Glochidia Infection of Endemic Fishes from Lake Prespa, N. Macedonia
Abstract
1. Introduction
- -
- Agriculture (through the introduction of agro-ecological practices);
- -
- Forestry (erosion control by reforestation and control of torrents);
- -
- River pollution (wetland restoration techniques would be used for flood control and water filtering of the Golema Reka River);
- -
- Wastewaters (use of wetlands to upgrade the technology of the existing municipal wastewater treatment plant for nutrient removal);
- -
- Solid waste (upgrade of the agricultural waste management systems).
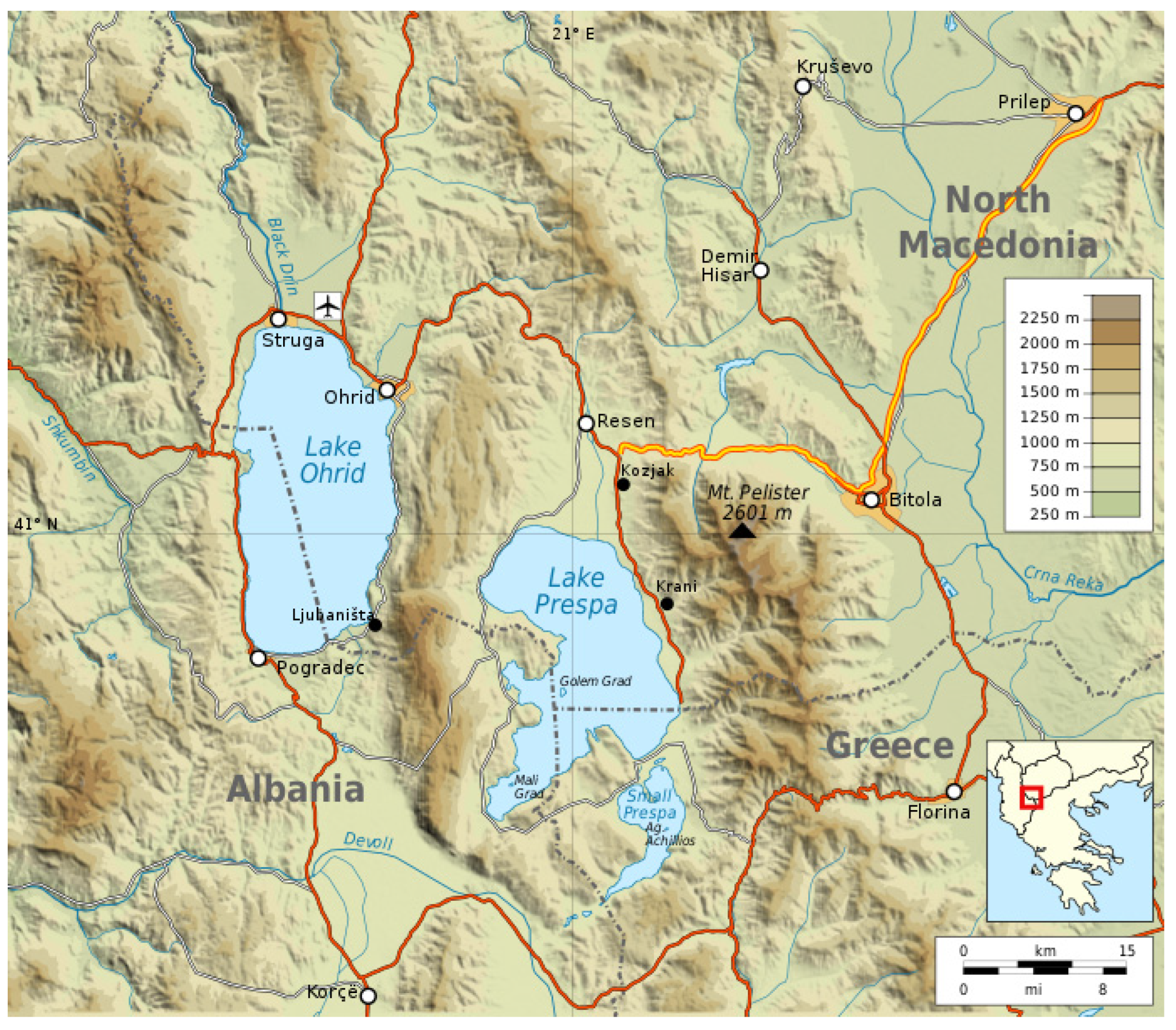
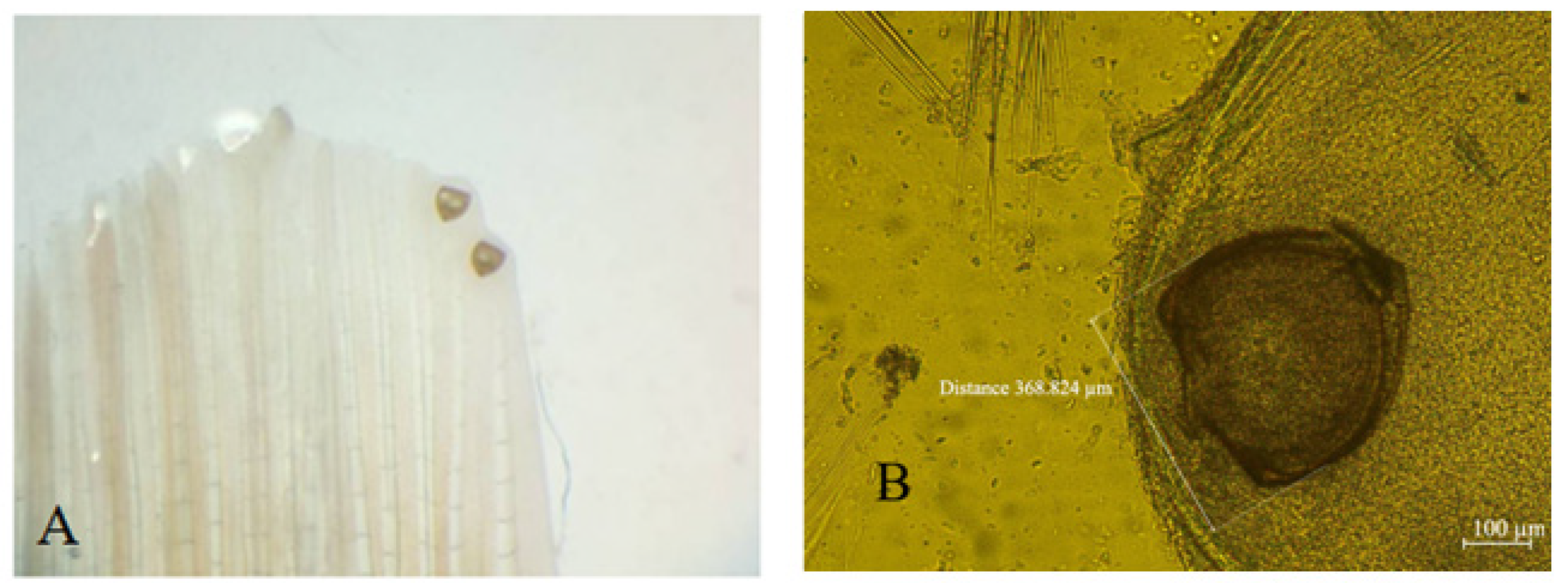
2. Materials and Methods
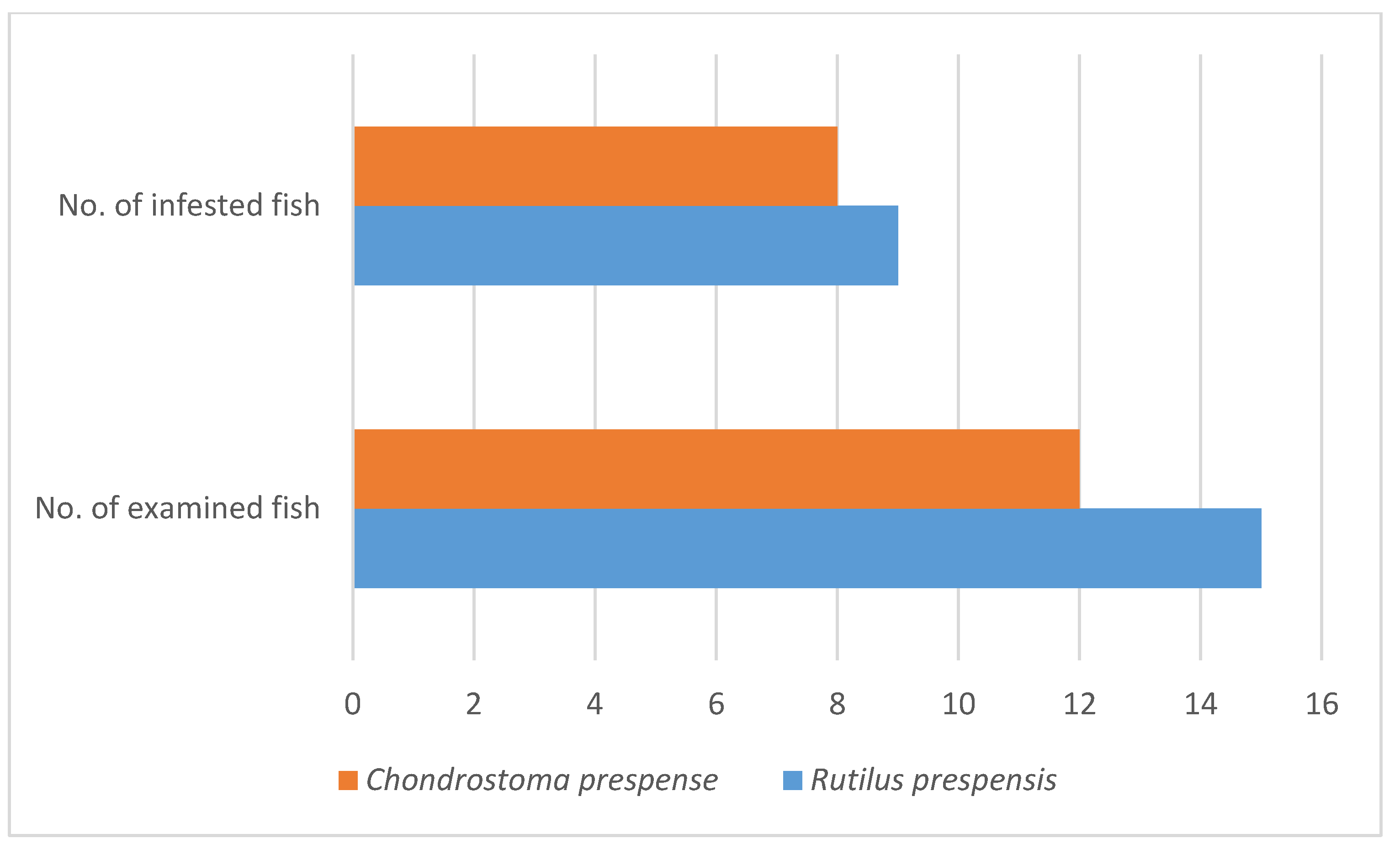
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- Petrovska, M.; Conevski, T.; Krstić, S.; Blinkov, I.; Stavric, V.; Cukaliev, O.; Longholt, J.; Ristovski, I.; Kočovski, Z.; Minčev, I.; et al. Prespa Lake Watershed Management Plan; Report; Ministry of Environment and Physical Planning: Skopje, North Macedonia, 2014.
- Restoration of the Prespa Lake Ecosystem-Implmention of the Prespa Lake Watershed Management Plan, UNDP Project Final Report, 2012.
- Benedict, A.; Geist, J. Early life-Cycle in Freshwater Mussel Conservation and Management; Book of Abstracts; The European Congress of Malacological Societes: Prague, Czech Republic, 2021; p. 55. [Google Scholar]
- Ćmiel, A.M.; Dołęga, J.; Aldridge, D.C.; Lipińska, A.; Tang, F.; Zając, K.; Lopes-Lima, M.; Zając, T. The size and shape of parasitic larvae of naiads (Unionidae) are not dependent on female size. Sci. Rep. 2021, 11, 23755. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.R.; Marjomäki, T.J.; Taskinen, J. Effect of glochidia infection on growth of fish: Freshwater pearl mussel Margaritifera margaritifera and brown trout Salmo trutta. Hydrobiologia 2019, 848, 3179–3189. [Google Scholar] [CrossRef]
- Horký, P.; Douda, K.; Maciak, M.; Závorka, L.; Slavik, O. Parasite-induced alterations of host behaviour in a riverine fish: The effects of glochidia on host dispersal. Freshw. Biol. 2014, 59, 1452–1461. [Google Scholar] [CrossRef]
- Taeubert, J.E.; Geist, J. Critical swimming speed of brown trout (Salmo trutta) infected with freshwater pearl mussel (Margaritifera margaritifera) glochidia and implications for artificial breeding of an endangered mussel species. Parasitol. Res. 2013, 112, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Douda, K.; Liu, H.-Z.; Yu, D.; Rouchet, R.; Liu, F.; Tang, Q.-Y.; Methling, C.; Smith, C.; Reichard, M. The role of local adaptation in shaping fish-mussel coevolution. Freshw. Biol. 2017, 62, 1858–1868. [Google Scholar] [CrossRef]
- Rogers-Lowery, C.L.; Dimock, R.V.; Kuhn, R.E. Antibody response of bluegill sunfish during development of acquired resistance against the larvae of the freshwater mussel Utterbackia imbecillis. Dev. Comp. Immunol. 2006, 31, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.; Vogel, C. The parasitic stage of the fresh- water pearl mussel (Margaritifera margaritifera L.) I. Host response to Glochidiosis. Arch. Für Hydrobiol. 1987, 76, 393–402. [Google Scholar]
- Ooue, K.; Terui, A.; Urabe, H.; Nakamura, F. A delayed effect of the aquatic parasite Margaritifera laevis on the growth of the salmonid host fish Oncorhynchus masou masou. Limnology 2017, 18, 345–351. [Google Scholar] [CrossRef]
- Thomas, G.R.; Taylor, J.; de Leaniz, C.G. Does the parasitic freshwater pearl mussel M. margaritifera harm its host? Hydrobiologia 2014, 735, 191–201. [Google Scholar] [CrossRef]
- Fritts, M.W.; Fritts, A.K.; Carleton, S.A.; Bringolf, R.B. Shifts in stable-isotope signatures confirm parasitic relationship of freshwater mussel glochidia attached to host fish. J. Molluscan Stud. 2013, 79, 163–167. [Google Scholar] [CrossRef][Green Version]
- Hastie, L.C.; Young, M.R. Freshwater pearl mussel (Margaritifera margaritifera) glochidiosis in wild and farmed salmonid stocks in Scotland. Hydrobiologia 2001, 445, 109–119. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Salonen, J.K.; Marjomäki, T.J.; Taskinen, J. Interaction between the endangered freshwater pearl mussel Margaritifera margaritifera, the duck mussel Anodonta anatina and the fish host (Salmo): Acquired and cross-immunity. Hydrobiologia 2017, 810, 273–281. [Google Scholar] [CrossRef]
- Denic, M.; Taeubert, J.-E.; Geist, J. Trophic relationships between the larvae of two freshwater mussels and their fish hosts. Invertebr. Biol. 2015, 134, 129–135. [Google Scholar] [CrossRef]
- Salonen, J.K.; Taskinen, J. Electrofishing as a new method to search for unknown populations of the endangered freshwater pearl mussel Margaritifera margaritifera. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 27, 115–127. [Google Scholar] [CrossRef]
- Österling, E.M.; Ferm, J.; Piccolo, J.J. Parasitic freshwater pearl mussel larvae (Margaritifera margaritifera L.) reduce the drift-feeding rate of juvenile brown trout (Salmo trutta L.). Environ. Biol. Fishes 2014, 97, 543–549. [Google Scholar] [CrossRef]
- Albrecht, C.; Hauffe, T.; Schreiber, K.; Wilke, T. Mollusc biodiversity in a European ancient lake system: Lakes Prespa and Mikri Prespa in the Balkans. Hydrobiologia 2011, 682, 47–59. [Google Scholar] [CrossRef]
- Vasiljkov, G.V. Gelmintozi ryb. Izdateljstvo “Kolos”; Moskva: Moscow, Russia, 1983; pp. 45–50. [Google Scholar]
- Modesto, V.; Ilarri, M.; Souza, A.T.; Lopes-Lima, M.; Douda, K.; Clavero, M.; Sousa, R. Fish and mussels: Importance of fish for freshwater mussel conservation. Fish Fish. 2017, 19, 244–259. [Google Scholar] [CrossRef]
- Kekäläinen, J.; Pirhonen, J.; Taskinen, J. Do highly ornamented and less parasitized males have high quality sperm?—An experimental test for parasite-induced reproductive trade-offs in European minnow (Phoxinus phoxinus). Ecol. Evol. 2014, 4, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- Nachev, M.; Hohenadler, M.; Bröckers, N.; Grabner, D.; Sures, B. Role of Invasive Gobies for Transmission of Acanthocephalans of the Genus Pomphorhynchus in the River Rhine. 2022; in press. [Google Scholar] [CrossRef]
- Brian, J.; Aldridge, D. Endosymbionts: An overlooked threat in the conservation of freshwater mussels? Biol. Conserv. 2019, 237, 155–165. [Google Scholar] [CrossRef]
- Jansen, W.; Bauer, G.; Zahner-Meike, E. Glochidial Mortality in Freshwater Mussels. In Ecology and Evolution of the Freshwater Mussels Unionoida; Bauer, G., Wächtler, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 145, pp. 185–211. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blazhekovikj-Dimovska, D.; Stojanovski, S.; Taskinen, J.; Smiljkov, S.; Rimcheska, B. Glochidia Infection of Endemic Fishes from Lake Prespa, N. Macedonia. Hydrobiology 2023, 2, 36-43. https://doi.org/10.3390/hydrobiology2010003
Blazhekovikj-Dimovska D, Stojanovski S, Taskinen J, Smiljkov S, Rimcheska B. Glochidia Infection of Endemic Fishes from Lake Prespa, N. Macedonia. Hydrobiology. 2023; 2(1):36-43. https://doi.org/10.3390/hydrobiology2010003
Chicago/Turabian StyleBlazhekovikj-Dimovska, Dijana, Stojmir Stojanovski, Jouni Taskinen, Stoe Smiljkov, and Biljana Rimcheska. 2023. "Glochidia Infection of Endemic Fishes from Lake Prespa, N. Macedonia" Hydrobiology 2, no. 1: 36-43. https://doi.org/10.3390/hydrobiology2010003
APA StyleBlazhekovikj-Dimovska, D., Stojanovski, S., Taskinen, J., Smiljkov, S., & Rimcheska, B. (2023). Glochidia Infection of Endemic Fishes from Lake Prespa, N. Macedonia. Hydrobiology, 2(1), 36-43. https://doi.org/10.3390/hydrobiology2010003






