Antioxidant and Anti-Inflammatory Potential of Seaweed Extracts as Functional Ingredients
Abstract
1. Introduction
2. Materials and Methods
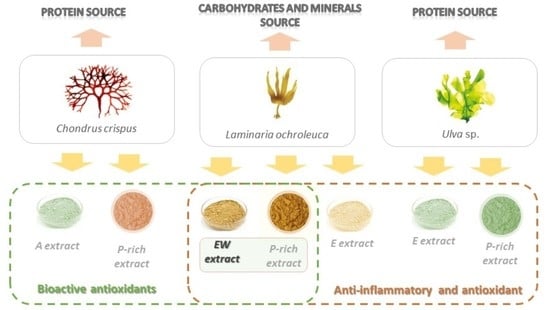
2.1. Seaweed Biomass Source
2.2. Nutritional Value of Seaweed Biomass
2.3. Bioactive Compound Extraction
2.4. Antioxidant Capacity Assessment
2.4.1. ABTS•+ Radical Scavenging
2.4.2. DPPH• Radical Scavenging
2.4.3. O2•− Radical Scavenging
2.4.4. NO Radical Scavenging
2.4.5. Oxygen Radical Absorbance Capacity (ORAC-FL) Assay
2.5. Anti-Inflammatory Capacity Potential Assessment
2.5.1. Human Red Blood Cell (HRBC) Membrane Stabilisation by Heat Induction
2.5.2. Cyclooxygenase (COX-2) Enzymatic Activity
2.6. Biochemical Characterisation of Extracts
2.6.1. Soluble Proteins
2.6.2. Lipids
2.6.3. Carbohydrates
2.6.4. Phenolic Compounds
2.7. Statistical Analysis
3. Results
3.1. Nutritional Characterisation of Seaweed Biomass
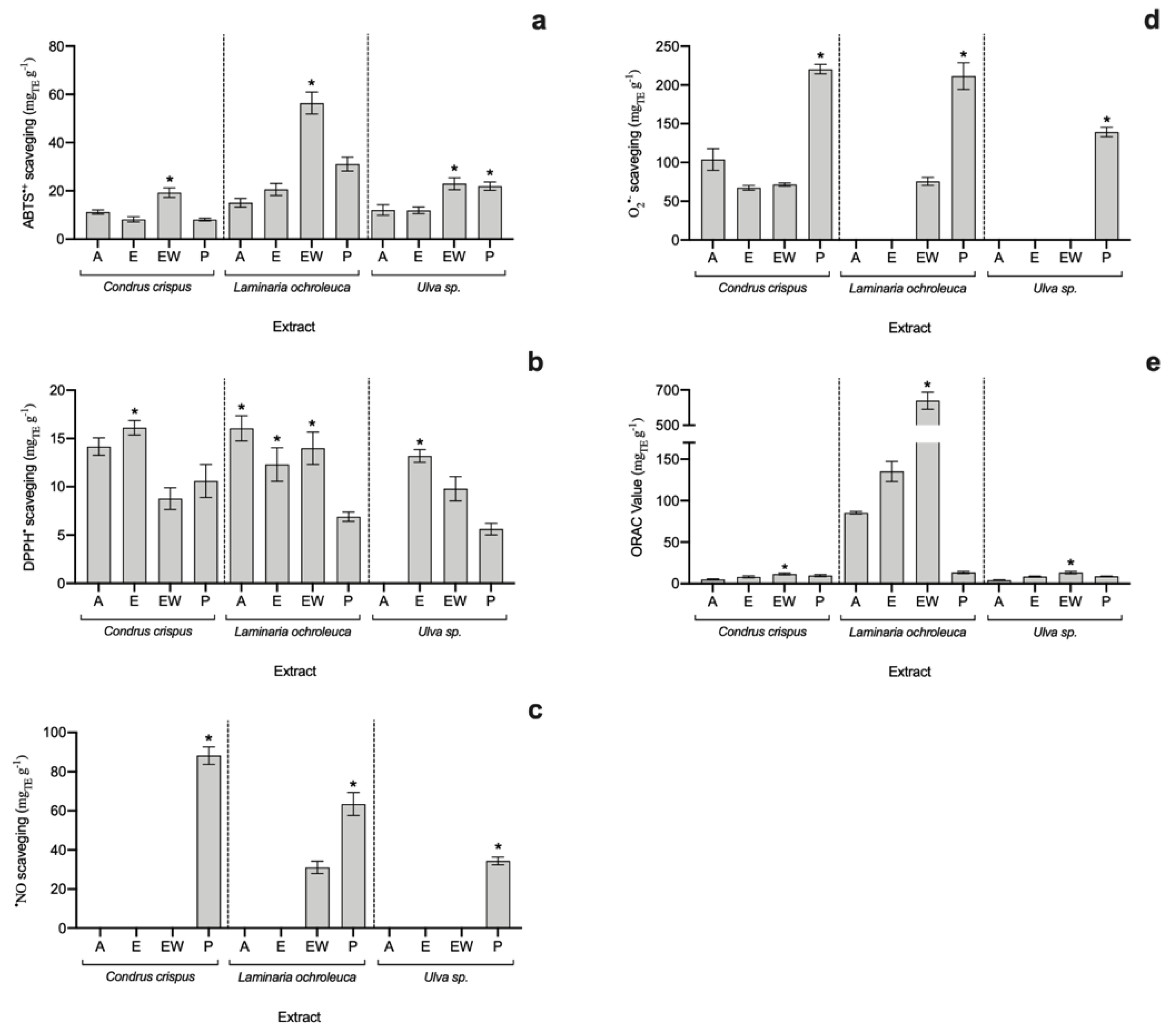
3.2. Antioxidant Capacity of Seaweed Extracts
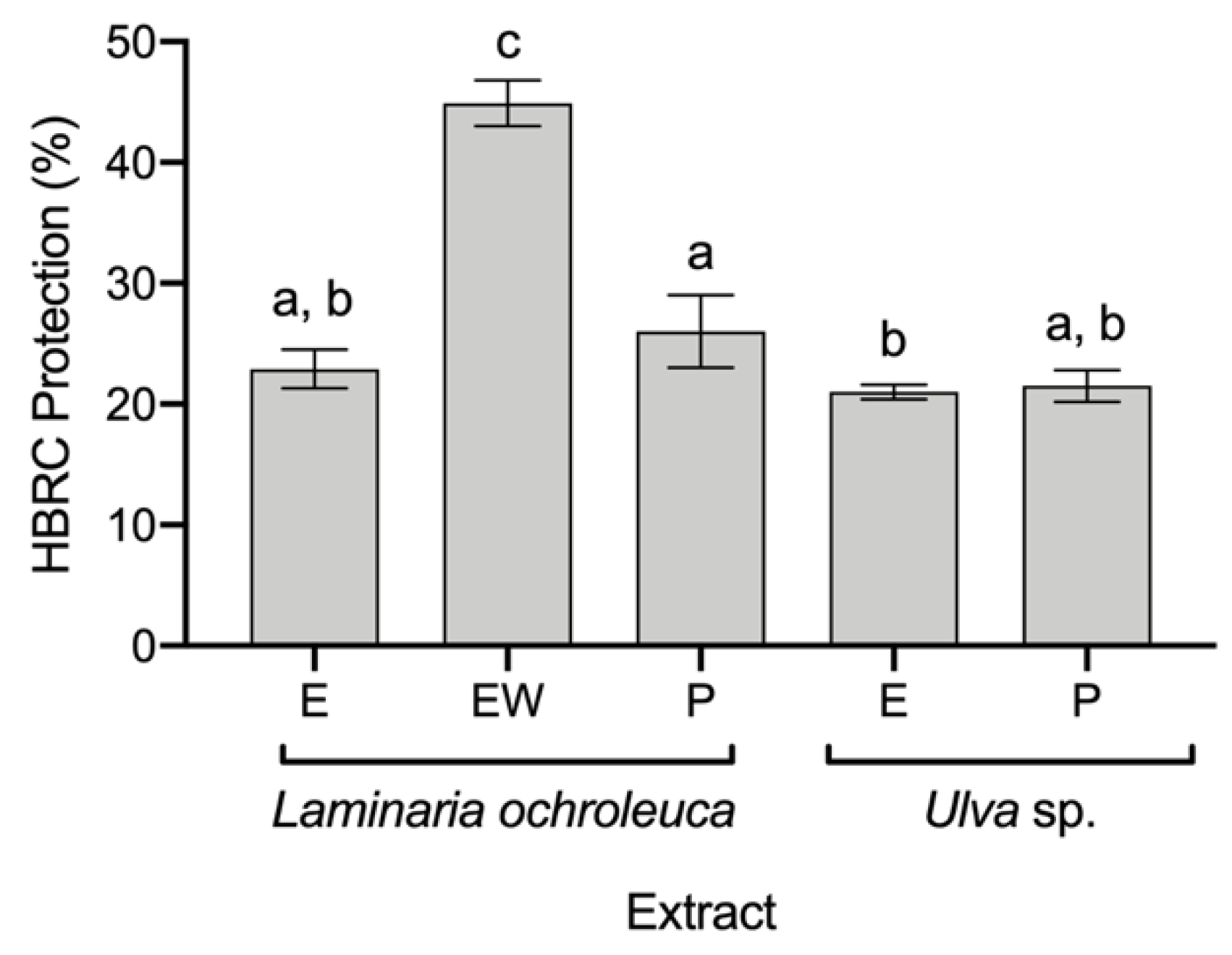
3.3. Anti-Inflammatory Potential of Seaweed Extracts
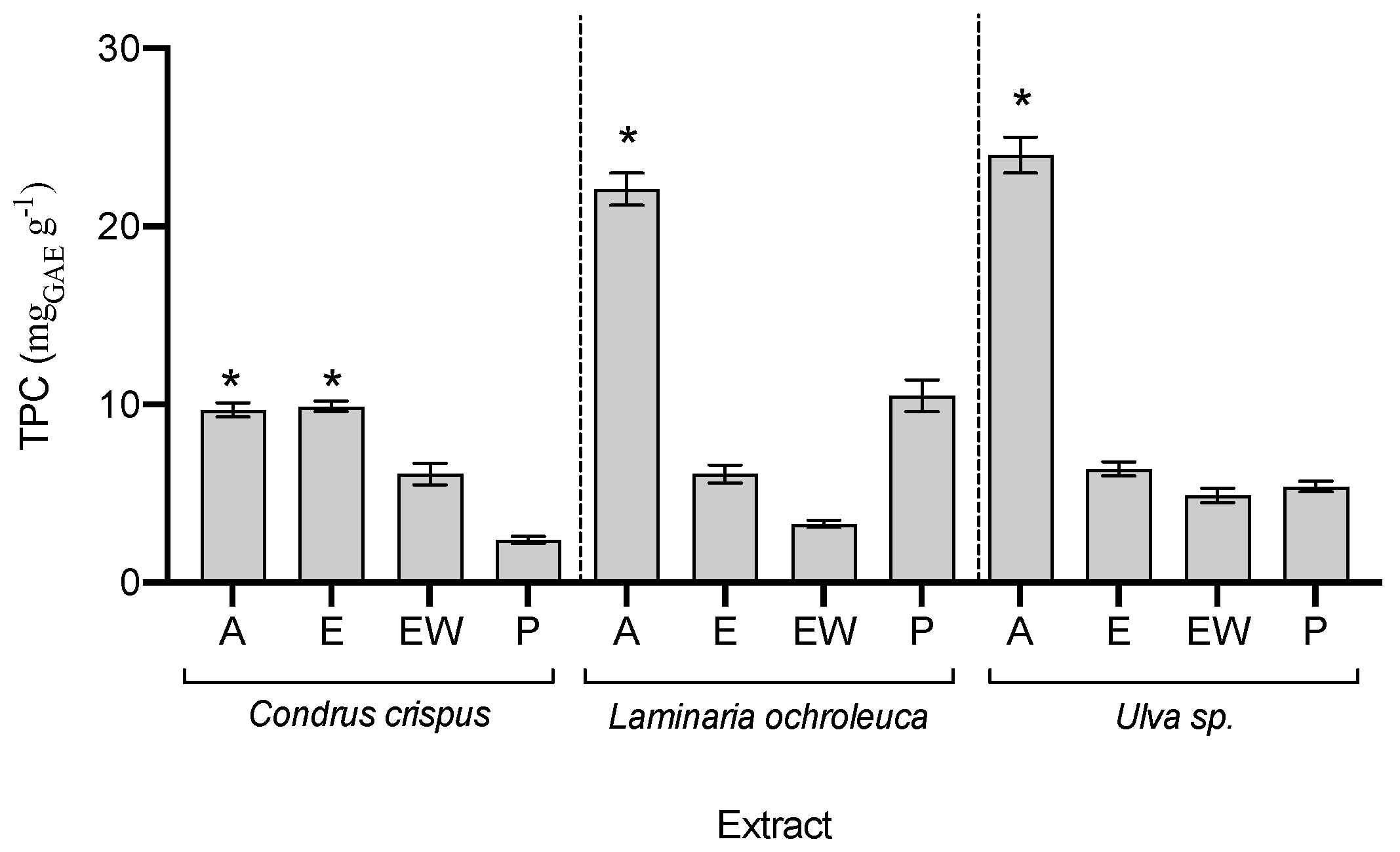
3.4. Biochemical Composition of Seaweed Extracts
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A Sustainable Functional Food for Complementary and Alternative Therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Bakir, S.; Catalkaya, G.; Ceylan, F.D.; Khan, H.; Guldiken, B.; Capanoglu, E.; Kamal, M.A. Role of Dietary Antioxidants in Neurodegenerative Diseases: Where Are We Standing? Curr. Pharm. Des. 2020, 26, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Dellarosa, N.; Laghi, L.; Martinsdóttir, E.; Jónsdóttir, R.; Sveinsdóttir, K. Enrichment of Convenience Seafood with Omega-3 and Seaweed Extracts: Effect on Lipid Oxidation. LWT—Food Sci. Technol. 2015, 62, 746–752. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of Antioxidant Potential of Seaweed Extracts for Enrichment of Convenience Food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In Vitro Antioxidant Properties of Crude Extracts and Compounds from Brown Algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Tenorio-Rodriguez, P.A.; Murillo-Álvarez, J.I.; Campa-Cordova, Á.I.; Angulo, C. Antioxidant Screening and Phenolic Content of Ethanol Extracts of Selected Baja California Peninsula Macroalgae. J. Food Sci. Technol. 2017, 54, 422–429. [Google Scholar] [CrossRef]
- Múzquiz de la Garza, A.R.; Tapia-Salazar, M.; Maldonado-Muñiz, M.; De La Rosa-Millán, J.; Gutiérrez-Uribe, J.A.; Santos-Zea, L.; Barba-Dávila, B.A.; Ricque-Marie, D.; Cruz-Suárez, L.E. Nutraceutical Potential of Five Mexican Brown Seaweeds. BioMed Res. Int. 2019, 2019, 3795160. [Google Scholar] [CrossRef]
- Melo, R.; Sousa-Pinto, I.; Antunes, S.C.; Costa, I.; Borges, D. Temporal and Spatial Variation of Seaweed Biomass and Assemblages in Northwest Portugal. J. Sea Res. 2021, 174, 102079. [Google Scholar] [CrossRef]
- Gaspar, R.; Pereira, L.; Sousa-Pinto, I. The Seaweed Resources of Portugal. Bot. Mar. 2019, 62, 499–525. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural Characterization and Antioxidant Activity of Water-Soluble Polysaccharides from the Tunisian Brown Seaweed Cystoseira Compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Granados-Guzman, G.; Salazar-Aranda, R.; Garza-Tapia, M.; Castro-Rios, R.; Waksman de Torres, N. Optimization and Validation of Two High-Throughput Methods Indicating Antiradical Activity. Curr. Anal. Chem. 2017, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-Laboratory Validation of Microplate Methods for Total Phenolic Content and Antioxidant Activity on Polyphenolic Extracts, and Comparison with Conventional Spectrophotometric Methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Pinho, B.R.; Sousa, C.; Valentão, P.; Andrade, P.B. Is Nitric Oxide Decrease Observed with Naphthoquinones in LPS Stimulated RAW 264.7 Macrophages a Beneficial Property? PLoS ONE 2011, 6, e24098. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Moualek, I.; Iratni Aiche, G.; Mestar Guechaoui, N.; Lahcene, S.; Houali, K. Antioxidant and Anti-Inflammatory Activities of Arbutus Unedo Aqueous Extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J Biol Chem 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid Microplate High-Throughput Methodology for Assessment of Folin-Ciocalteu Reducing Capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef]
- Barbosa, M.; Fernandes, F.; Pereira, D.M.; Azevedo, I.C.; Sousa-Pinto, I.; Andrade, P.B.; Valentão, P. Fatty Acid Patterns of the Kelps Saccharina Latissima, Saccorhiza Polyschides and Laminaria Ochroleuca: Influence of Changing Environmental Conditions. Arab. J. Chem. 2020, 13, 45–58. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58d–68d. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Bank, G.; Schauss, A. Antioxidant Testing: An ORAC Update. Nutraceuticals World 2004, 7, 2003–2004. [Google Scholar]
- Yan, X.; Nagata, T.; Fan, X. Antioxidative Activities in Some Common Seaweeds. Plant Foods Hum. Nutr. 1998, 52, 253–262. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Recovery of Bioactive and Gelling Extracts from Edible Brown Seaweed Laminaria Ochroleuca by Non-Isothermal Autohydrolysis. Food Chem. 2019, 277, 353–361. [Google Scholar] [CrossRef]
- Vázquez-Freire, M.J.; Lamela, M.; Calleja, J.M. Laminaria Ochroleuca: A Preliminary Study of Its Effect on the Central Nervous System. Phytother. Res. 1994, 8, 422–425. [Google Scholar] [CrossRef]
- Lamela, M.; Anca, J.; Villar, R.; Otero, J.; Calleja, J.M. Hypoglycemic Activity Op Several Seaweed Extracts. J. Ethnopharmacol. 1989, 27, 35–43. [Google Scholar] [CrossRef]
- Bonneville, M.; Saint-Mezard, P.; Benetiere, J.; Hennino, A.; Pernet, I.; Denis, A.; Nicolas, N.F. Laminaria Ochroleuca Extract Reduces Skin Inflammation. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1124–1125. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Cui, C.; Lu, J.; Sun-Waterhouse, D.; Mu, L.; Sun, W.; Zhao, M.; Zhao, H. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. LWT 2016, 73, 602–608. [Google Scholar] [CrossRef]
- Ahmad, T.; Eapen, M.S.; Ishaq, M.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N.; Sohal, S.S.; Fitton, J.H.; Guven, N.; Caruso, V.; et al. Anti-Inflammatory Activity of Fucoidan Extracts In Vitro. Mar. Drug 2021, 19, 702. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Dissanayake, A.A.; Xiao, C.; Gao, J.; Zhao, M.; Nair, M.G. The edible seaweed Laminaria japonica contains cholesterol analogues that inhibit lipid peroxidation and cyclooxygenase enzymes. PLoS ONE 2022, 17, e0258980. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Busquets-Cortés, C.; Capó, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 Inhibitors as a Ther-apeutic Target in Inflammatory Diseases. Curr. Med. Chem. 2018, 26, 3225–3241. [Google Scholar] [CrossRef]
- Adnan, A.Z.; Armin, F.; Sudji, I.R.; Novida, M.D.; Roesma, D.I.; Ali, H.A.; Fauzana, A. In Vitro Anti-Inflammatory Activity Test of Tinocrisposide and Freeze-Dried Aqueous Extract of Tinospora Crispa Stems on Human Red Blood Cell by Increasing Membrane Stability Experiment. Asian J. Pharm. Clin. Res. 2019, 12, 125–129. [Google Scholar] [CrossRef]
- Chippada, S.C.; Volluri, S.S.; Bammidi, S.R.; Vangalapati, M. In Vitro Anti Inflammatory Activity of Methanolic Extract of Centella Asiatica by HRBC Membrane Stabilisation. Rasayan J. Chem. 2011, 4, 457–460. [Google Scholar]
- Lee, K.S.; Cho, E.; Weon, J.B.; Park, D.; Fréchet, M.; Chajra, H.; Jung, E. Inhibition of UVB-Induced Inflammation by Laminaria Japonica Extract via Regulation of Nc886-PKR Pathway. Nutrients 2020, 12, 1958. [Google Scholar] [CrossRef]
- Pacheco, D.; Miranda, G.; Rocha, C.P.; Pato, R.L.; Cotas, J.; Gonçalves, A.M.M.; Dias Santos, S.M.; Bahcevandziev, K.; Pereira, L. Portuguese Kelps: Feedstock Assessment for the Food Industry. Appl. Sci. 2021, 11, 10681. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral Content of Edible Marine Seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant Activities of Enzymatic Extracts from Brown Seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]

| Algae | Content (%) | ||||
|---|---|---|---|---|---|
| Moisture | Protein | Fat | Ash | Carbohydrates | |
| Chondrus crispus | 8.1 ± 0.1 a | 24.4 ± 0.5 a | 0.4 ± 0.1 a | 19.4 ± 0.1 a | 47.7 ± 0.6 a |
| Laminaria ochroleuca | 5.6 ± 0.1 b | 8.9 ± 0.2 b | 0.5 ± 0.1 a | 38.2 ± 0.4 b | 46.7 ± 0.6 a |
| Ulva sp. | 11.7 ± 0.5 c | 25.0 ± 0.1 a | 0.3 ± 0.1 a | 27.2 ± 0.2 c | 35.8 ± 0.2 b |
| Algae | Extract | Content (%Dry Extract) | ||
|---|---|---|---|---|
| Proteins | Carbohydrates | Lipids | ||
| Chondrus crispus | A | 8.9 ± 0.6 a | 10.2 ± 1.0 a | 30.0 ± 0.8 a |
| E | 10.4 ± 0.3 b | 11.3 ± 1.0 a | 19.0 ± 0.2 b | |
| EW | 13.2 ± 0.7 c | 40.2 ± 1.5 b | 24.8 ± 2.5 c | |
| P | 8.2 ± 0.8 a | 73.3 ± 6.0 c | 1.0 ± 0.1 d | |
| Laminaria ochroleuca | A | 11.3 ± 0.9 b | 10.7 ± 0.8 a | 52.4 ± 5.1 e |
| E | 8.9 ± 0.5 a | 5.7 ± 0.5 d | 14.2 ± 1.0 f | |
| EW | 13.1 ± 0.5 c | 27.3 ± 0.6 e | 1.2 ± 0.1 d | |
| P | 6.4 ± 0.3 d | 68.4 ± 3.9 c | 0.1 ± 0.1 g | |
| Ulva sp. | A | 9.2 ± 0.6 a | 10.7 ± 1.0 a | 61.0 ± 0.1 h |
| E | 9.8 ± 0.5 a, b | 12.8 ± 1.2 a | 24.5 ± 0.3 c | |
| EW | 13.5 ± 0.9 c | 23.8 ± 2.0 f | 3.2 ± 0.1 i | |
| P | 5.7 ± 0.3 e | 67.8 ± 3.1 c | 0.1 ± 0.1 g | |
| Algae | Concentration (mgTE g−1) | HRBC (%) | COX (%) | ||
|---|---|---|---|---|---|
| ABTS•+ | DPPH• | ORAC | |||
| Laminaria ochroleuca | EW: 56.41 ± 4.56 | EW: 13.99 ± 1.68 | EW: 638.90 ± 48.30 | EW: 44.9 ± 1.6 | EW: 32.8 ± 2.42 |
| Laminaria sp. | W: 32 1 | EW: 5–20 2 | P: 312.12 3 | FE: 52–88 4 | SE: 47 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro, H.M.; Pagels, F.; Tavares, T.G.; Costa, I.; Sousa-Pinto, I.; Guedes, A.C. Antioxidant and Anti-Inflammatory Potential of Seaweed Extracts as Functional Ingredients. Hydrobiology 2022, 1, 469-482. https://doi.org/10.3390/hydrobiology1040028
Amaro HM, Pagels F, Tavares TG, Costa I, Sousa-Pinto I, Guedes AC. Antioxidant and Anti-Inflammatory Potential of Seaweed Extracts as Functional Ingredients. Hydrobiology. 2022; 1(4):469-482. https://doi.org/10.3390/hydrobiology1040028
Chicago/Turabian StyleAmaro, Helena M., Fernando Pagels, Tânia G. Tavares, Isabel Costa, Isabel Sousa-Pinto, and A. Catarina Guedes. 2022. "Antioxidant and Anti-Inflammatory Potential of Seaweed Extracts as Functional Ingredients" Hydrobiology 1, no. 4: 469-482. https://doi.org/10.3390/hydrobiology1040028
APA StyleAmaro, H. M., Pagels, F., Tavares, T. G., Costa, I., Sousa-Pinto, I., & Guedes, A. C. (2022). Antioxidant and Anti-Inflammatory Potential of Seaweed Extracts as Functional Ingredients. Hydrobiology, 1(4), 469-482. https://doi.org/10.3390/hydrobiology1040028