Sulfate (SO42−) Decline Supported Lake Kinneret (Israel) Invasion of N2-Fixing Cyanobacterium Aphanizomenon ovalisporum
Abstract
:1. Introduction
2. Material and Methods
2.1. Statistical Methods
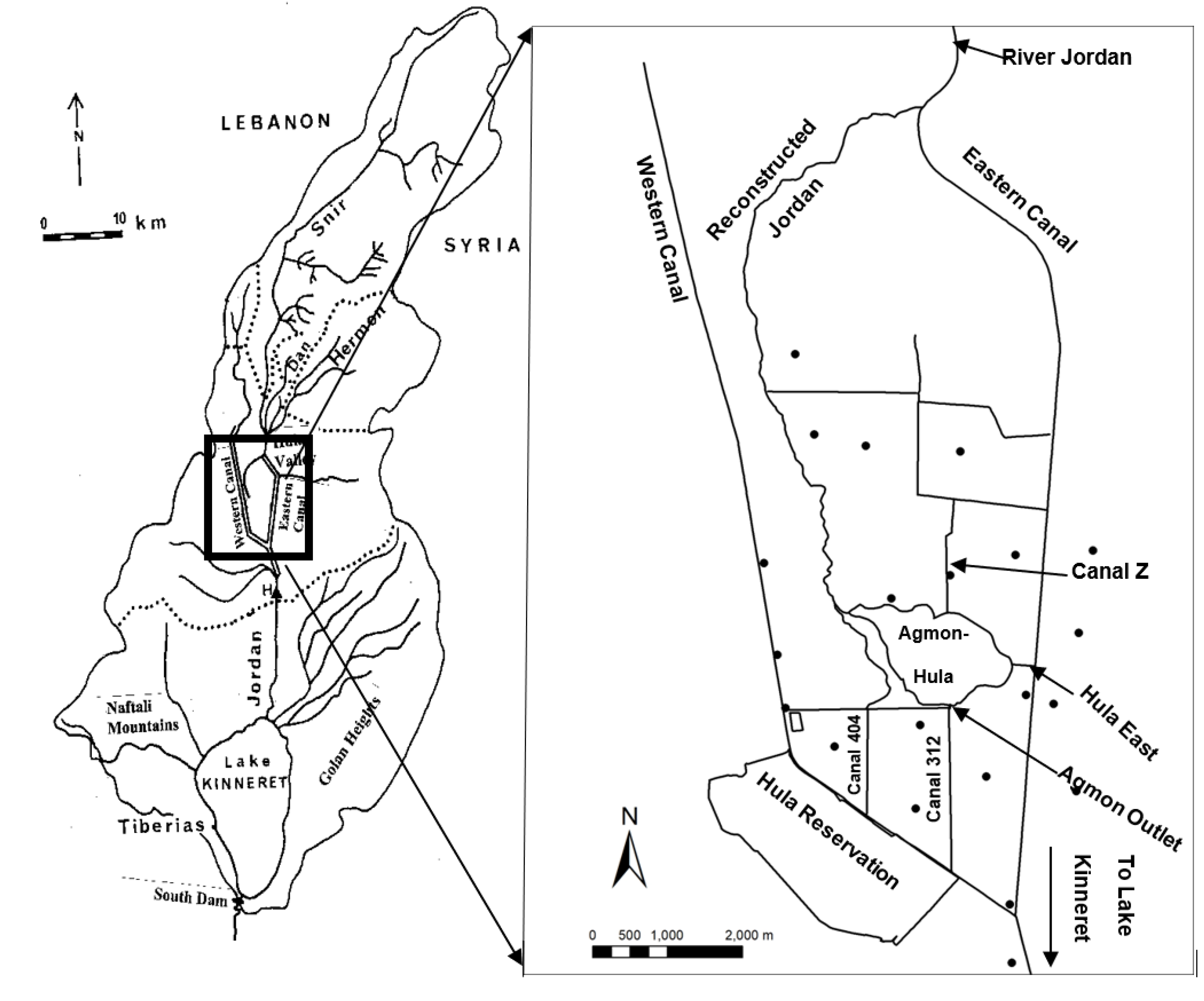
2.2. Ground Water Table Mapping
3. Results
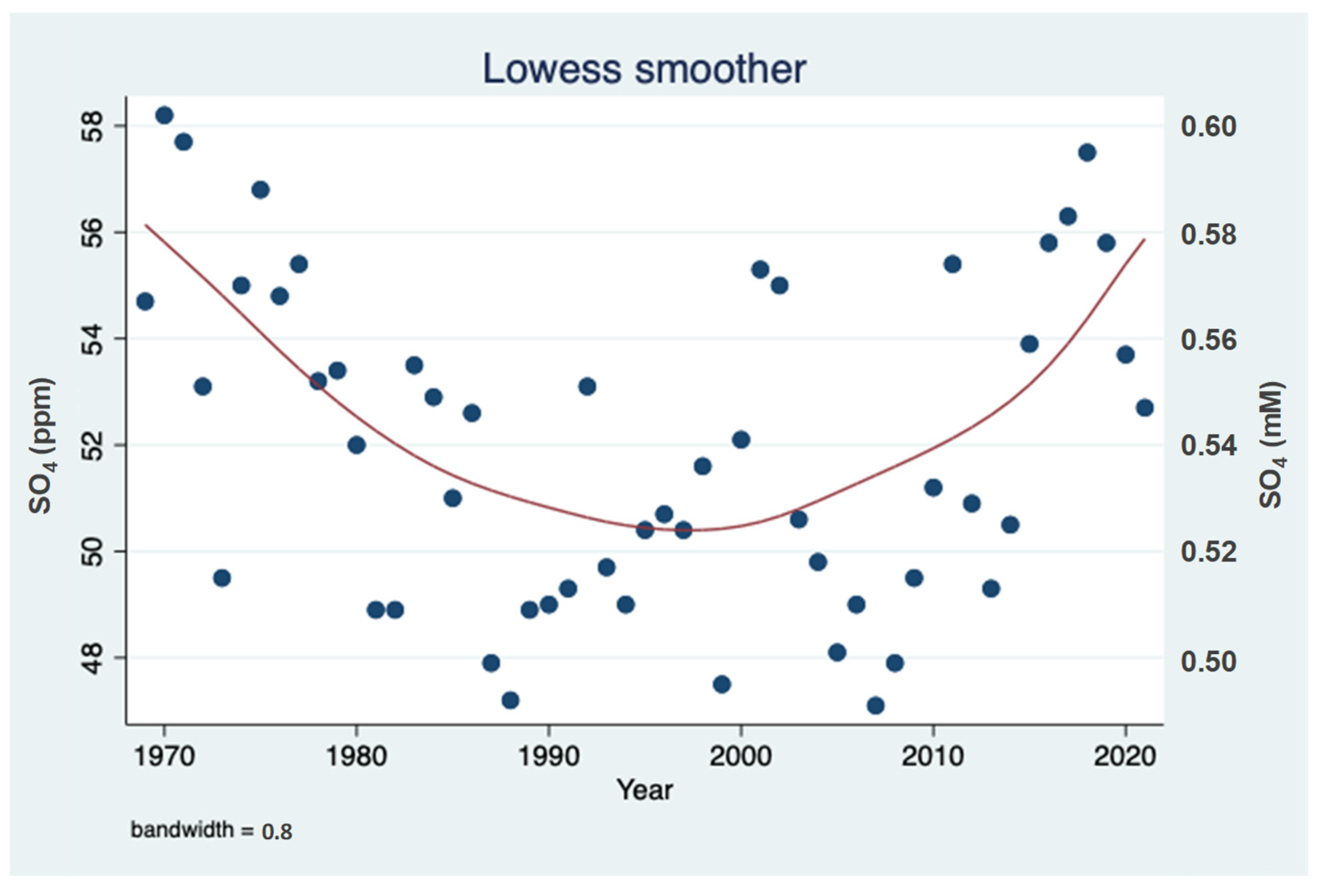
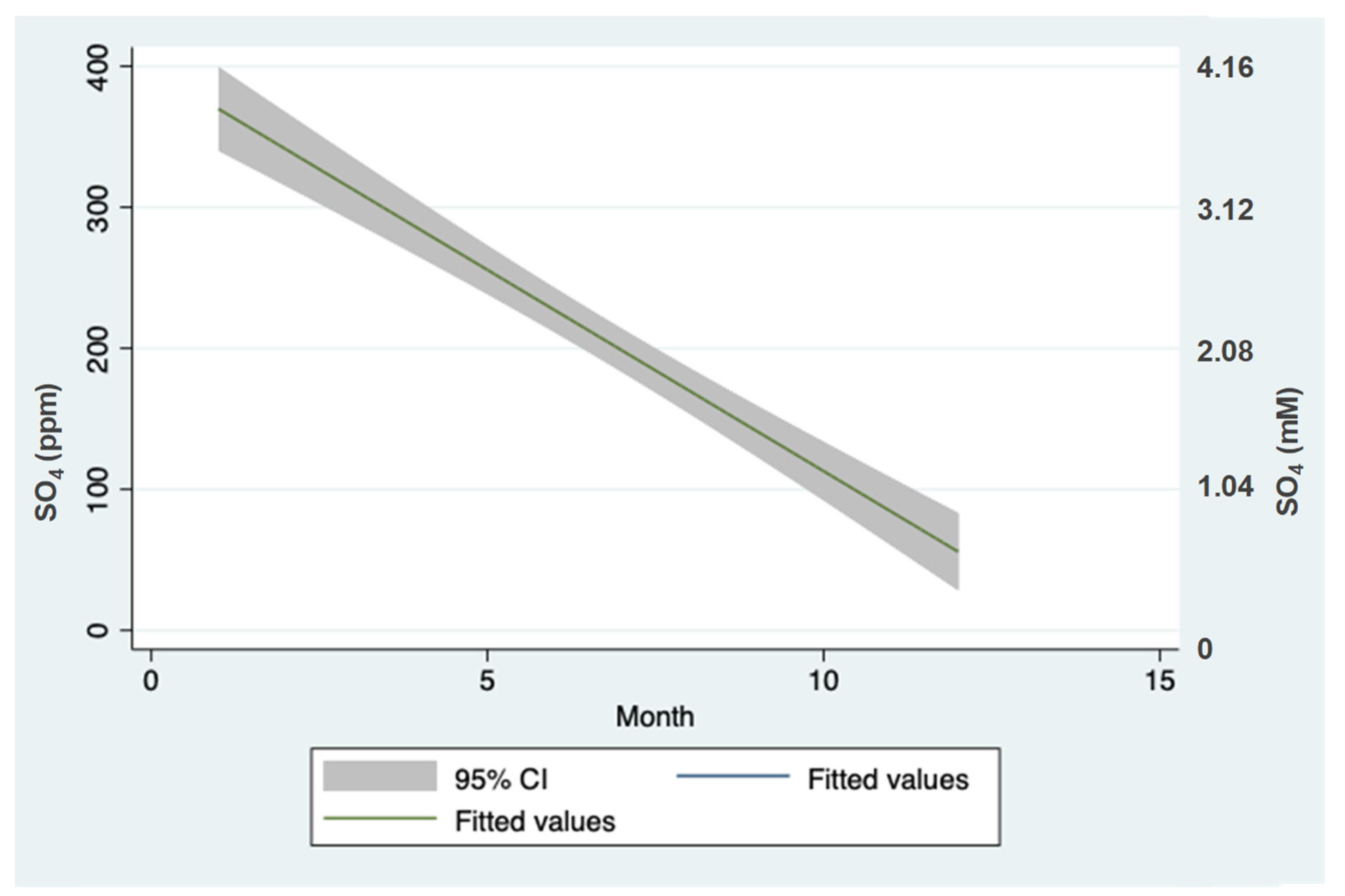
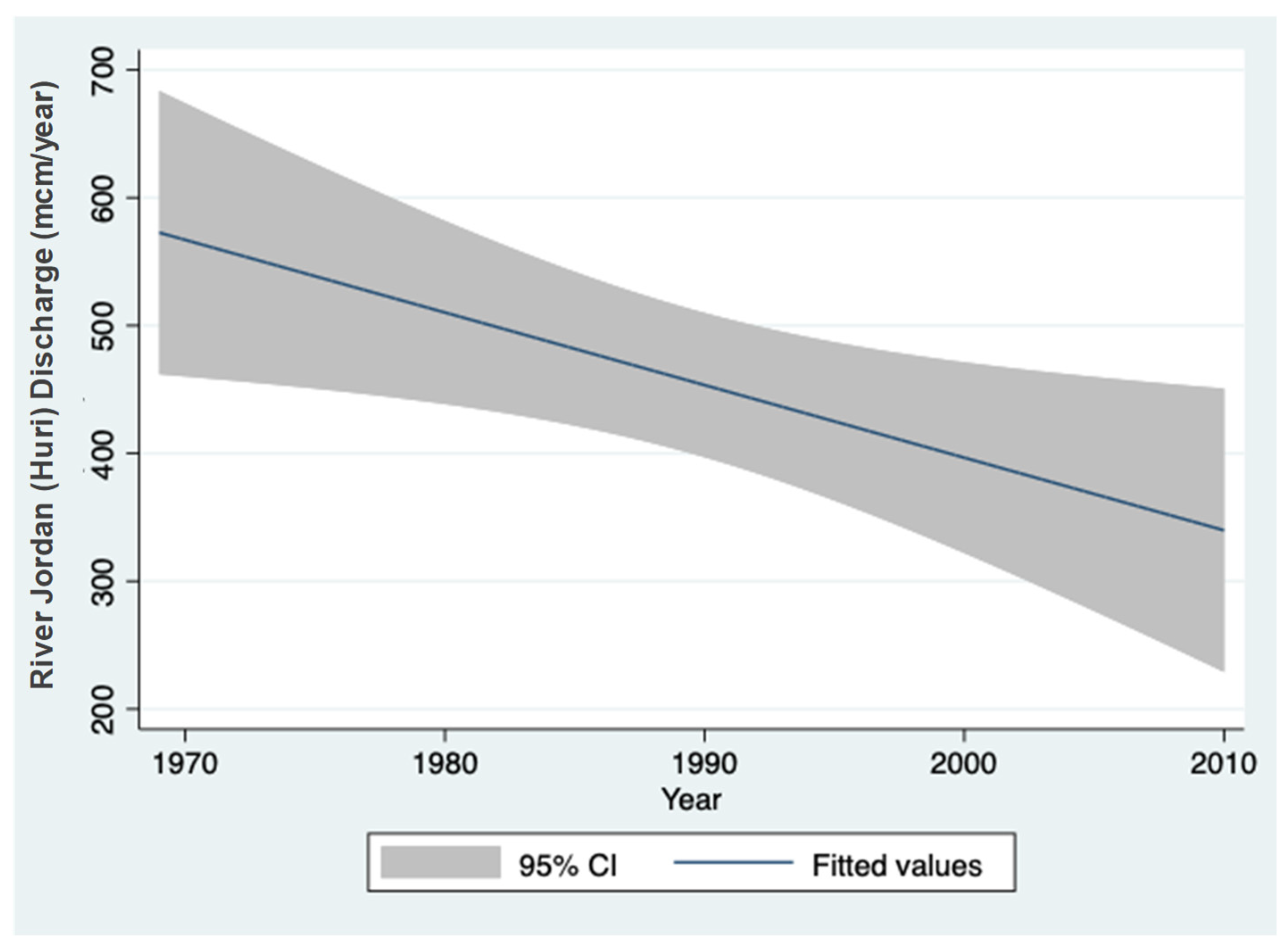
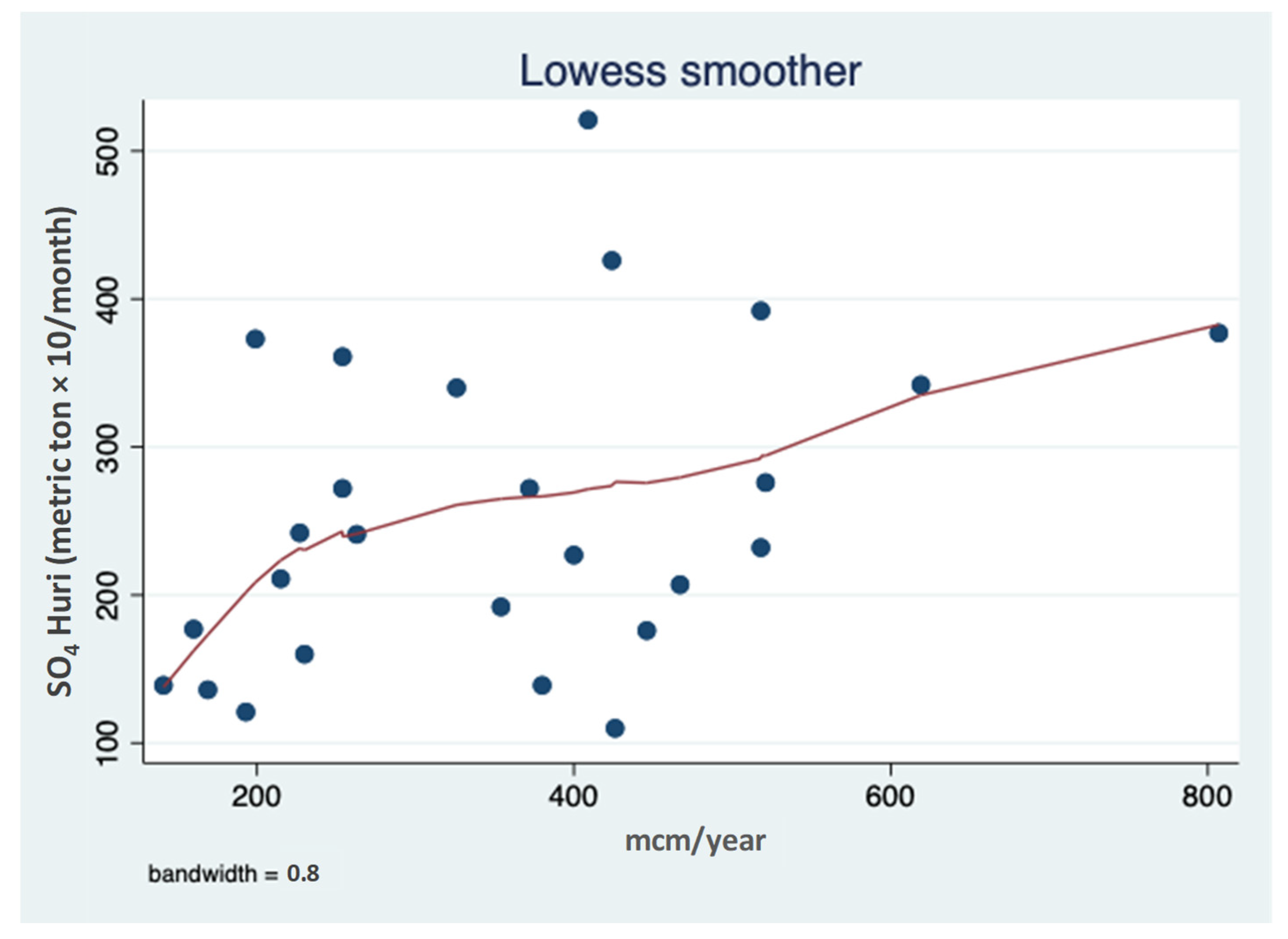
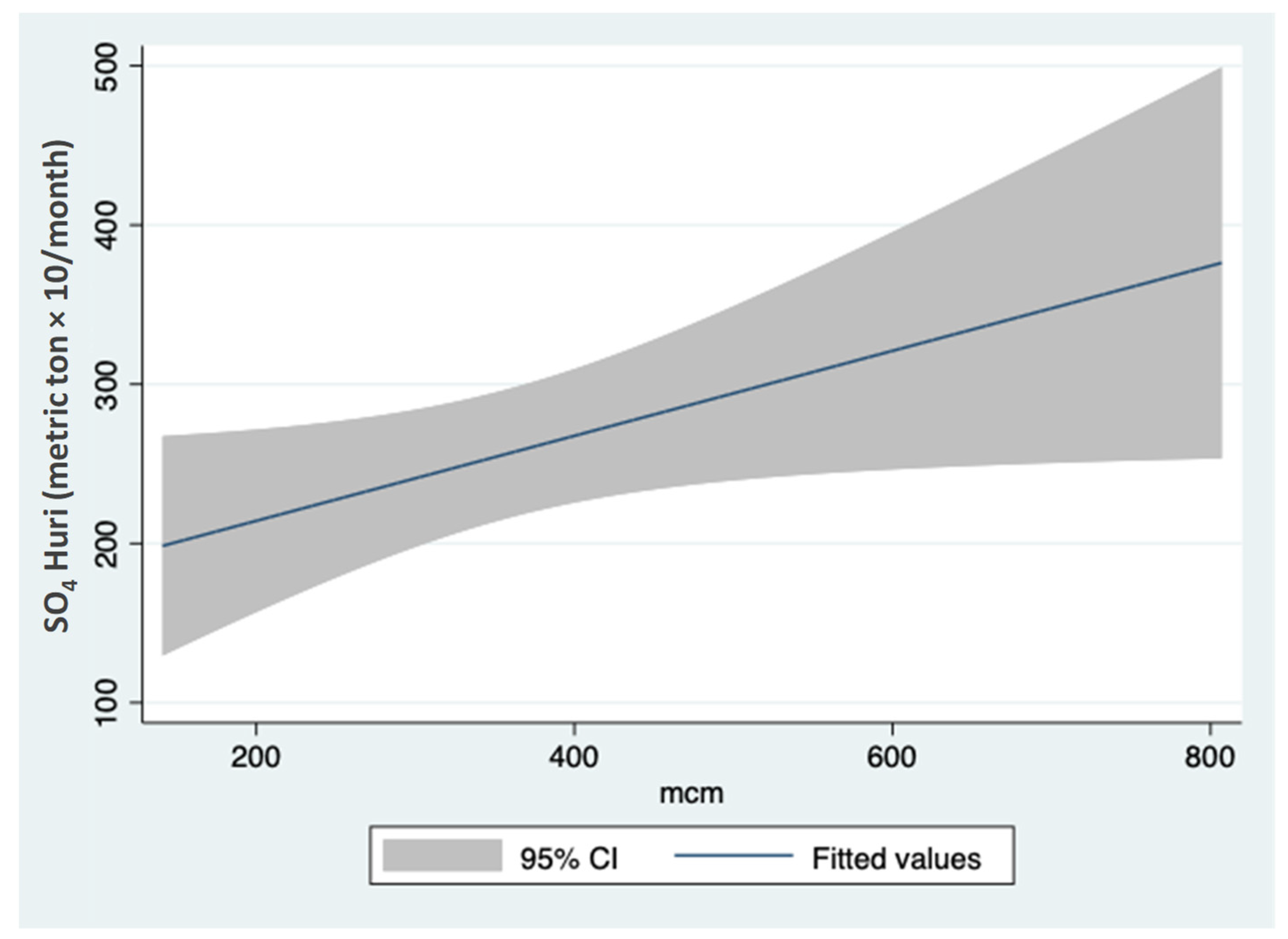
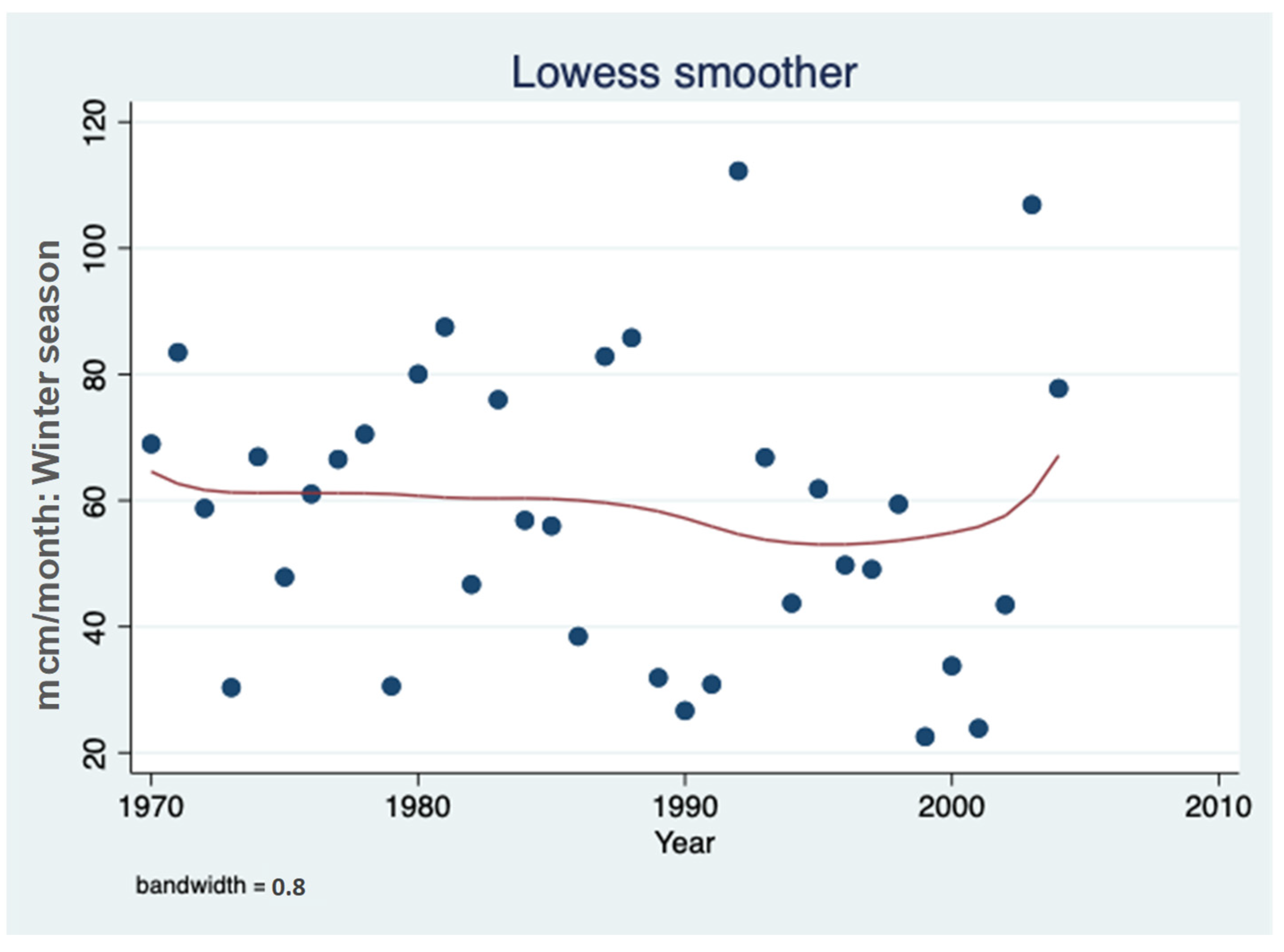
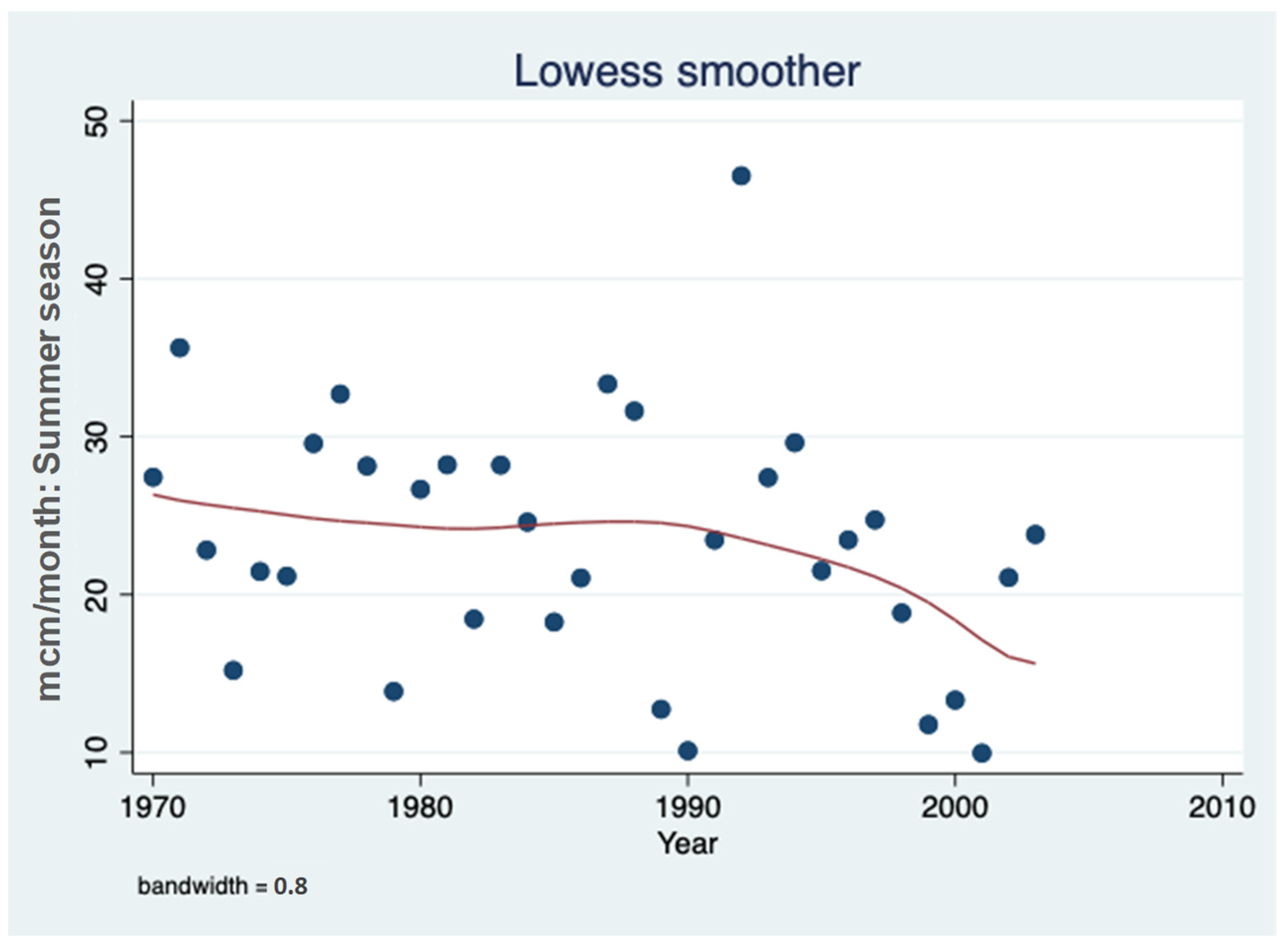
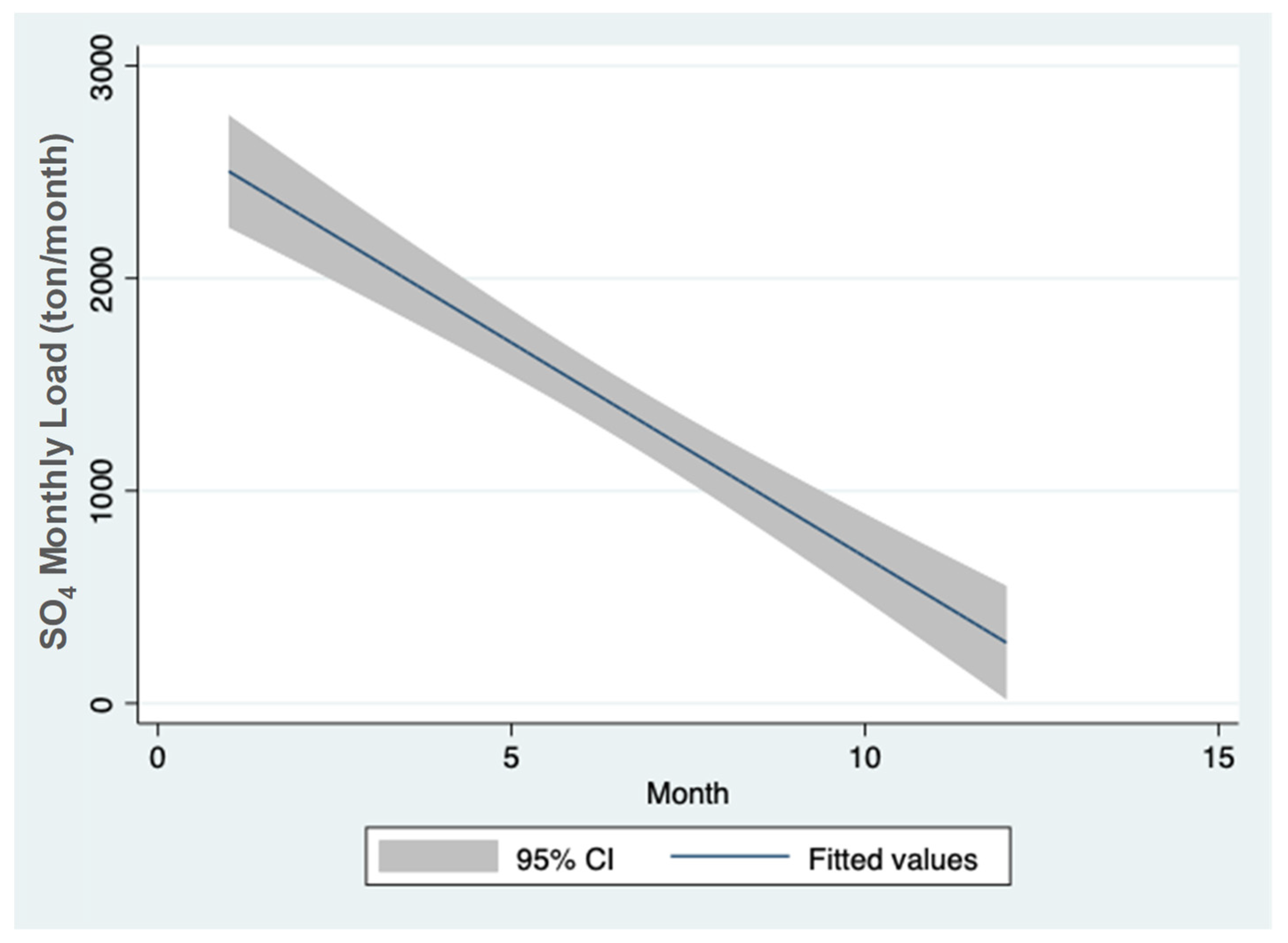
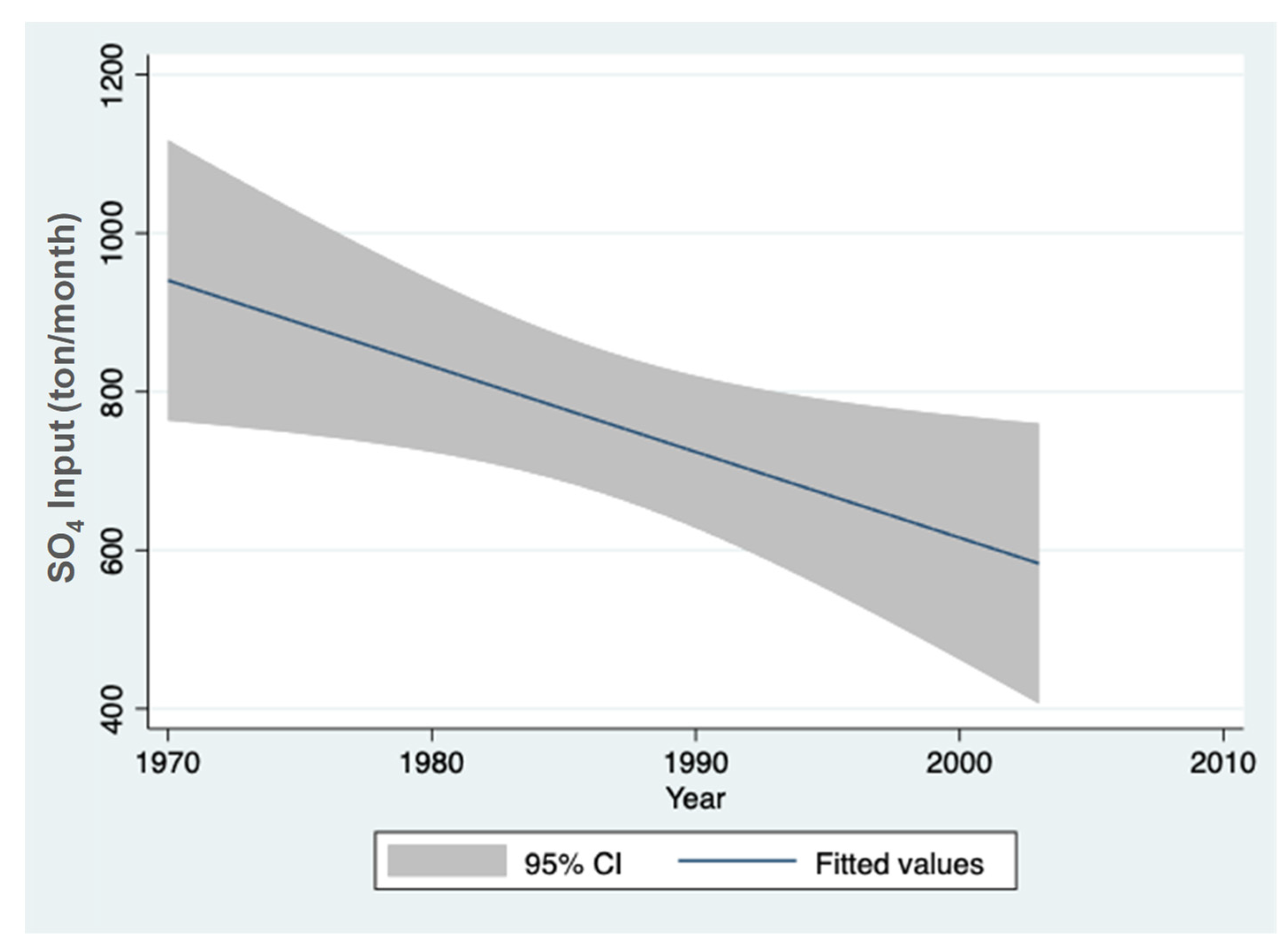
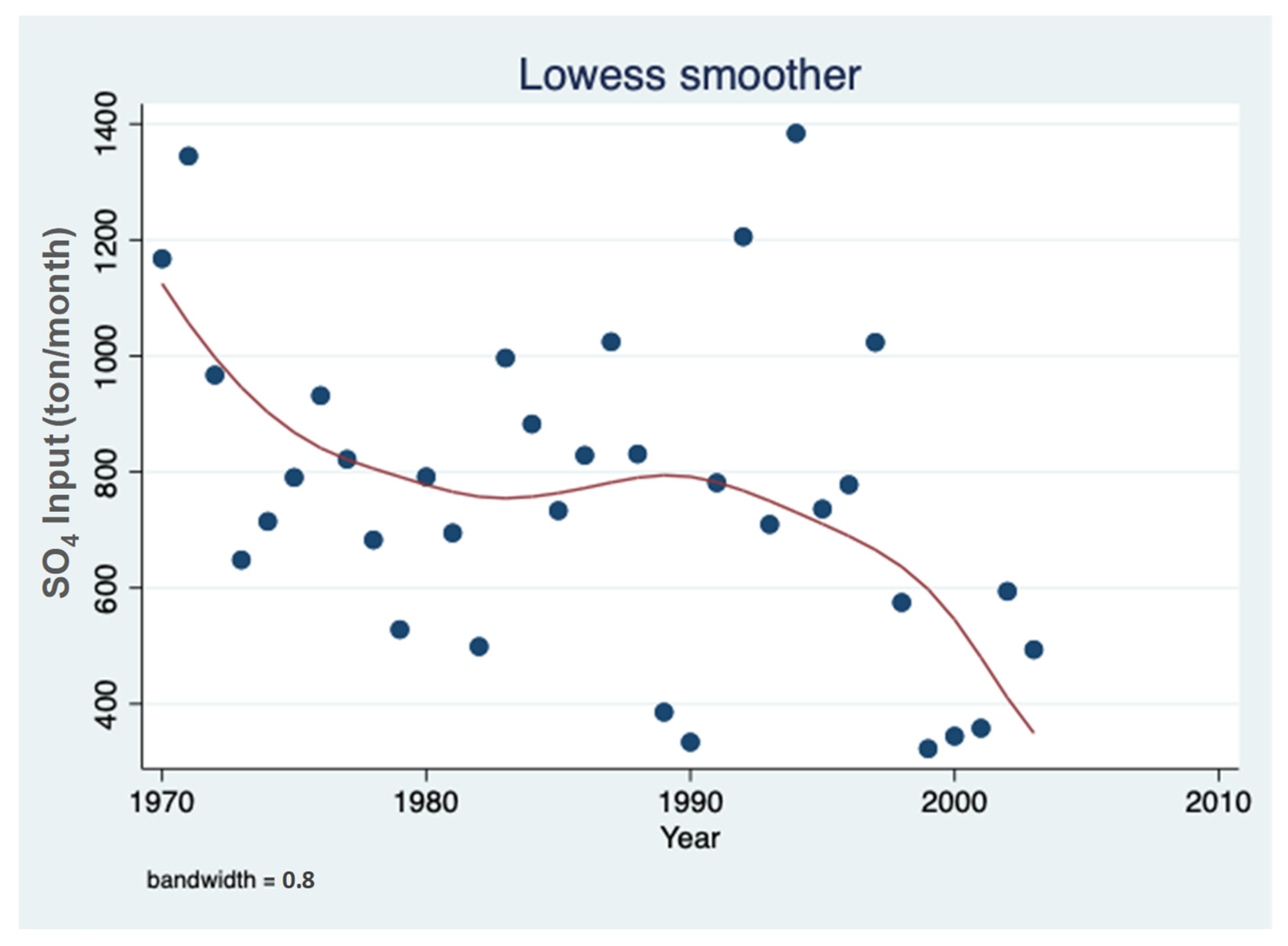
3.1. Sulfate (SO42−) Resources
3.2. Sulfate (SO42−) Outsources in the Hula Valley
4. Discussion
4.1. Sulfur Cycle in Lake Kinneret
4.2. Molybdenum (MoO42−) in Lake Kinneret
4.3. SO42− Flushing from the Peat Soil
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hadas, O.; Pinkas, R.; Malinsky-Rushansky, N.; Nishri, A.; Kaplan, A.; Rimmer, A.; Sukenik, A. Appearance and establishment of diazotrophic cyanobacteria in Lake Kinneret, Israel. Freshw. Biol. 2012, 57, 1214–1227. [Google Scholar] [CrossRef]
- Zohary, T.; Sukenik, A.; Berman, T.; Nishri, A. (Eds.) Lake Kinneret-Ecology and Management; Springer: Berlin/Heidelberg, Germany, 2014; p. 683. [Google Scholar]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Peterson, M.J.; Beaty, K.L.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.N. Nitrogen in aquatic ecosystems. Ambio 2002, 31, 102–112. [Google Scholar] [CrossRef]
- Cotner, J.B. Nitrogen is not a ‘house of cards’. Environ. Sci. Technol. 2017, 51, 3. [Google Scholar] [CrossRef] [Green Version]
- Gophen, M.; Smith, V.H.; Nishri, A.; Threlkeld, S.T. Nitrogen Deficiency, Phosphorus Sufficiency, and the Invasion of Lake Kinneret, Israel, by N2-Fixing Cyanobacterium Aphanizomenon ovalisporum. Aquat. Sci. 1999, 61, 293–306. [Google Scholar] [CrossRef]
- Smith, V.H. Low Nitrogen to Phosphorus Ratios Favor Dominance by Blue-Green Algae in Lake Phytoplankton. Science 1983, 221, 669–671. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.H. The nitrogen and phosphorus dependence of algal biomass in lakes: An experimental and theoretical analysis. Limnol. Oceanogr. 1982, 27, 1101–1111. [Google Scholar] [CrossRef]
- Higgins, S.N.; Paterson, M.J.; Hecky, R.E.; Schindler, D.W.; Venkiteswaran, J.J.; Findlay, J.J. Biological nitrogen fixation prevents the response of a eutrophic lake to reduced loading of nitrogen: Evidence from a 46-year whole-lake experiment. Ecosystems 2017, 21, 1088–1100. [Google Scholar] [CrossRef]
- Haworth, R.W.; Cole, J.J. Molybdenum availability, nitrogen limitation, and phytoplankton growth in natural waters. Science 1985, 229, 653–655. [Google Scholar] [CrossRef]
- Marino, R.; Haworth, R.W. Why is Planktonic Nitrogen Fixation So Rare in Coastal Marine Ecosystems? Insight from a Cross-Systems Approach. In Aquatic Microbial Ecology and Biochemistry: A Dual Perspective; Gilbert, P., Kana, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 127–139. [Google Scholar]
- Cole, J.J.; Lane, J.M.; Marino, R.; Haworth, R.W. Molybdenum assimilation by cyanobacteria and phytoplankton in freshwater and salt water. Limnol. Oceanogr. 1993, 38, 25–35. [Google Scholar] [CrossRef] [Green Version]
- LKDB 1970–2020; Annual Reports; Lake Kinneret Date Base, Kinneret Limnological Laboratory, IOLR: Haifa, Israel.
- Serruya, C. Lake Kinneret. In Monographgiae Biologcae; Springer: Berlin, Germany, 1978; Volume 32. [Google Scholar]
- Gophen, M. Ecological Research in the Lake Kinneret and Hula Valley (Israel) Ecosystems; Scientific Research Publishing, Inc.: USA, 2018; 335p, Available online: https://www.scirp.org/book/detailedinforofabook.aspx?bookid=2574 (accessed on 16 January 2022).
- Mazor, E. Mineral waters of the Kinneret basin and possible origin. In Lake Kinneret; Serruya, C., Ed.; Springer: Berlin, Germany, 1978; pp. 103–117. [Google Scholar]
- Meron, M.; Degani, G.; Lahav, U.; Dosoretz, C.; Shaviv, A. Suitability of Shamir Borehole Drillings for Agricultural Utilization. In Annual Report, Research Program No. 596-0382-08; Agriculture Ministry Foundation: Kiryat Shmone, Israel, 2010; 60p. (In Hebrew) [Google Scholar]
- Gophen, M.; Levanon, D. (Eds.) 1993-2006 Hula Project, Annual Reports; Jewish National Fund (Keren Kayemet LeIsrael), Migal-Scientific Research Institute, and Israeli Water Authority: Kiryat Shmone, Israel, 2006. [Google Scholar]
- Gonen, E. (Ed.) Hula Project Annual Report; Jewish National Fund (Keren Kayemet LeIsrael), Migal-Scientific Research Institute, and Israeli Water Authority: Kiryat Shmone, Israel, 2007; 133p. [Google Scholar]
- Barnea, I. (Ed.) Hula Project Annual Report; Jewish National Fund (Keren Kayemet LeIsrael) Migal-Scientific Research Institute and Israeli Water Authority: Kiryat Shmone, Israel, 2008; 159p. [Google Scholar]
- Barnea, I. (Ed.) 2008–2018 Hula Project Annual Report; Jewish National Fund (Keren Kayemet LeIsrael) Migal-Scientific Research Institute and Israeli Water Authority: Kiryat Shmone, Israel, 2022. [Google Scholar]
- Nishri, A.; Halitz, L.; Yofe, O. Metal Concentrations in Lake Knneret. In Kinneret Limnological Laboratory, IOLR-T16/20092 and Geological Survey of Israel- TR-GSI/17/2002; Interim Report; IOLR: Haifa, Israel, 2002; 9p. (In Hebrew) [Google Scholar]
- Manheim, F.T.; Landergren, S. Molybdenum 42. In Handbook of Geochemistry; Part 5; Springer: Berlin/Heidelberg, Germany, 1978; Volume 2. [Google Scholar]
- Dahl, T.W.; Anbar, D.A.; Gordon, G.; Rosing, M.T.; Frei, R.; Canfield, D. The behaviour of molybdenum and its iso-topes across the chemocline and in the sediments of sulfidic Lake Cadagno, Switzerland. Geochim. Cosmochim. Acta 2010, 74, 144–163. [Google Scholar] [CrossRef]
- Magyar, B.; Moorand, H.C.; Sigg, L. Vertical Distribution and Transport of Molybdenum in a Lake with Seasonally Anoxic Hypolimnion. Limnol. Oceanogr. 1993, 38, 521–531. [Google Scholar] [CrossRef]
- Howarth, R.W.; Marino, R.; Lane, J.; Cole, D.R. Nitrogen Fixation in Freshwater, Estuarine and Marine Ecosystems. 1. Rates and Importance. Limnol. Oceanogr. 1988, 33, 669–687. [Google Scholar] [CrossRef]
- Frevert, T. Heavy metals in Lake Kinneret Israel, II, Hydrogen sulfide dependent precipitation of copper cadmium lead and zinc. Arch. Fuer Hydrobiol. 1987, 109, 1–24. [Google Scholar]
- Frevert, T.; Sollman, C. Heavy metals in Lake Kinneret Israel, III, Lead and Copper in interstitial water and sediment dry weights. Arch. Fuer Hydrobiol. 1987, 109, 191–205. [Google Scholar]
- Shaked, Y.; Erel, Y.; Sukenik, A. The Biochemical cycle of Iron and Associated Elements in Lake Kinneret. Geochim. Cosmochim. Acta 2004, 68, 1439–1451. [Google Scholar] [CrossRef]
- Marino, R.; Howarth, R.W.; Shamess, J.; Prepas, E. Molybdenum and sulfate as controls on the abundance of nitro-gen-fixing cyanobacteria in saline lakes in Alberta. Limnol. Oceanogr. 1990, 35, 245–259. [Google Scholar] [CrossRef]
- Marino, R.; Howarth, R.W.; Chan, F.; Cole, J.J.; Likens, G.E. Sulfate inhibition of molybdenum-dependent nitrogen fixation by planktonic cyanobacteria under sea water conditions: A non-reversible effect. In Aquatic Biodiversity; Springer: Dordrecht, The Netherlands, 2003; pp. 277–293. [Google Scholar]
- Glass, J.B.; Axler, R.P.; Chandra, S.; Goldman, C.R. Molybdenum limitation of microbial nitrogen fixation in aquatic ecosystems and pure cultures. Front. Microbiol. 2012, 3, 331. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.J.P.; da Silva, J.F. The Involvement of Molybdenum in Life. Biochem. Biophys. Res. Commun. 2002, 292, 293–299. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.; Spinks, S.; Andrews, S.; Thayalan, W.; Bowden, S. High Molybdenum availability for evolution in a Mesoproterozoic lacustrine environment. Nat. Commun. 2015, 6, 6996. [Google Scholar] [CrossRef] [Green Version]
- Romero, I.C.; Klein, N.J.; Sanudo, S.A.; Capone, D.G. Potential trace metal co-limitation controls on N2 fixation and NO3 uptake in lakes with varying trophic status. Front. Microbiol. 2013, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Kawakubo, S.; Hashi, S.; Iwatsuki, M. Physicochemical speciation of molybdenum in rain water. Water Res. 2001, 35, 2489–2495. [Google Scholar] [CrossRef]
- Goldman, C.R. Molybdenum as a Factor Limiting Primary Productivity in Castle Lake, California. Science 1960, 132, 1016–1017. [Google Scholar] [CrossRef] [PubMed]
- Kroneck, P.M.H.; Abt, D.J. Molybdenum in Nitrate Reductase and Nitrate Oxidoreductase. In Metal Ions in Biological Systems; Sigel, H., Sigel, A.R., Eds.; Marcell Dekker, Inc.: New York, NY, USA, 2002; pp. 369–403. [Google Scholar]




| Source | SO4 (ton/year) | |
|---|---|---|
| Input | Reconstructed Jordan | 40 |
| Input | “Canal Z” | 1564 |
| Input | Hula East | 105 |
| Total Input | 1709 | |
| Output | Agmon Outlet | 1302 |
| Output | Irrigation | 135 |
| Total Output | 1437 |
| 1969–1978 | 1985–1995 | |
|---|---|---|
| SO42− (ppm) Mo (ppb) | 56 0.5–1.5 | 46 0.5–1.5 |
| SO42− (mM) Mo (µM) | 0.583 0.00521–0.0156 | 0.479 0.00521–0.0156 |
| SO42− (mM)/Mo (µm) ratio | 1.11–0.37 × 105 | 0.91–0.31 × 105 |
| GWT (m) | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| 1 | 2 | 6 | 8 | 13 | 6 | 34 | 9 | 12 | 1 |
| 1.5 | 393 | 266 | 528 | 507 | 495 | 494 | 461 | 319 | 154 |
| 2 | 1303 | 1596 | 1555 | 826 | 1488 | 1118 | 1409 | 1098 | 979 |
| 2.5 | 682 | 450 | 321 | 639 | 369 | 660 | 526 | 919 | 765 |
| 3 | 27 | 77 | 5 | 363 | 55 | 99 | 13 | 61 | 481 |
| 3.5 | 7 | 12 | 0 | 61 | 5 | 11 | 0 | 6 | 35 |
| 4 | 4 | 6 | 0 | 8 | 0 | 3 | 0 | 0 | 3 |
| 4.5 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 2418 | 2418 | 2417 | 2417 | 2418 | 2419 | 2418 | 2418 | 2418 |
| Rain | 671 | 352 | 607 | 467 | 477 | 474 | 848 | 798 | 535 |
| Year | Dry Layer (106 m3) | Wet Layer (106 m3) (%) |
|---|---|---|
| 2013 | 50.2 | 70.7 (58.5) |
| 2014 | 70.5 | 50.4 (41.7) |
| 2015 | 47.3 | 73.6 (60.9) |
| 2016 | 67.3 | 53.6 (44.3) |
| 2017 | 72.6 | 48.3 (40.0) |
| 2018 | 70.9 | 50.0 (41.4) |
| 2019 | 48.7 | 72.2 (59.7) |
| 2020 | 51.9 | 69.0 (57.1) |
| 2021 | 56.8 | 64.1 (53.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gophen, M.; Levin-Orlov, V. Sulfate (SO42−) Decline Supported Lake Kinneret (Israel) Invasion of N2-Fixing Cyanobacterium Aphanizomenon ovalisporum. Hydrobiology 2022, 1, 146-163. https://doi.org/10.3390/hydrobiology1020012
Gophen M, Levin-Orlov V. Sulfate (SO42−) Decline Supported Lake Kinneret (Israel) Invasion of N2-Fixing Cyanobacterium Aphanizomenon ovalisporum. Hydrobiology. 2022; 1(2):146-163. https://doi.org/10.3390/hydrobiology1020012
Chicago/Turabian StyleGophen, Moshe, and Valerie Levin-Orlov. 2022. "Sulfate (SO42−) Decline Supported Lake Kinneret (Israel) Invasion of N2-Fixing Cyanobacterium Aphanizomenon ovalisporum" Hydrobiology 1, no. 2: 146-163. https://doi.org/10.3390/hydrobiology1020012
APA StyleGophen, M., & Levin-Orlov, V. (2022). Sulfate (SO42−) Decline Supported Lake Kinneret (Israel) Invasion of N2-Fixing Cyanobacterium Aphanizomenon ovalisporum. Hydrobiology, 1(2), 146-163. https://doi.org/10.3390/hydrobiology1020012







