Resiliency and Recovery of Aquatic Vegetation Following Scouring Floods in Two First-Magnitude Springs, Missouri, USA
Abstract
:1. Introduction
2. Materials and Methods
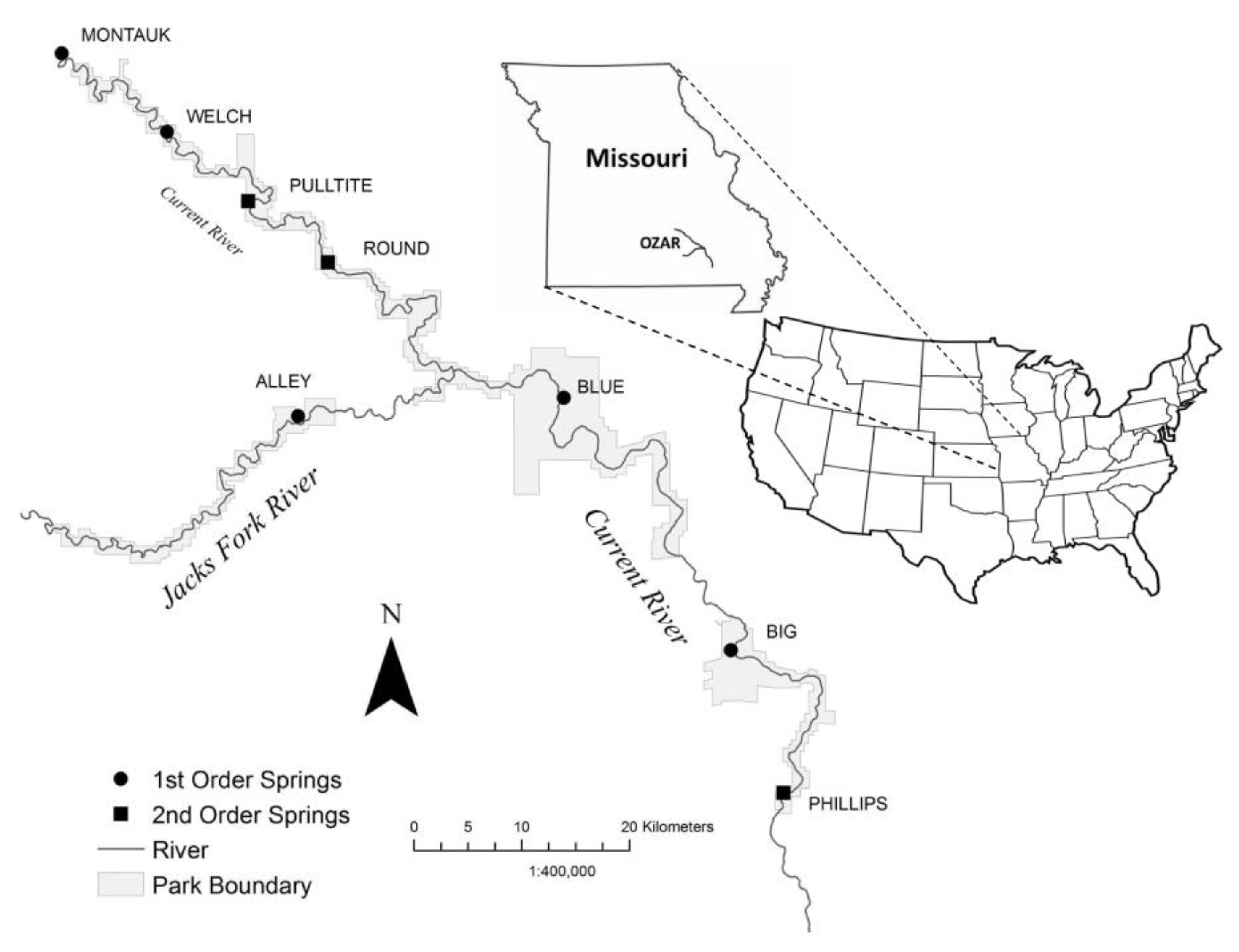
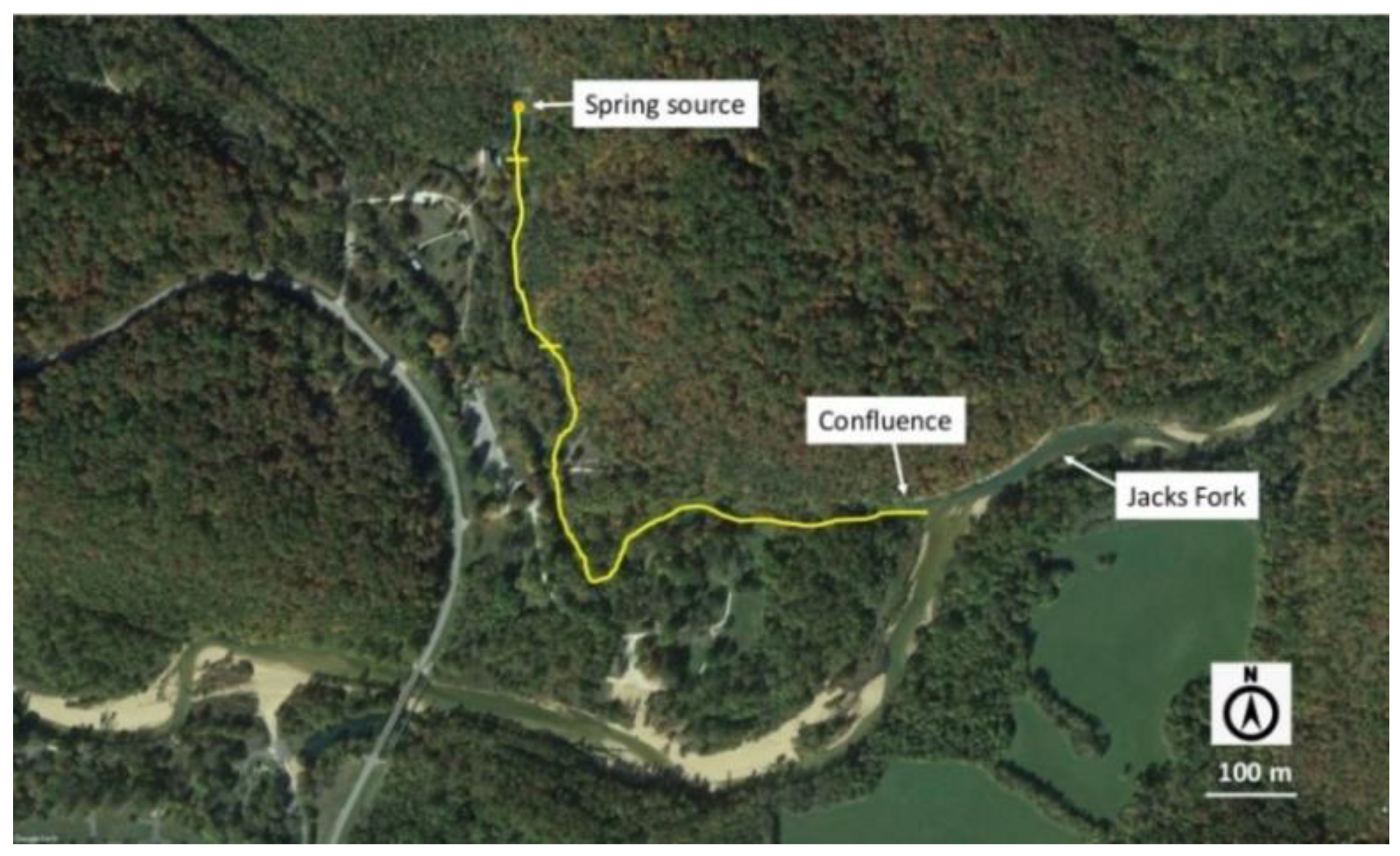
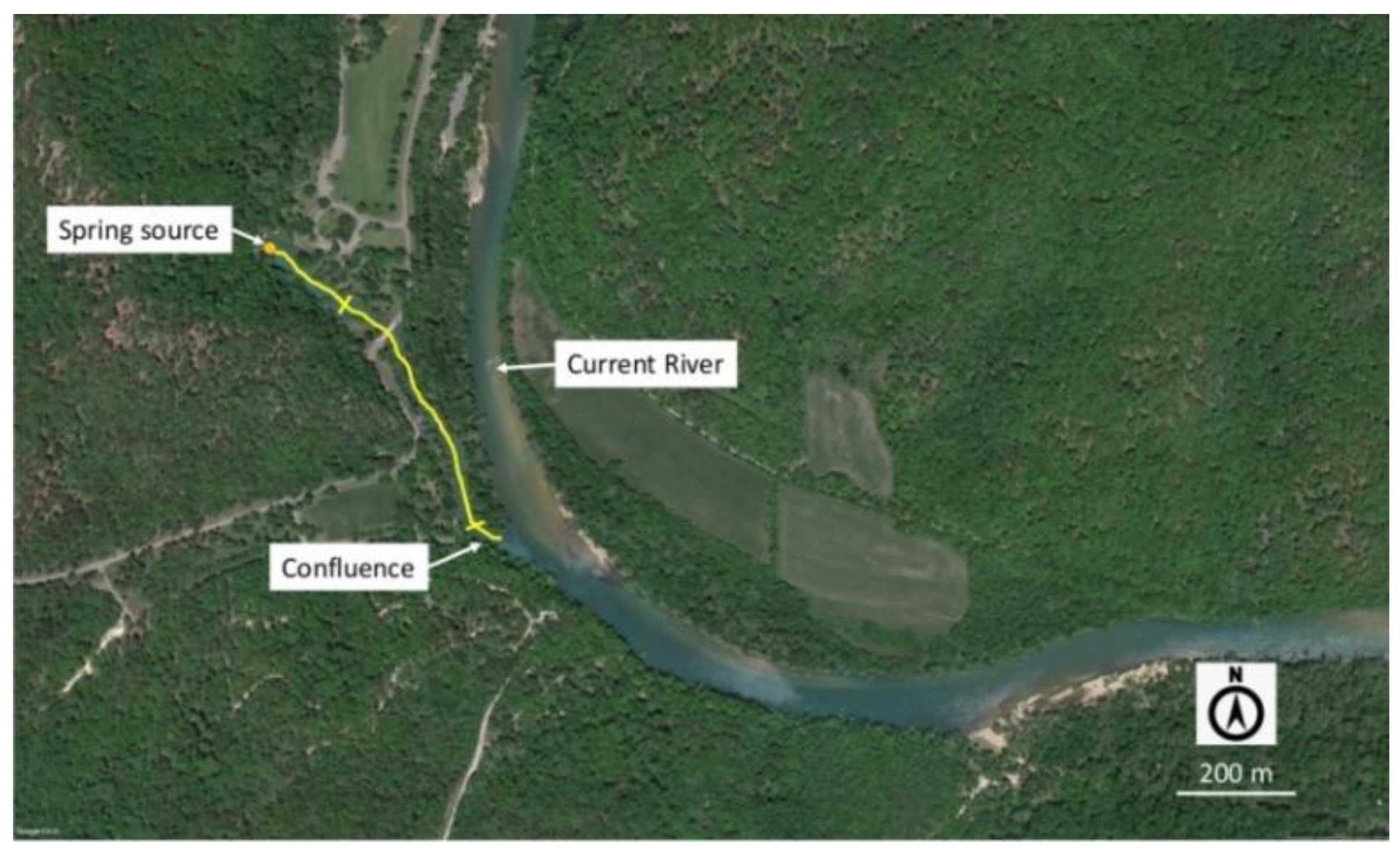
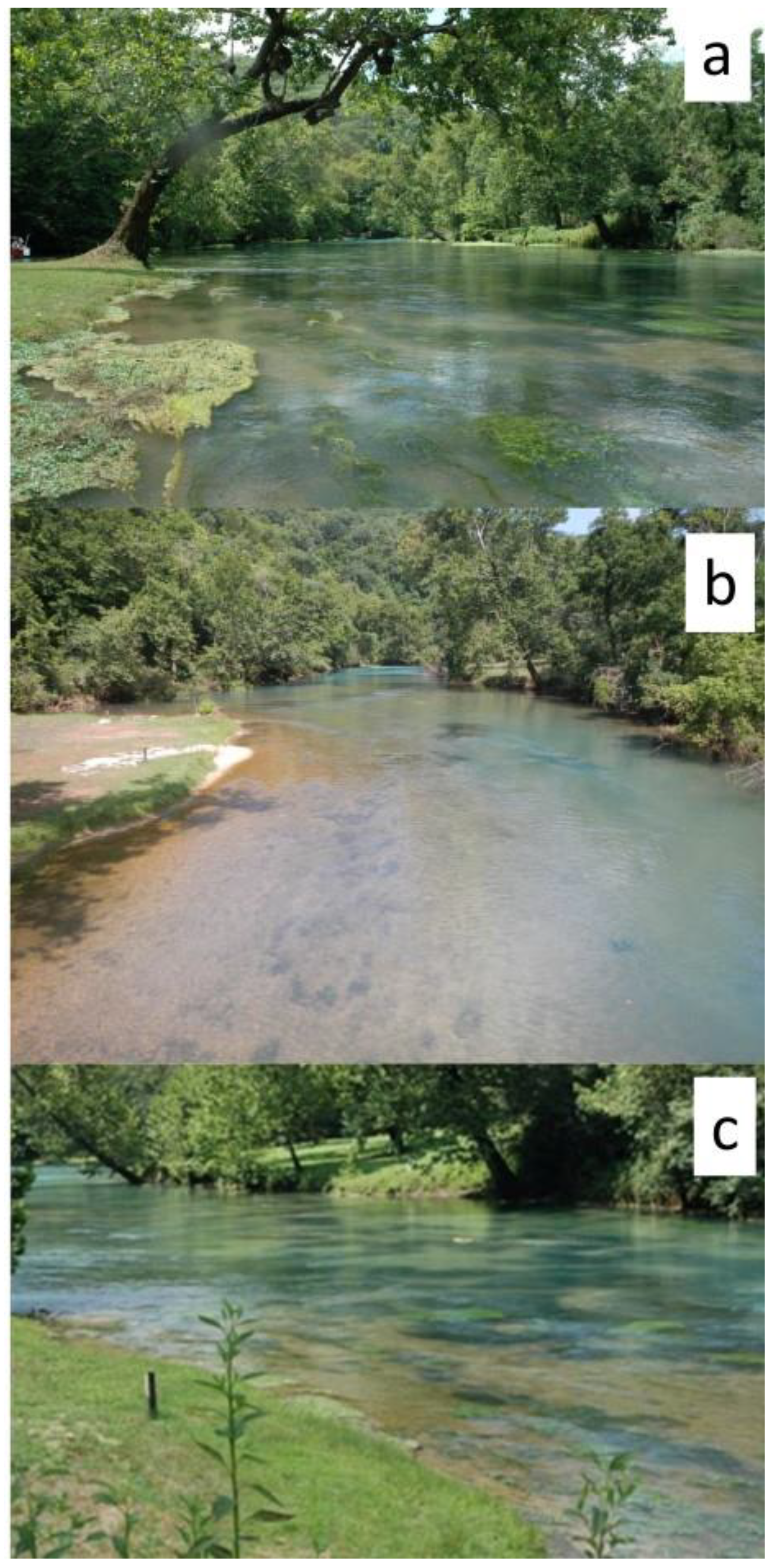
2.1. Study Sites
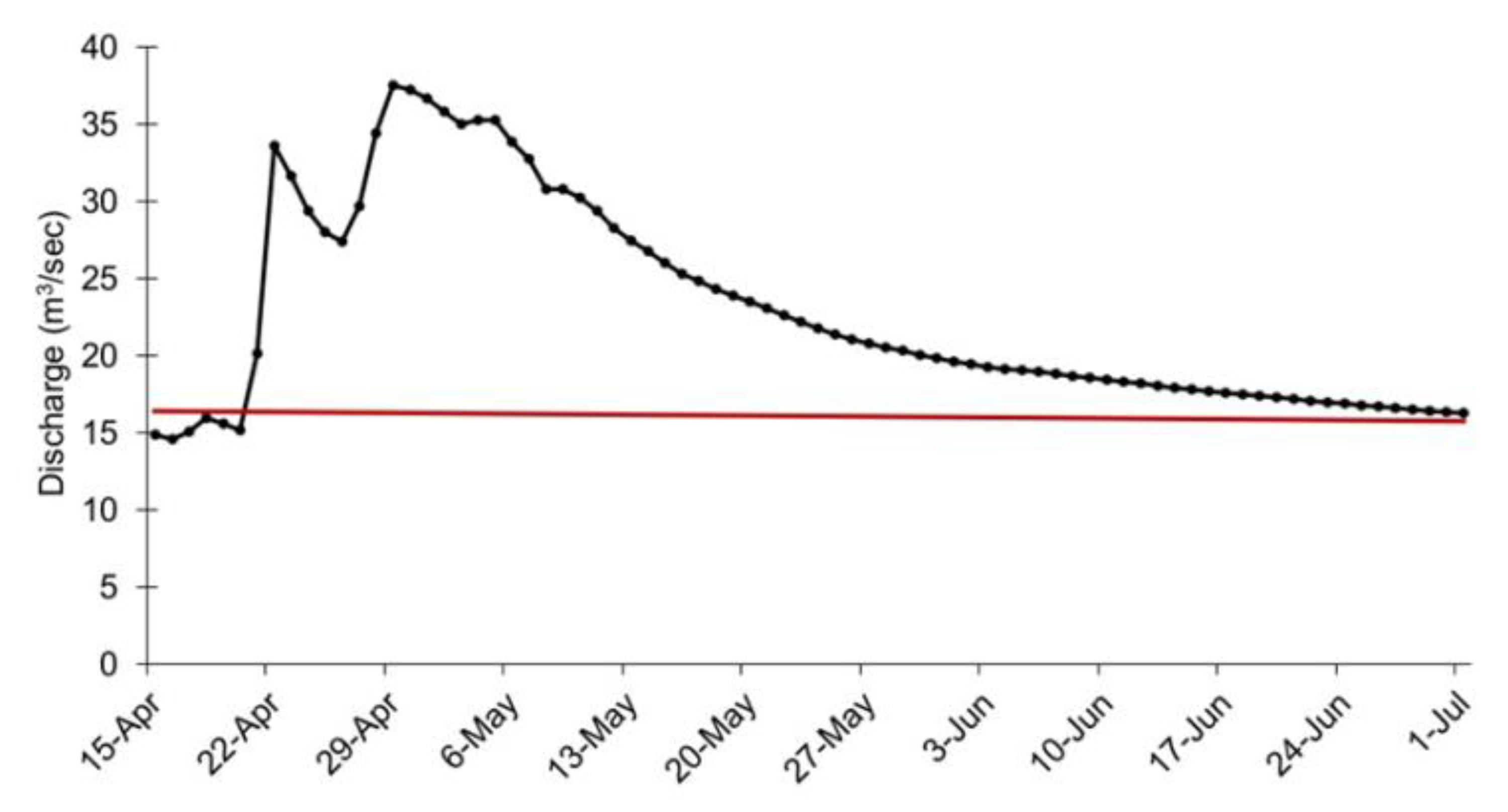
2.2. The Flood Event
2.3. Vegetation Assessment
2.4. Metrics and Statistical Analyses
3. Results
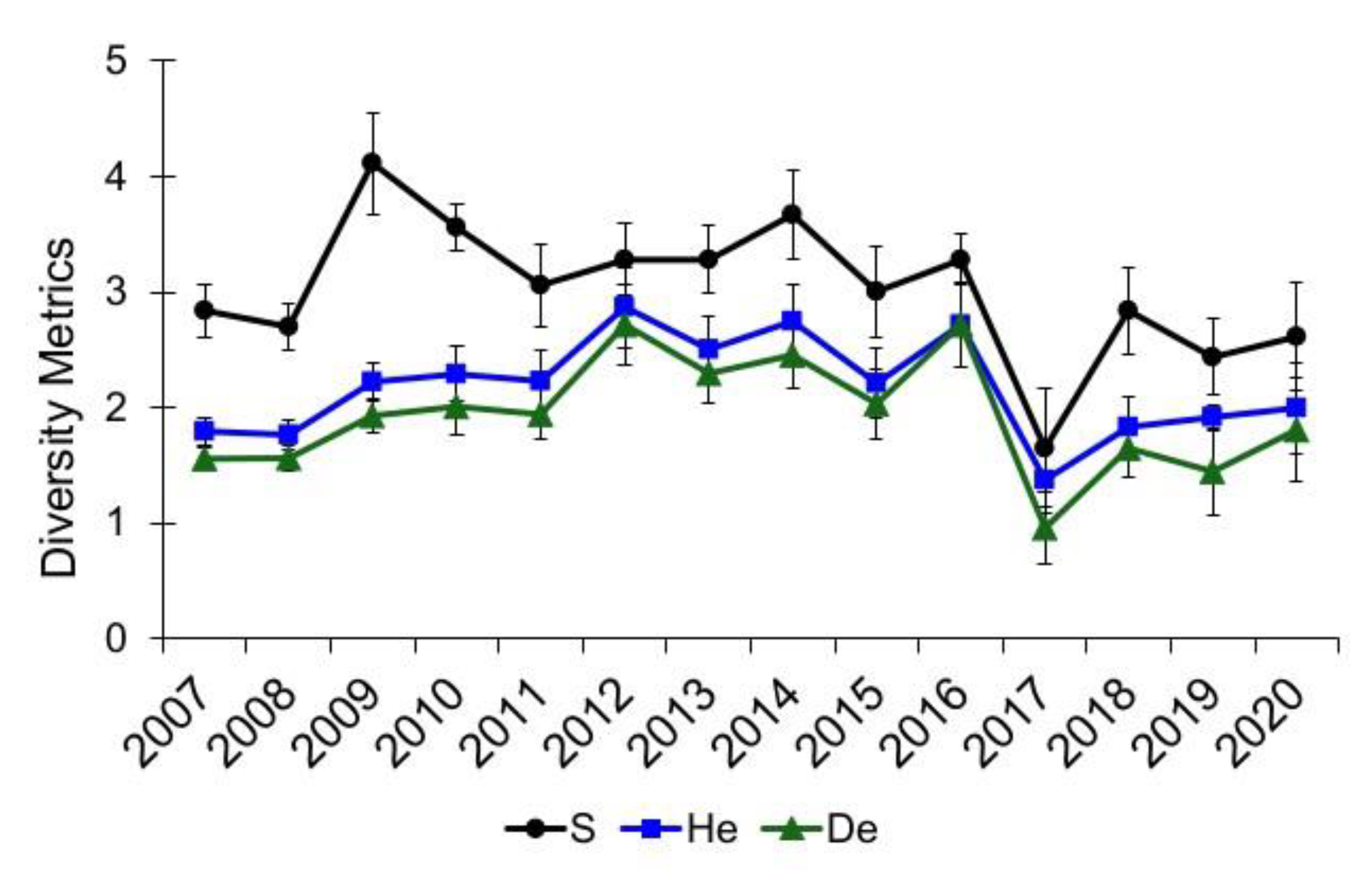
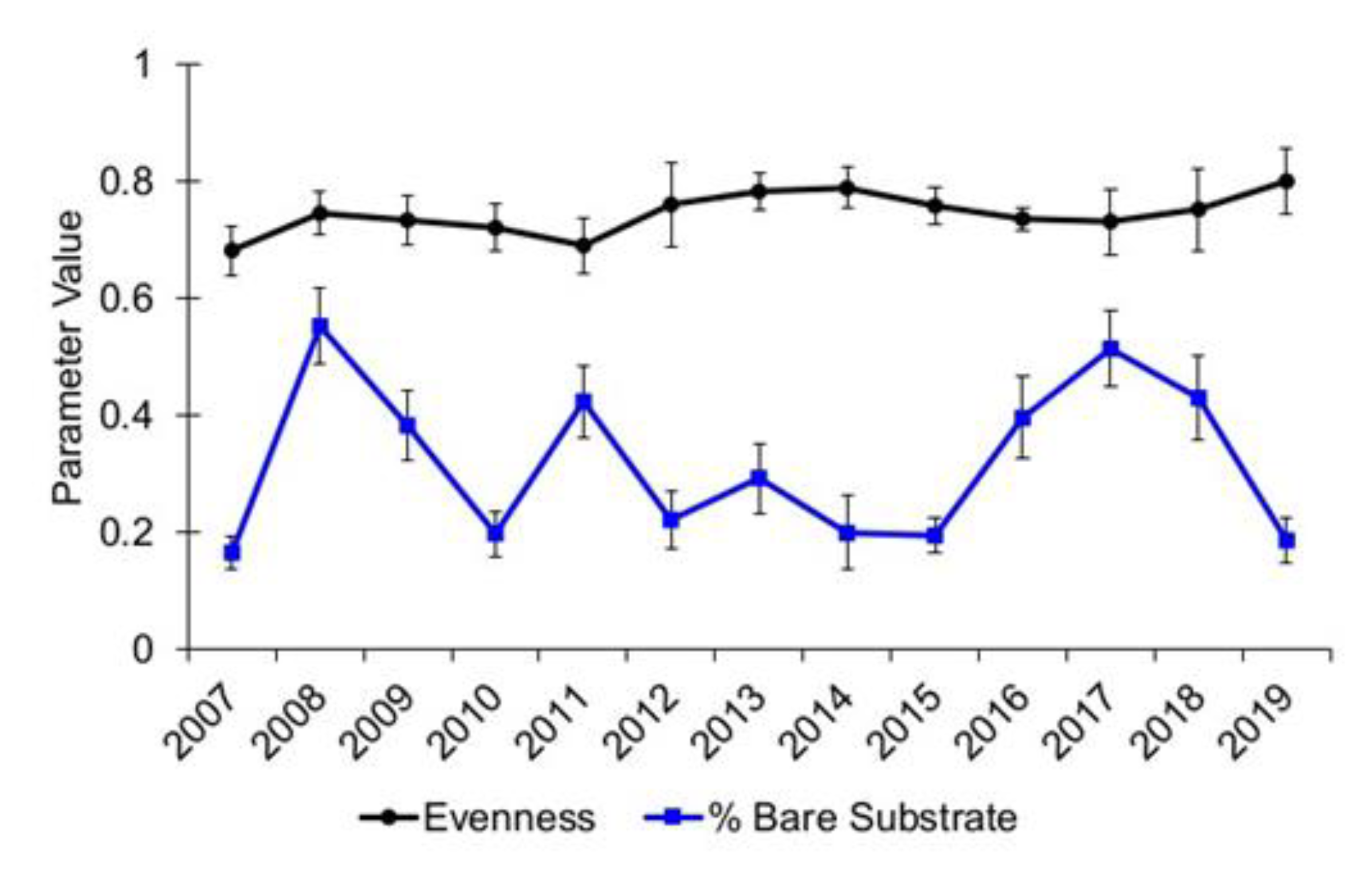
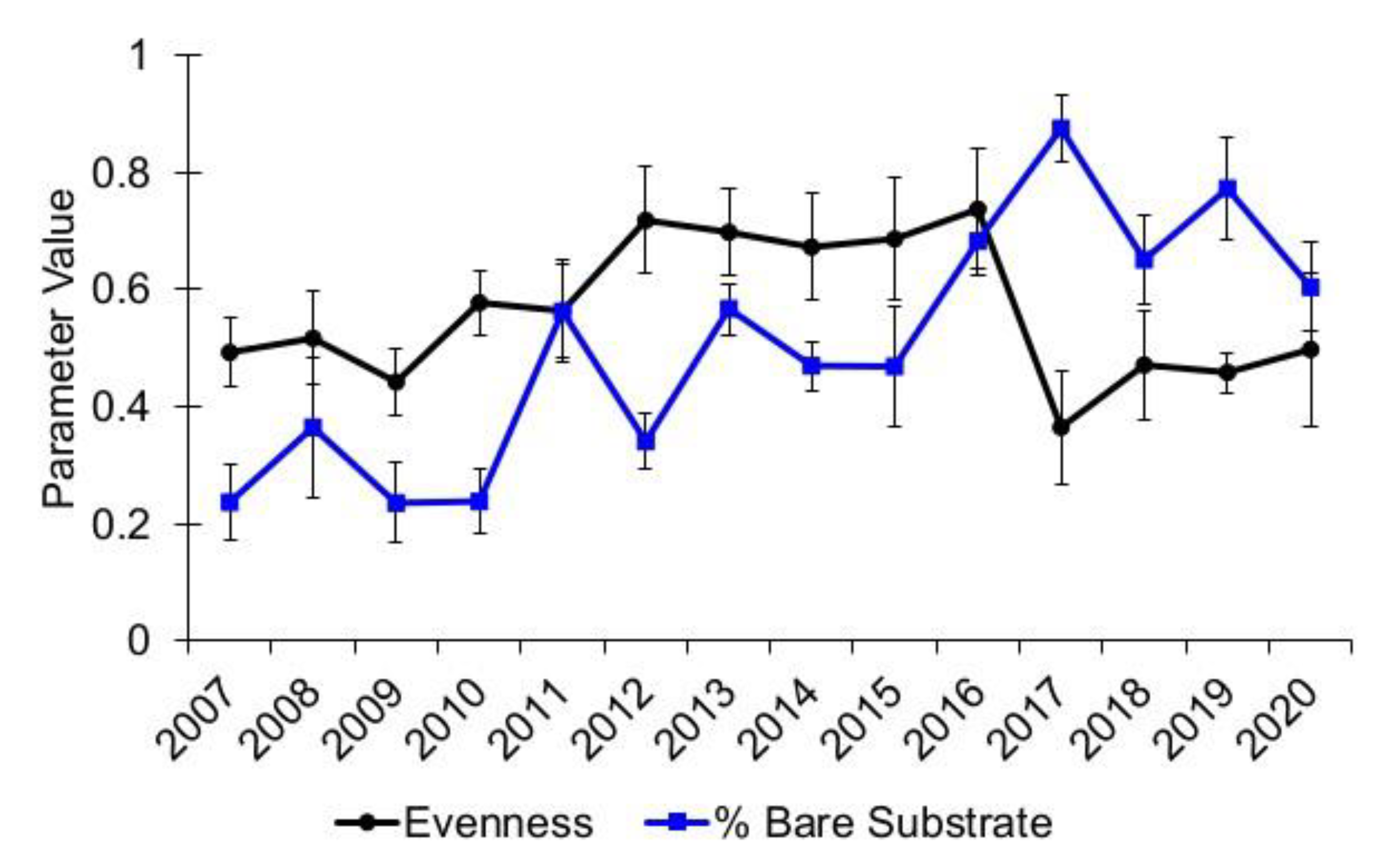
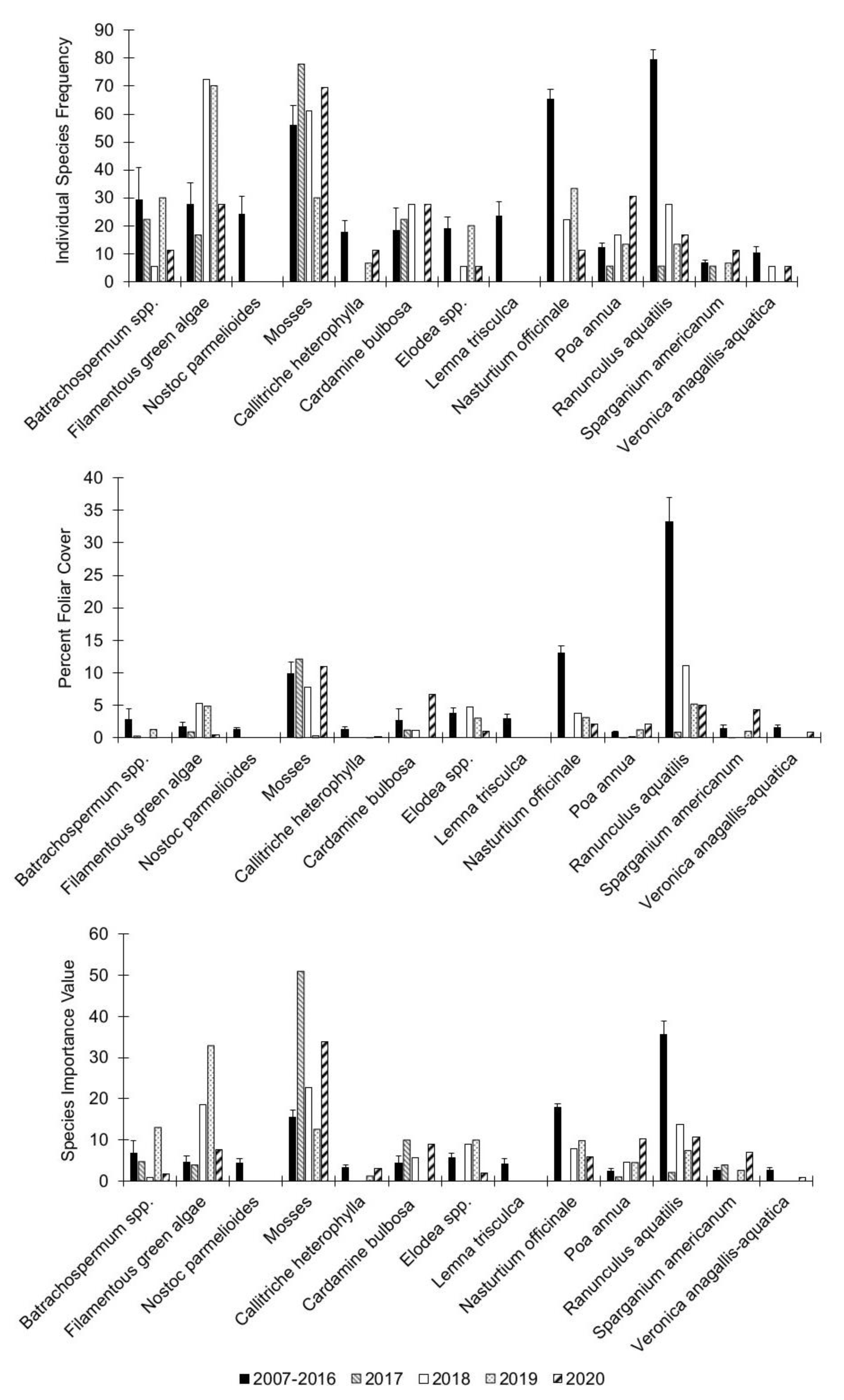
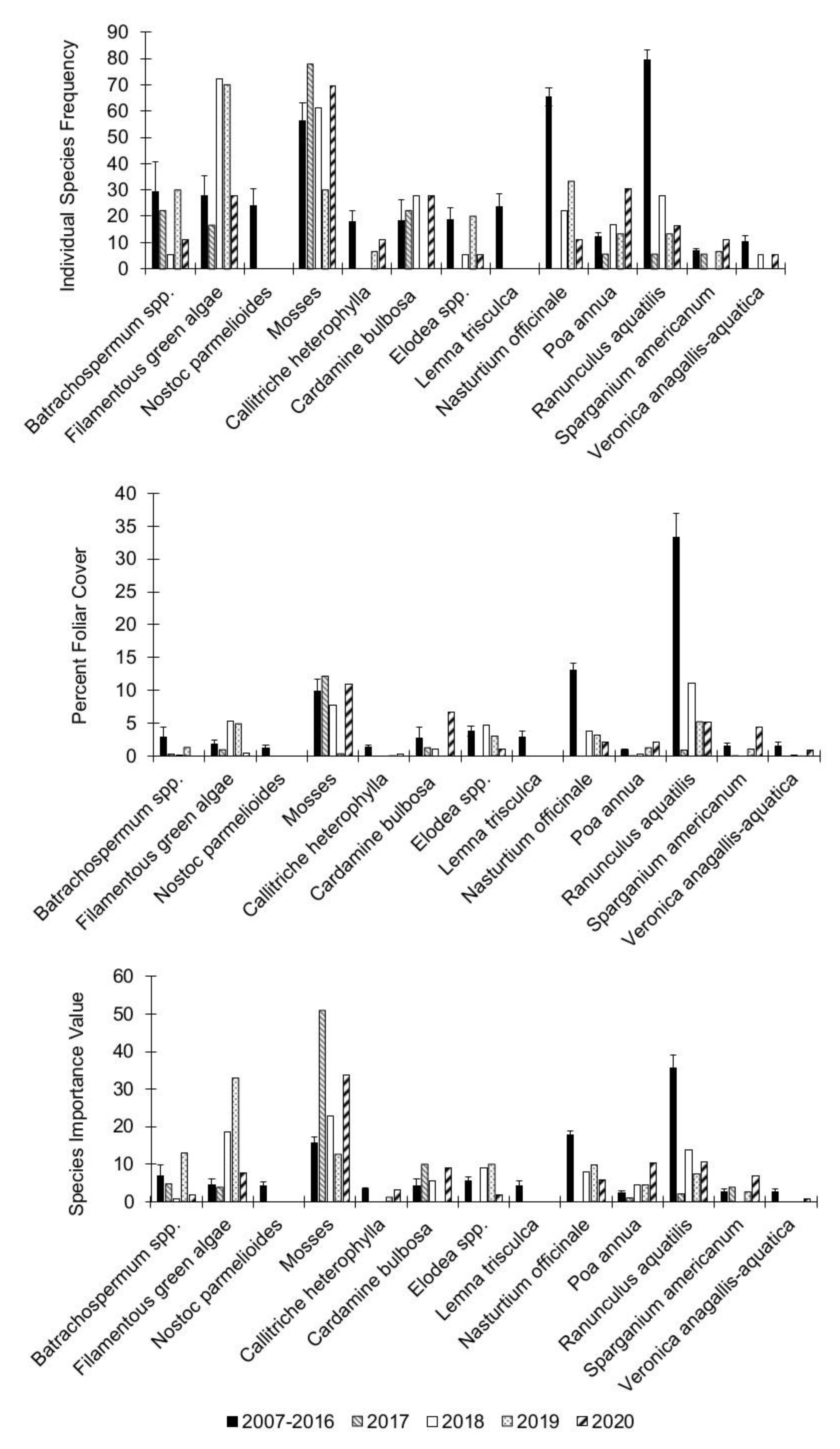
3.1. Community Diversity
3.2. Individual Species Dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haslam, S.M. River Plants, the Macrophytic Vegetation of Watercourses, 2nd revised ed.; Forrest Text: Sŵn y Nant, UK, 2006; p. 438. [Google Scholar]
- Jackson, M.B.; Colmer, T.D. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, L.; Voesnenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B.; Ishizawa, K.; Ito, O. Evolution and mechanisms of plant tolerance to flooding stress. Ann. Bot. 2009, 103, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta, P.; Armstrong, W.; Voesenek, L.A.C.J. Plants and flooding stress. New Phytol. 2011, 190, 269–273. [Google Scholar] [CrossRef]
- Caudle, K.L.; Maricle, B.R. Effects of flooding on photosynthesis, chlorophyll fluorescence, and oxygen stress in plants of varying flooding tolerance. Trans. Kansas Acad. Sci. 2012, 115, 5–18. [Google Scholar]
- Jones, J.I.; Collins, A.L.; Naden, P.S.; Sear, D.A. The relationship between fine sediment and macrophytes in rivers. River Res. Appl. 2012, 28, 1006–1018. [Google Scholar] [CrossRef]
- Herrera, A. Responses to flooding of plant water relations and leaf gas exchange in tropical tolerant trees of a black-water wetland. Front. Plant Sci. 2013, 4, 106. [Google Scholar] [CrossRef] [Green Version]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Henriques, J. Aquatic macrophytes. In Aquatic Biology and Hydroelectric Power Development in New Zealand; Henriques, P.R., Ed.; Oxford University Press: Auckland, New Zealand, 1987; pp. 207–222. [Google Scholar]
- Chambers, P.A.; Prepas, E.E.; Hamilton, H.R.; Bothwell, M.L. Current velocity and its effect on aquatic macrophytes in flowing waters. Ecol. Appl. 1991, 1, 249–257. [Google Scholar] [CrossRef]
- Tremolieres, M.; Carbiener, R.; Ortscheit, A.; Klein, J.-P. Changes in aquatic vegetation in Rhine floodplain streams in Alsace in relation to disturbance. J. Veg. Sci. 1994, 5, 169–178. [Google Scholar] [CrossRef]
- Sangmek, P.; Meksumpun, C. Influence of eco-hydrological factors on aquatic plant succession in a regulated river: A case study of the Petchburi River, Thailand. Water Environ. J. 2015, 29, 243–251. [Google Scholar] [CrossRef]
- Czarnecka, B.; Rysiak, A.; Chabudziński, L. Spring flora diversity versus ecosystem stressors: Case studies from the Western Roztocze and Lublin Upland border, South-East Poland. Ecohydrol. Hydrobiol. 2020, 20, 693–706. [Google Scholar] [CrossRef]
- Steyermark, J.A. Studies of the vegetation of Missouri—II. Phanerogamic flora of the freshwater springs in the Ozarks of Missouri. Publ. Field Mus. Nat. Hist. Bot. Ser. 1941, 9, 476–641. [Google Scholar]
- Lemke, D. Aquatic macrophytes of the upper San Marcos River, Hays Co., Texas. Southwest. Nat. 1989, 34, 289–291. [Google Scholar] [CrossRef]
- Bowles, D.E.; Dodd, H.R. The floristics and community ecology of aquatic vegetation occurring in seven large springs at Ozark National Scenic Riverways, Missouri. J. Bot. Res. Inst. Tex. 2015, 9, 235–249. [Google Scholar]
- Bowles, D.E.; Cheri, C.R. Aquatic vegetation of springs at Buffalo National River, Arkansas. Castanea 2019, 84, 224–237. [Google Scholar] [CrossRef]
- Rafferty, M.D. The Ozarks, Land and Life, 2nd ed.; University of Arkansas Press: Fayetteville, AR, USA, 2001; p. 368. [Google Scholar]
- Meinzer, O.E. Large Springs in the United States; Water-Supply Paper 557; U.S. Geological Survey: Washington, DC, USA, 1927; p. 94.
- Vineyard, J.D.; Feder, G.; Pflieger, W.L.; Lipscomb, R.G. Springs of Missouri with Sections on Fauna and Flora; Water Resources Report No. 29; Missouri Geological Survey and Water Resources: Rolla, MO, USA, 1974; p. 220.
- Bowles, D.E. Vascular plants of Mammoth Spring, Arkansas. J. Torrey Bot. Soc. 2020, 147, 87–93. [Google Scholar] [CrossRef]
- Bowles, D.E.; Dodd, H.R.; Williams, M.H.; Morrison, L.M.; James, K.; DeBacker, M.D.; Ciak, C.E.; Hinsey, J.A.; Rowell, G.A.; Haack, J.L. Protocol for Monitoring Spring Communities at Ozark National Scenic Riverways, Missouri; Natural Resource Report NPS/HTLN/NRR—2008/029; National Park Service: Fort Collins, CO, USA, 2008; p. 214.
- Bowles, D.E.; Dodd, H.R.; Hinsey, J.A.; Cribbs, J.T.; Luraas, J.A. Spring Communities Monitoring at Ozark National Scenic Riverways, Missouri: 2007–2009 Status Report; Natural Resource Technical Report NPS/OZAR/NRTR—2011/511; National Park Service: Fort Collins, CO, USA, 2011; p. 57.
- Bowles, D.E.; Dodd, H.R. Aquatic Vegetation Monitoring in Springs at Ozark National Scenic Riverways, 2007–2015; Natural Resource Data Series NPS/OZAR/NRDS—2016/1044; National Park Service: Fort Collins, CO, USA, 2016; p. 21.
- United States Geological Survey (USGS). National Water Information System, USGS Gages 07067500, 07065495, 07066000, Missouri. Available online: https://waterdata.usgs.gov/nwis/uv/ (accessed on 28 April 2020).
- Mugel, D.N.; Richards, J.M.; Schumacher, J.G. Geohydrologic Investigations and Landscape Characteristics of Areas Contributing Water to Springs, the Current River, and Jacks Fork, Ozark National Scenic Riverways, Missouri; Scientific Investigations Report 2009-5138; U.S. Geological Survey: Reston, VA, USA, 2009; p. 92.
- Daubenmire, R.F. Canopy coverage method of vegetation analysis. Northwest Sci. 1959, 33, 43–64. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423, 623–656. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Joust, L. Entropy and diversity. Oikos 2006, 113, 2. [Google Scholar]
- Epps, T.W.; Singleton, K.J. An omnibus test for the two-sample problem using the empirical characteristic function. J. Stat. Comput. Sim. 1986, 26, 177–203. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 228. [Google Scholar]
- Goerg, S.J.; Kaiser, J. Nonparametric testing of distributions—the Epps–Singleton two-sample test using the empirical characteristic function. Stata J. 2009, 9, 454–465. [Google Scholar] [CrossRef] [Green Version]
- Bendix, J.; Hupp, C.R. Hydrological and geomorphological impacts on riparian plant communities. Hydrol. Process. 2000, 14, 2977–2990. [Google Scholar] [CrossRef]
- Bilby, R. Effects of a spate on the macrophyte vegetation of a stream pool. Hydrobiologia 1977, 56, 109–112. [Google Scholar] [CrossRef]
- Henry, C.P.; Bornette, G.; Amoros, C. Differential effects of floods on the aquatic vegetation of braided channels of the Rhone River. J. N. Am. Benthol. Soc. 1994, 13, 439–467. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Macrophytes: Ecology of Aquatic Plants; Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2009; p. 11. [Google Scholar]
- Bowles, D.E.; Dodd, H.R.; Hinsey, J.A.; Cribbs, J.T.; Williams, J.M. Aquatic Invertebrate Community Structure in Springs at Ozark National Scenic Riverways, Missouri, 2007–2016; Natural Resource Data Series NPS/HTLN/NRDS—2018/1161; National Park Service: Fort Collins, CO, USA, 2018; p. 37.
- Kim, J.-H.; Choi, I.-K. Vegetation behavior and its habitat at region against flood flow in urban streams. J. Eng. Sci. Technol. 2013, 8, 306–315. [Google Scholar]
- Puijalon, S.; Bornette, G.; Sagnes, P. Adaptations to increasing hydraulic stress: Morphology, hydrodynamics and fitness of two higher aquatic plant species. J. Exp. Bot. 2005, 56, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Puijalon, S.; Bouma, T.J.; Douay, C.J.; van Groenendael, J.; Anten, N.P.R.; Martel, E.; Bornette, G. Plant resistance to mechanical stress: Evidence of an avoidance–tolerance trade-off. New Phytol. 2011, 191, 1141–1149. [Google Scholar] [CrossRef]
- Schutten, J.; Dainty, J.; Davy, A.J. Root anchorage and its significance for submerged plants in shallow lakes. J. Ecol. 2005, 93, 556–571. [Google Scholar] [CrossRef]
- Riis, T.; Biggs, B.J.F. Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnol. Oceanogr. 2003, 48, 1488–1497. [Google Scholar] [CrossRef] [Green Version]
- Koehl, M.A.R. The interaction of moving water and sessile organisms. Sci. Am. 1982, 247, 110–120. [Google Scholar] [CrossRef]
- Hickey, J.T.; Salas, J.E. Environmental Effects of Extreme Floods; U.S.-Italy Research Workshop on the Hydrometeorology, Impacts, and Management of Extreme Floods: Perugia, Italy, 1995; p. 23. [Google Scholar]
- Maltchik, L.; Pedro, F. Responses of aquatic macrophytes to disturbance by flash floods in a Brazilian semiarid intermittent stream. Biotropica 2001, 33, 566–572. [Google Scholar] [CrossRef]
- Keruzoré, A.A.; Willby, N.J. Avoidance of hydrological disturbance by aquatic vegetation in the floodplain of a large upland river. Aquat. Bot. 2014, 116, 19–26. [Google Scholar] [CrossRef]
- Padial, A.A.; Carvalho, P.; Thomaz, S.M.; Boschilia, S.M.; Rodrigues, R.B.; Kobayashi, J.T. The role of an extreme flood disturbance on macrophyte assemblages in a Neotropical floodplain. Aquat. Sci. 2009, 71, 389–398. [Google Scholar] [CrossRef]
- Barrat-Segretain, M.-H.; Henry, C.P.; Bornette, G. Regeneration and colonization of aquatic plant fragments in relation to the disturbance frequency of their habitats. Arch. Hydrobiol. 1999, 145, 111–127. [Google Scholar] [CrossRef]
- Barrat-Segretain, M.-H.; Bornette, G.; Hering-Vilas-Bôas, A. Comparative abilities of vegetative regeneration among aquatic plants growing in disturbed habitats. Aquat. Bot. 1998, 60, 201–211. [Google Scholar] [CrossRef]
- Blom, C.W.P.M.; Voesenek, L.A.C.J. Flooding: The survival strategies of plants. Trends Ecol. Evol. 1996, 11, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Combroux, I.; Bornette, G.; Willby, N.J.; Amoros, C. Regenerative strategies of aquatic plants in disturbed habitats: The role of the propagule bank. Arch. Hydrobiol. 2001, 152, 215–235. [Google Scholar]
- Mony, C.; Puijalon, S.; Bornette, G. Resprouting response of aquatic clonal plants to cutting may explain their resistance to spate flooding. Folia Geobot. 2011, 46, 155–164. [Google Scholar] [CrossRef]
- Strauss, V.; Janauer, G.A. Impact of the 2002 extreme flood on aquatic macrophytes in a former side channel of the river Danube (Austria). Belg. J. Bot. 2007, 140, 17–24. [Google Scholar]
- Kitamura, K.; Yoshimura, J.; Tainaka, K. Potential impacts of flooding events and stream modification on an endangered endemic plant, Schoenoplectus gemmifer (Cyperaceae). Ecol. Res. 2009, 24, 533–546. [Google Scholar] [CrossRef] [Green Version]




| Variable | Alley | Big |
|---|---|---|
| Recharge area (km2) | 382.20 | 1118.00 |
| Mean annual discharge m3/s | 3.78 | 12.49 |
| Forested (km2) | 227.50 | 899.60 |
| Cultivated (km2) | 2.86 | 6.24 |
| Open (km2) | 148.46 | 199.16 |
| Urban (km2) | 3.90 | 10.40 |
| Public owned land (km2) | 80.60 | 663.00 |
| Human population in recharge area | 1767.00 | 5355.00 |
| Channel width (mean) (m) | 13.98 | 32.77 |
| Depth (cm) | 55.75 | 71.27 |
| Length of spring run (m) | 988.00 | 656.00 |
| Source elevation (m asl) | 217.93 | 137.77 |
| Mouth elevation (m asl) | 205.74 | 130.45 |
| Overall channel slope (%) | 0.94 | 0.95 |
| Current velocity (m/s) | 0.46 | 0.38 |
| Dominant substrate size (mm) | 58.91 | 36.50 |
| Substrate embeddedness (%) | 0.32 | 0.54 |
| Canopy cover (%) | 0.24 | 0.23 |
| Water temperature (°C) | 13.78 | 14.24 |
| Specific conductance (μS/cm) | 266.71 | 311.53 |
| Dissolved oxygen (mg/L) | 9.41 | 9.32 |
| pH | 7.24 | 7.27 |
| Spring | W | p |
|---|---|---|
| Alley | ||
| S | 3.71 | 0.45 |
| He | 2.6 | 0.63 |
| De | 3.79 | 0.43 |
| Evenness | 0.86 | 0.93 |
| Bare Substrate | 3.87 | 0.42 |
| Big | ||
| S | 11.54 | 0.02 |
| He | 8.37 | 0.08 |
| De | 6.86 | 0.14 |
| Evenness | 9.41 | 0.05 |
| Bare Substrate | 12.24 | 0.02 |
| Taxa | PFC | ISF | SIV | |||
|---|---|---|---|---|---|---|
| W | p | W | p | W | p | |
| Alley Spring | ||||||
| Batrachospermum spp. | 14.83 | 0.005 | 3.17 | 0.53 | 5.88 | 0.21 |
| Filamentous green algae (Chlorophyta) | 3.24 | 0.52 | 17.34 | 0.002 | 3.41 | 0.49 |
| Nostoc parmelioides Kütz. ex Bornet and Flahault | 2.97 | 0.56 | 3.44 | 0.49 | 9.98 | 0.04 |
| Mosses (Bryophyta) | 5.06 | 0.28 | 10.5 | 0.03 | 3.61 | 0.46 |
| Lemna trisculca L. | 8.6 | 0.07 | 2.74 | 0.6 | 1.71 | 0.79 |
| Callitriche heterophylla Pursh | 17.4 | 0.002 | 6.88 | 0.14 | 7.38 | 0.12 |
| Cardamine bulbosa (Schreb. ex Muhl.) Britton, Sterns and Poggenb. | 1.34 | 0.85 | 2.1 | 0.72 | 2.63 | 0.62 |
| Elodea spp. | 7.21 | 0.12 | 1.43 | 0.84 | 2.17 | 0.7 |
| Glyceria striata (Lam.) Hitchc. | 4.32 | 0.36 | 1.06 | 0.9 | 6.33 | 0.18 |
| Nasturtium officinale W.T. Aiton | 0.81 | 0.94 | 2.67 | 0.99 | 1.25 | 0.87 |
| Sparganium americanum Nutt. | 4.07 | 0.4 | 3.7 | 0.45 | 2.2 | 0.69 |
| Veronica anagallis-aquatica L. | 4.96 | 0.29 | 21.26 | 0.0003 | 15.35 | 0.004 |
| Big Spring | ||||||
| Batrachospermum spp. | 2.37 | 0.67 | 1.76 | 0.78 | 0.98 | 0.91 |
| Filamentous green algae (Chlorophyta) | 9.03 | 0.06 | 3.18 | 0.53 | 4.22 | 0.38 |
| Nostoc parmelioides Kütz. ex Bornet and Flahault | no test | no test | no test | no test | no test | no test |
| Mosses (Bryophyta) | 4.42 | 0.36 | 6.79 | 0.15 | 2.15 | 0.71 |
| Callitriche heterophylla Pursh | 16.2 | 0.003 | 5.16 | 0.27 | 3.55 | 0.47 |
| Cardamine bulbosa (Schreb. ex Muhl.) Britton, Sterns and Poggenb. | 0.59 | 0.96 | 9161.2 | 0 | 67.01 | <0.0001 |
| Elodea spp. | 1.12 | 0.89 | 2.24 | 0.69 | 7.38 | 0.12 |
| Lemna trisculca L. | no test | no test | no test | no test | no test | |
| Nasturtium officinale W.T. Aiton | 143.57 | <0.0001 | 106.93 | <0.0001 | 72.49 | <0.0001 |
| Poa annua L. | 1.41 | 0.84 | 2.28 | 0.68 | 6.14 | 0.19 |
| Ranunculus aquatilis L. | 31.06 | 0.0003 | 221.64 | <0.0001 | 35.71 | <0.0003 |
| Sparganium americanum Nutt. | 1.97 | 0.75 | 9.1 | 0.06 | 4.11 | 0.39 |
| Veronica anagallis-aquatica L. | 6.31 | 0.18 | 31.99 | 0.0001 | 50.29 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowles, D.E. Resiliency and Recovery of Aquatic Vegetation Following Scouring Floods in Two First-Magnitude Springs, Missouri, USA. Hydrobiology 2022, 1, 164-182. https://doi.org/10.3390/hydrobiology1020013
Bowles DE. Resiliency and Recovery of Aquatic Vegetation Following Scouring Floods in Two First-Magnitude Springs, Missouri, USA. Hydrobiology. 2022; 1(2):164-182. https://doi.org/10.3390/hydrobiology1020013
Chicago/Turabian StyleBowles, David E. 2022. "Resiliency and Recovery of Aquatic Vegetation Following Scouring Floods in Two First-Magnitude Springs, Missouri, USA" Hydrobiology 1, no. 2: 164-182. https://doi.org/10.3390/hydrobiology1020013
APA StyleBowles, D. E. (2022). Resiliency and Recovery of Aquatic Vegetation Following Scouring Floods in Two First-Magnitude Springs, Missouri, USA. Hydrobiology, 1(2), 164-182. https://doi.org/10.3390/hydrobiology1020013






