.D.; Hughes, G.M. Gross and fine structure of the pseudo branch of the climbing perch, Anabas testudineus (Bloch). J. Fish Biol. 1981, 19, 427–438. [Google Scholar] [CrossRef]
- Olson, K.R.; Munshi, J.S.D.; Ghosh, T.K.; Ojha, J. Gill microcirculation of the air-breathing climbing perch, Anabas testudineus (Bloch): Relationships with the accessory respiratory organs and systemic circulation. Am. J. Anat. 1986, 176, 305–320. [Google Scholar] [CrossRef]
- Van Trieu, N.; Long, D.N. Seed Production Technology of Climbing Perch (Anabas Testudineus): A Study on the Larval Rearing. In The 2001 Annual Workshop of JIRCAS; 2001. [Google Scholar]
- Wang, T.-Y.; Tzeng, C.-S.; Shen, S.-C. Conservation and Phylogeography of Taiwan Paradise Fish, Macropodus Opercularis Linnaeus. Acta Zool. Taiwanica 1999, 2, 121–134. [Google Scholar]
- Ahmed, K.; Parvin, E.; Arif, M.; Akter, M.S.; Khan, M.S.; Islam, M. Measurements of genotoxic potential of cadmium in different tissues of fresh water climbing perch Anabas testudineus (Bloch), using the comet assay. Environ. Toxicol. Pharmacol. 2010, 30, 80–84. [Google Scholar] [CrossRef]
- Farhad Hossain, M.; Rahman, M.M.; Sayed, M. Experimental Infection of Indigenous Climbing Perch Anabas testudineus with Aeromonas hydrophila Bacteria. Progress. Agric. 2013, 22, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Tam, N.T.; Berg, H.; Tuyen, P.T.B.; van Cong, N. Effect of Chlorpyrifos Ethyl on Acetylcholinesterase Activity in Climbing Perch (Anabas testudineus, Bloch, 1972). Arch. Environ. Contam. Toxicol. 2015, 69, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Zuryani, H.; Muslimin, B.; Khotimah, K. Feminization of Climbing Perch, Anabas testudineus (Bloch, 1792) through Larvae Immersion Milk Solutions and Soy Milk. J. Iktiologi Indones. 2018, 17, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Al-Rasheed, A.; Handool, K.O.; Garba, B.; Noordin, M.; Bejo, S.K.; Kamal, F.M.; Daud, H.H.M. Crude extracts of epidermal mucus and epidermis of climbing perch Anabas testudineus and its antibacterial and hemolytic activities. Egypt. J. Aquat. Res. 2018, 44, 125–129. [Google Scholar] [CrossRef]
- Chakraborty, B.K. Sustainable Aquaculture Practice of Climbing Perch Koi, Anabas testudineus (Bloch, 1792) Under Semi Intensive Aquaculture System in Bangladesh. Proc. Zool. Soc. 2015, 69, 133–140. [Google Scholar] [CrossRef]
- Zworykin, D.D. Reproduction and spawning behavior of the climbing perch Anabas testudineus (Perciformes, Anabantidae) in an aquarium. J. Ichthyol. 2012, 52, 379–388. [Google Scholar] [CrossRef]
- Nasri, A.; Mezni, A.; Lafon, P.-A.; Wahbi, A.; Cubedo, N.; Clair, P.; Harrath, A.H.; Beyrem, H.; Rossel, M.; Perrier, V. Ethinylestradiol (EE2) residues from birth control pills impair nervous system development and swimming behavior of zebrafish larvae. Sci. Total Environ. 2021, 770, 145272. [Google Scholar] [CrossRef] [PubMed]
- Ojoghoro, J.; Scrimshaw, M.; Sumpter, J. Steroid hormones in the aquatic environment. Sci. Total Environ. 2021, 792, 148306. [Google Scholar] [CrossRef]
- Barber, B.J.; Blake, N.J. Chapter 6 Reproductive Physiology. In Scallops: Biology, Ecology and Aquaculture; 2006; pp. 357–416. [Google Scholar] [CrossRef]
- Sumon, K.A.; Yesmin, M.F.; Brink, P.J.V.D.; Bosma, R.H.; Peeters, E.T.H.M.; Rashid, H. Effects of long-term chlorpyrifos exposure on mortality and reproductive tissues of Banded Gourami (Trichogaster fasciata). J. Environ. Sci. Health Part B 2019, 54, 549–559. [Google Scholar] [CrossRef]
- Sarma, P.R. Red Cell Indices. Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths Publishers: Salem, UK, 1990. [Google Scholar]
- Carrasco, K.R.; Tilbury, K.L.; Myers, M.S. Assessment of the Piscine Micronucleus Test as an in situ Biological indicator of Chemical Contaminant Effects. Can. J. Fish. Aquat. Sci. 1990, 47, 2123–2136. [Google Scholar] [CrossRef]
- Mahboob, S.; Sheri, A.N. Relationships among Gonad Weight, Liver Weight and Body Weight of Major, Common and Some Chinese Carps under Composite Culture System with Special Reference to Pond Fertilization. Asian-Australas. J. Anim. Sci. 2002, 15, 740–744. [Google Scholar] [CrossRef]
- Van den Belt, K.; Wester, P.W.; van der Ven, L.T.M.; Verheyen, R.; Witters, H. Effects of Ethynylestradiol on the Reproductive Physiology in Zebrafish (Danio rerio): Time Dependency and Reversibility. Environ. Toxicol. Chem. 2002, 21, 767–775. [Google Scholar] [CrossRef]
- Zha, J.; Wang, Z.; Wang, N.; Ingersoll, C. Histological alternation and vitellogenin induction in adult rare minnow (Gobiocypris rarus) after exposure to ethynylestradiol and nonylphenol. Chemosphere 2006, 66, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.L.; Blunt, B.R. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environ. Toxicol. 2005, 20, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Bessho, H.; Hara, A.; Nakamura, M.; Iguchi, T.; Fujita, K. Elevated serum vitellogenin levels and gonadal abnormalities in wild male flounder (Pleuronectes yokohamae) from Tokyo Bay, Japan. Mar. Environ. Res. 2000, 49, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.J.; Yokota, H.; Oshima, Y.; Tsuruda, Y.; Shimasaki, Y.; Honjo, T. The effects of methyltestosterone on the sexual development and reproduction of adult medaka (Oryzias latipes). Aquat. Toxicol. 2008, 87, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Oropesa, A.L.; Jiménez, B.; Gil, M.C.; Osswald, J.; Fallola, C.; Pula, H.J.; Cuesta, J.M.; Gómez, L. Histological alterations in the structure of the testis in tench (Tinca tinca) after exposure to 17 alpha-ethynylestradiol. Environ. Toxicol. 2013, 29, 1182–1192. [Google Scholar] [CrossRef]
- Depiereux, S.; Liagre, M.; Danis, L.; de Meulder, B.; Depiereux, E.; Segner, H.; Kestemont, P. Intersex Occurrence in Rainbow Trout (Oncorhynchus mykiss) Male Fry Chronically Exposed to Ethynylestradiol. PLoS ONE 2014, 9, e98531. [Google Scholar] [CrossRef] [Green Version]
- Miller, H.D.; Clark, B.W.; Hinton, D.E.; Whitehead, A.; Martin, S.; Kwok, K.W.; Kullman, S.W. Anchoring Ethinylestradiol Induced Gene Expression Changes with Testicular Morphology and Reproductive Function in the Medaka. PLoS ONE 2012, 7, e52479. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, C.; Malarvizhi, A.; Kumaran, S.S.; Ramesh, M. Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem. Toxicol. 2010, 48, 2848–2854. [Google Scholar] [CrossRef]
- Simonato, J.D.; Guedes, C.; Martinez, C. Biochemical, physiological, and histological changes in the neotropical fish Prochilodus lineatus exposed to diesel oil. Ecotoxicol. Environ. Saf. 2008, 69, 112–120. [Google Scholar] [CrossRef]
- Saravanan, M.; Karthika, S.; Malarvizhi, A.; Ramesh, M. Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: Hematological, biochemical, ionoregulatory and enzymological responses. J. Hazard. Mater. 2011, 195, 188–194. [Google Scholar] [CrossRef]
- Winkaler, E.U.; Santos, T.R.; Machado-Neto, J.G.; Martinez, C.B. Acute lethal and sublethal effects of neem leaf extract on the neotropical freshwater fish Prochilodus lineatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Lusková, V.; Drastichová, J.; Žlábek, V. The Effect of Diazinon on Haematological Indices of Common Carp (Cyprinus carpio L.). Acta Veter-Brno 2001, 70, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Talas, Z.S.; Gulhan, M.F. Effects of various propolis concentrations on biochemical and hematological parameters of rainbow trout (Oncorhynchus mykiss). Ecotoxicol. Environ. Saf. 2009, 72, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, B.; Pathiratne, A. Multiple biomarker responses of Nile tilapia (Oreochromis niloticus) exposed to textile industry effluents reaching Bolgoda North Lake, Sri Lanka. Sri Lanka J. Aquat. Sci. 2013, 15, 1–11. [Google Scholar] [CrossRef]
- Washburn, B.S.; Krantz, J.S.; Avery, E.H.; Freedland, R.A. Effects of estrogen on gluconeogenesis and related parameters in male rainbow trout. Am. J. Physiol. Integr. Comp. Physiol. 1993, 264, R720–R725. [Google Scholar] [CrossRef]
- Washburn, B.S.; Bruss, M.L.; Avery, E.H.; Freedland, R.A. Effects of estrogen on whole animal and tissue glucose use in female and male rainbow trout. Am. J. Physiol. Integr. Comp. Physiol. 1992, 263, R1241–R1247. [Google Scholar] [CrossRef]
- Pinto, P.I.S.; Estêvão, M.D.; Power, D.M. Effects of Estrogens and Estrogenic Disrupting Compounds on Fish Mineralized Tissues. Mar. Drugs 2014, 12, 4474–4494. [Google Scholar] [CrossRef] [Green Version]
- Chaves-Pozo, E.; García-Ayala, A.; Cabas, I. Effects of Sex Steroids on Fish Leukocytes. Biology 2018, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Pirsaheb, M. Investigation of steroid hormone residues in fish: A systematic review. Process Saf. Environ. Prot. 2021, 152, 14–24. [Google Scholar] [CrossRef]
- Schwaiger, J.; Spieser, O.; Bauer, C.; Ferling, H.; Mallow, U.; Kalbfus, W.; Negele, R. Chronic toxicity of nonylphenol and ethinylestradiol: Haematological and histopathological effects in juvenile Common carp (Cyprinus carpio). Aquat. Toxicol. 2000, 51, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.P.; Hill, R.L.; Janz, D.M. Developmental estrogenic exposure in zebrafish (Danio rerio): II. Histological evaluation of gametogenesis and organ toxicity. Aquat. Toxicol. 2003, 63, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Reproductive endocrine function in female atlantic croaker exposed to pollutants. Mar. Environ. Res. 1988, 24, 179–183. [Google Scholar] [CrossRef]
- Flores-Valverde, A.M.; Horwood, J.; Hill, E.M. Disruption of the Steroid Metabolome in Fish Caused by Exposure to the Environmental Estrogen 17α-Ethinylestradiol. Environ. Sci. Technol. 2010, 44, 3552–3558. [Google Scholar] [CrossRef]



| Parameters | Treatments | Sampling Days | |||
|---|---|---|---|---|---|
| Male | Female | ||||
| Day 45 | Day 60 | Day 45 | Day 60 | ||
| GSI (± S.D.) | T0 | 0.42 ± 0.12 a | 0.62 ± 0.35 a | 3.57 ± 0.12 a | 5.92 ± 1.59 a |
| T3 | 0.68 ± 0.09 b | 1.12 ± 0.29 c | 6.34 ± 0.15 b | 7.64 ± 0.45 b | |
| T30 | 0.74 ± 0.15 b | 0.88 ± 0.26 b | 7.78 ± 0.11 b | 9.54 ± 0.92 b | |
| T300 | 0.57 ± 0.11 a | 0.67 ± 0.34 a | 9.03 ± 0.19 c | 13.33 ± 0.44 c | |
| HSI (± S.D.) | T0 | 1.04 ± 0.53 a | 1.15 ± 0.59 a | 1.28 ± 0.08 a | 1.34 ± 0.04 b |
| T3 | 1.48 ± 0.11 b | 1.54 ± 0.27 b | 1.22 ± 0.12 a | 1.03 ± 0.09 a | |
| T30 | 1.73 ± 0.36 c | 1.74 ± 0.23 c | 1.19 ± 0.06 a | 1.19 ± 0.24 a | |
| T300 | 1.53 ± 0.07 b | 1.49 ± 0.29 b | 1.12 ± 0.01 a | 1.11 ± 0.40 a | |
| Alterations | Treatment | Exposure Duration (Days) | |
|---|---|---|---|
| 45 | 60 | ||
| Degenerated granulose layer (DGL) | T0 | − | − |
| T3 | − | + | |
| T30 | + | ++ | |
| T300 | +++ | +++ | |
| Atretic follicle (AF) | T0 | − | − |
| T3 | + | + | |
| T30 | − | ++ | |
| T300 | ++ | +++ | |
| Inter follicular space (IFS) | T0 | − | − |
| T3 | + | + | |
| T30 | ++ | + | |
| T300 | +++ | +++ | |
| Adhesion (AD) | T0 | − | − |
| T3 | − | + | |
| T30 | + | ++ | |
| T300 | ++ | +++ | |
| Cytoplasmic clumping (CC) | T0 | − | − |
| T3 | − | − | |
| T30 | + | ++ | |
| T300 | + | ++ | |
| Cysts (CT) | T0 | − | − |
| T3 | − | + | |
| T30 | ++ | + | |
| T300 | ++ | ++ | |
| Degenerated oocyte wall (DOW) | T0 | − | − |
| T3 | + | + | |
| T30 | ++ | ++ | |
| T300 | ++ | ++ | |
| Necrosis (NE) | T0 | − | − |
| T3 | − | + | |
| T30 | + | + | |
| T300 | ++ | ++ | |
| Alterations | Treatment | Exposure Duration (Days) | |
|---|---|---|---|
| 45 | 60 | ||
| Ruptured interstitial connective tissue (rIST) | T0 | − | − |
| T3 | + | ++ | |
| T30 | ++ | ++ | |
| T300 | ++ | ++ | |
| Deformed seminiferous tubule (dST) | T0 | − | − |
| T3 | + | + | |
| T30 | + | + | |
| T300 | ++ | ++ | |
| Empty tubular lumen (eL) | T0 | − | − |
| T3 | ++ | ++ | |
| T30 | ++ | ++ | |
| T300 | +++ | +++ | |
| Loss of spermatozoa (sSZ) | T0 | − | − |
| T3 | + | ++ | |
| T30 | ++ | ++ | |
| T300 | +++ | +++ | |
| Parameters | Treatments | Sampling Days | |||
|---|---|---|---|---|---|
| Male | Female | ||||
| 45 Day | 60 Day | 45 Day | 60 Day | ||
| Blood glucose (mg/dL) | T0 | 168.3 ± 0.03 c | 181.3 ± 0.03 c | 86.0± 0.05 a | 120.3 ± 0.03 b |
| T3 | 101.3 ± 0.04 b | 122.0 ± 0.07 b | 131.3 ± 0.07 b | 111.3 ± 0.05 b | |
| T30 | 117.6 ± 0.06 b | 110.3 ± 0.09 a | 120.3 ± 0.11 b | 104.3 ± 0.07 a | |
| T300 | 85.0 ± 0.07 a | 112.3 ± 0.11 a | 153.3 ± 0.01 c | 118.0 ± 0.03 b | |
| Blood hemoglobin (Hb %) | T0 | 7.5 ± 0.01 b | 11.9 ± 0.03 b | 6.6 ± 0.01 b | 13.0 ± 0.01 c |
| T3 | 8.0 ± 0.07 b | 11.6 ± 0.05 b | 6.9 ± 0.03 b | 12.8 ± 0.03 b | |
| T30 | 6.4 ± 0.11 a | 10.5 ± 0.19 a | 5.3 ± 0.05 a | 11.9 ± 0.05 a | |
| T300 | 6.1 ± 0.18 a | 12.3 ± 0.28 c | 5.6 ± 0.17 a | 11.6 ± 0.19 a | |
| RBC (×106/mm3) | T0 | 0.19 ± 0.01 a | 0.39 ± 0.01 c | 0.25 ± 0.01 b | 0.31 ± 0.01 c |
| T3 | 0.31 ± 0.04 b | 0.33 ± 0.05 b | 0.25 ± 0.07 b | 0.24 ± 0.05 b | |
| T30 | 0.19 ± 0.04 a | 0.25 ± 0.04 a | 0.17 ± 0.04 a | 0.20 ± 0.04 a | |
| T300 | 0.18 ± 0.11 a | 0.27 ± 0.17 a | 0.18 ± 0.09 a | 0.17 ± 0.07 a | |
| WBC (×103/mm3) | T0 | 1.19 ± 0.03 b | 1.12 ± 0.03 a | 0.96 ± 0.05 a | 1.01 ± 0.07 a |
| T3 | 1.01 ± 0.05 a | 1.37 ± 0.04 b | 0.88 ± 0.03 a | 1.13 ± 0.09 b | |
| T30 | 1.08 ± 0.04 a | 1.42 ± 0.05 b | 1.15 ± 0.04 b | 1.35 ± 0.11 b | |
| T300 | 1.47 ± 0.13 c | 1.55 ± 0.17 c | 1.29 ± 0.04 c | 1.50 ± 0.13 c | |
| Hematocrit (%) | T0 | 22.5 ± 0.03 b | 35.7 ± 0.03 b | 19.8 ± 0.05 b | 39.0 ± 0.05 c |
| T3 | 24.0 ± 0.05 c | 34.8 ± 0.09 b | 20.7 ± 0.07 b | 35.1 ± 0.05 a | |
| T30 | 19.2 ± 0.06 a | 31.5 ± 0.06 a | 15.9 ± 0.09 a | 35.7 ± 0.09 a | |
| T300 | 18.3 ± 0.09 a | 36.9 ± 0.11 b | 16.8 ± 0.07 a | 34.8 ± 0.09 a | |
| Mean corpuscular volume (MCV) | T0 | 89.9 ± 0.05 | 90.0 ± 0.09 | 90.1 ± 0.03 | 89.8 ± 0.09 |
| T3 | 90.0 ± 0.03 | 89.9 ± 0.03 | 90.1 ± 0.04 | 90.0 ± 0.03 | |
| T30 | 90.1 ± 0.06 | 90.2 ± 0.05 | 90.4 ± 0.17 | 89.9 ± 0.07 | |
| T300 | 90.4 ± 0.09 | 90.0 ± 0.11 | 90.0 ± 0.15 | 90.1 ± 0.03 | |
| Mean corpuscular hemoglobin (MCH) | T0 | 29.9 ± 0.11 | 30 ± 0.09 | 30.0 ± 0.19 | 29.9 ± 0.03 |
| T3 | 30.0 ± 0.15 | 29.9 ± 0.14 | 30.0 ± 0.05 | 30.0 ± 0.09 | |
| T30 | 30.0 ± 0.07 | 30.1 ± 0.11 | 30.1 ± 0.06 | 29.9 ± 0.08 | |
| T300 | 30.1 ± 0.06 | 30 ± 0.01 | 30 ± 0.09 | 30.0 ± 0.06 | |
| Mean corpuscular hemoglobin concentration (MCHC) | T0 | 33.3 ± 0.13 | 33.3 ± 0.09 | 33.3 ± 0.14 | 33.2 ± 0.16 |
| T3 | 33.3 ± 0.18 | 33.2 ± 0.07 | 33.3 ± 0.12 | 33.3 ± 0.17 | |
| T30 | 33.3 ± 0.14 | 33.4 ± 0.17 | 33.4 ± 0.09 | 33.2 ± 0.03 | |
| T300 | 33.4 ± 0.12 | 33.3 ± 0.12 | 33.3 ± 0.07 | 33.3 ± 0.09 | |
| ECA | Treatments | Percentage of ECA | |||
|---|---|---|---|---|---|
| Male | Female | ||||
| 45 Day | 60 Day | 45 Day | 60 Day | ||
| Teardrop | T0 | 0.21 ± 0.01 a | 0.51 ± 0.01 a | 0.12 ± 0.01 a | 0.22 ± 0.01 a |
| T3 | 0.63 ± 0.04 b | 0.93 ± 0.05 b | 0.55 ± 0.07 b | 0.45 ± 0.05 b | |
| T30 | 1.15 ± 0.04 c | 1.25 ± 0.04 c | 0.55 ± 0.04 b | 0.59 ± 0.04 b | |
| T300 | 1.63 ± 0.11 c | 1.73 ± 0.17 c | 0.83 ± 0.09 c | 0.98 ± 0.07 c | |
| Fusion | T0 | 0.38 ± 0.03 a | 0.35 ± 0.03 a | 0.36 ± 0.05 a | 0.39 ± 0.05 a |
| T3 | 0.47 ± 0.05 b | 0.75 ± 0.09 b | 0.61 ± 0.07 b | 0.64 ± 0.05 b | |
| T30 | 0.87 ± 0.06 c | 0.79 ± 0.06 b | 1.11 ± 0.09 c | 0.91 ± 0.09 b | |
| T300 | 0.98 ± 0.09 c | 0.89 ± 0.11 c | 1.63 ± 0.07 c | 1.85 ± 0.09 c | |
| Elongated | T0 | 0.68 ± 0.03 a | 0.81 ± 0.03 a | 0.86 ± 0.05 a | 0.80 ± 0.03 a |
| T3 | 1.11 ± 0.04 b | 1.22 ± 0.07 a | 1.01 ± 0.07 b | 1.11 ± 0.05 a | |
| T30 | 1.47 ± 0.06 c | 1.56 ± 0.09 b | 1.11 ± 0.11 b | 1.14 ± 0.07 b | |
| T300 | 1.55 ± 0.07 c | 1.72 ± 0.11 c | 1.53 ± 0.01 c | 1.18 ± 0.03 b | |
| Echinocytic | T0 | 0.11 ± 0.01 a | 0.17 ± 0.03 a | 0.21 ± 0.01 a | 0.41 ± 0.01 a |
| T3 | 0.43 ± 0.07 a | 0.65 ± 0.05 b | 0.51 ± 0.03 b | 0.73 ± 0.03 b | |
| T30 | 0.95 ± 0.11 b | 1.15 ± 0.19 c | 0.89 ± 0.05 b | 0.98 ± 0.05 b | |
| T300 | 1.43 ± 0.18 b | 1.73 ± 0.28 c | 1.53 ± 0.17 c | 1.89 ± 0.19 c | |
| Twin | T0 | 0.31 ± 0.01 a | 0.91 ± 0.01 a | 0.12 ± 0.01 a | 0.22 ± 0.01 a |
| T3 | 0.63 ± 0.04 b | 1.13 ± 0.05 b | 0.65 ± 0.07 b | 0.45 ± 0.05 b | |
| T30 | 1.05 ± 0.04 b | 1.45 ± 0.04 c | 0.45 ± 0.04 b | 0.55 ± 0.04 b | |
| T300 | 1.93 ± 0.11 c | 1.98 ± 0.17 c | 0.93 ± 0.09 c | 0.83 ± 0.07 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerasinghe, C.; Akhtar, N.; Uddin, M.H.; Rachamalla, M.; Sumon, K.A.; Islam, M.J.; Bhandari, R.K.; Rashid, H. Contraceptive-Pill-Sourced Synthetic Estrogen and Progestogen in Water Causes Decrease in GSI and HSI and Alters Blood Glucose Levels in Climbing Perch (Anabas testudineus). Hydrobiology 2023, 2, 19-35. https://doi.org/10.3390/hydrobiology2010002
Weerasinghe C, Akhtar N, Uddin MH, Rachamalla M, Sumon KA, Islam MJ, Bhandari RK, Rashid H. Contraceptive-Pill-Sourced Synthetic Estrogen and Progestogen in Water Causes Decrease in GSI and HSI and Alters Blood Glucose Levels in Climbing Perch (Anabas testudineus). Hydrobiology. 2023; 2(1):19-35. https://doi.org/10.3390/hydrobiology2010002
Chicago/Turabian StyleWeerasinghe, Chathuri, Noreen Akhtar, Md Helal Uddin, Mahesh Rachamalla, Kizar Ahmed Sumon, Md. Jakiul Islam, Ramji Kumar Bhandari, and Harunur Rashid. 2023. "Contraceptive-Pill-Sourced Synthetic Estrogen and Progestogen in Water Causes Decrease in GSI and HSI and Alters Blood Glucose Levels in Climbing Perch (Anabas testudineus)" Hydrobiology 2, no. 1: 19-35. https://doi.org/10.3390/hydrobiology2010002
APA StyleWeerasinghe, C., Akhtar, N., Uddin, M. H., Rachamalla, M., Sumon, K. A., Islam, M. J., Bhandari, R. K., & Rashid, H. (2023). Contraceptive-Pill-Sourced Synthetic Estrogen and Progestogen in Water Causes Decrease in GSI and HSI and Alters Blood Glucose Levels in Climbing Perch (Anabas testudineus). Hydrobiology, 2(1), 19-35. https://doi.org/10.3390/hydrobiology2010002