Crustaceans (Malacostraca and Thecostraca) from the International Minho River, Iberian Peninsula
Abstract
:1. Introduction
2. Materials and Methods
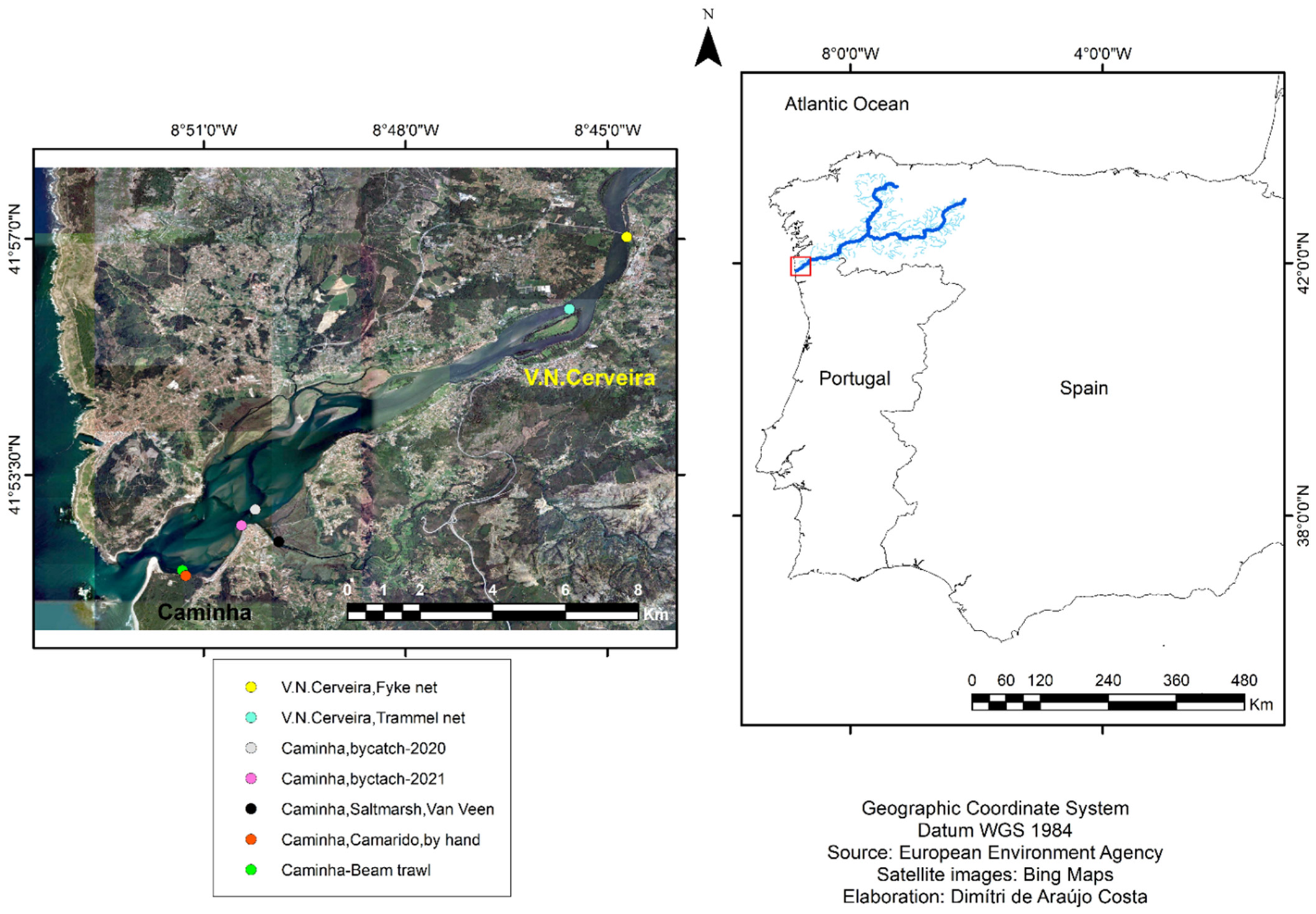
2.1. Study Area
2.2. Sampling, Identification and Preservation of Specimens
2.3. DNA Extraction, Amplification and Sequencing
2.4. Data Analysis
3. Results
- Subphyllum Crustacea Brünnich, 1772
- Superclass Multicrustacea Regier, Shultz, Zwick, Hussey, Ball, Wetzer, Martin & Cunningham, 2010
- Class Malacostraca Latreille, 1802
- Subclass Eumalacostraca Grobben, 1892
- Superorder Eucarida Calman, 1904
- Order Decapoda Latreille, 1802
- Suborder Pleocyemata Burkenroad, 1963
- Infraorder Astacidea Latreille, 1802
- Superfamily Astacoidea Latreille, 1802
- Family Cambaridae Hobbs, 1942
- Genus Procambarus Ortmann, 1905
- Infraorder Brachyura Latreille, 1802
- Superfamily Portunoidea Rafinesque, 1815
- Family Carcinidae MacLeay, 1838
- Genus Carcinus Leach, 1814
- Infraorder Caridea Dana, 1852
- Superfamily Atyoidea De Haan, 1849
- Family Atyidae De Haan, 1849
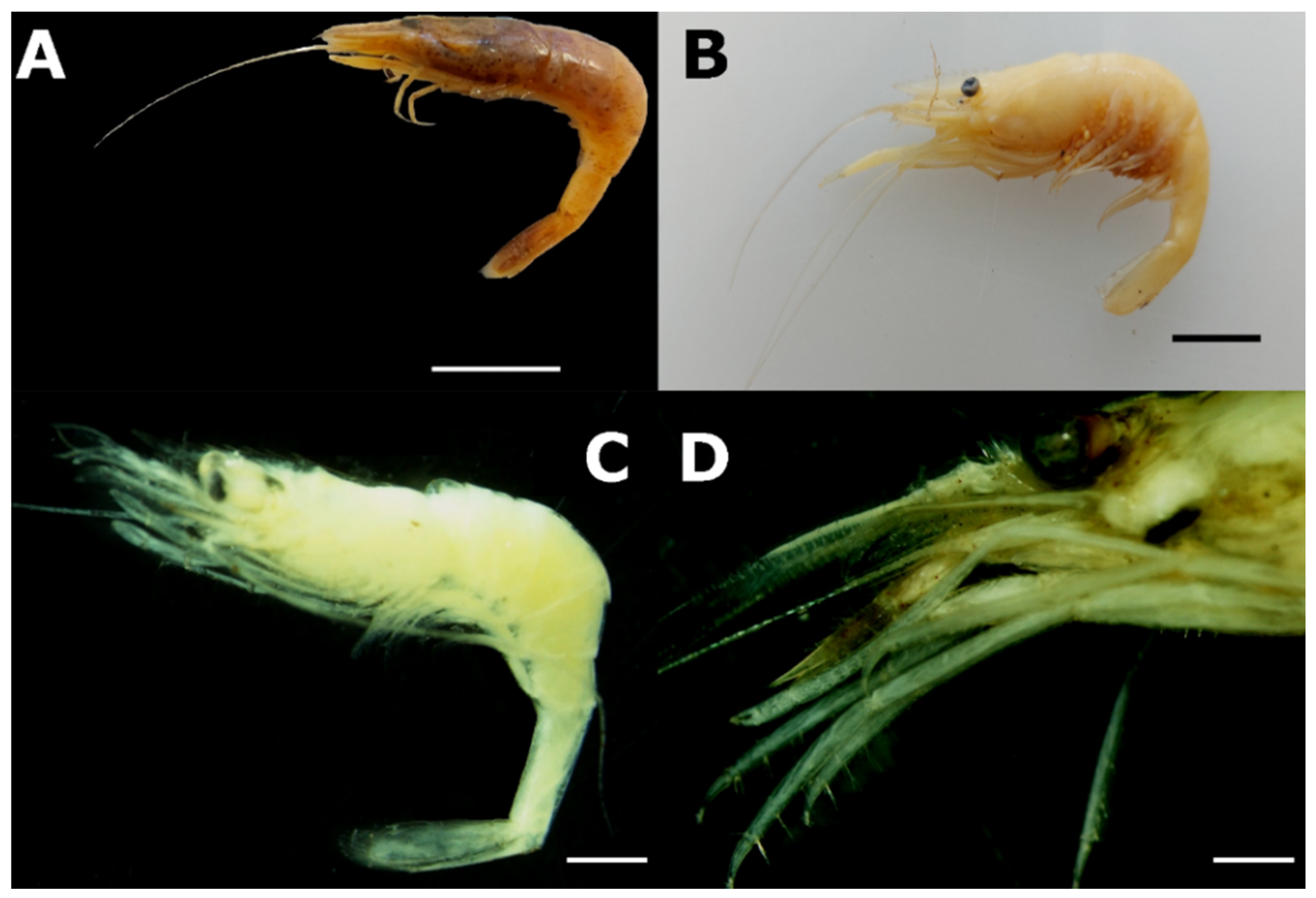
- Genus Atyaephyra de Brito Capello, 1866
- Superfamily Crangonoidea Haworth, 1825
- Family Crangonidae Haworth, 1825
- Genus Crangon Fabricius, 1798
- Superfamily Palaemonoidea Rafinesque, 1815
- Family Palaemonidae Rafinesque, 1815
- Genus Palaemon Weber, 1795
- Superfamily Processoidea Ortmann, 1896
- Family Processidae Ortmann, 1896
- Genus Processa Leach, 1815
- Superorder Peracarida Calman, 1904
- Order Cumacea Krøyer, 1846
- Family Bodotriidae Scott, 1901
- Genus Iphinoe Bate, 1856
- Family Diastylidae Bate, 1856
- Genus Diastylis Say, 1818
- Order Mysida Boas, 1883
- Family Mysidae Haworth, 1825
- Genus Gastrosaccus Norman, 1868
- Genus Neomysis Czerniavsky, 1882
- Genus Praunus Leach, 1814
- Order Tanaidacea Dana, 1849
- Suborder Tanaidomorpha Sieg, 1980
- Family Leptocheliidae Lang, 1973
- Genus Heterotanais Sars, 1882
- Subclass Phyllocarida Packard, 1879
- Order Leptostraca Claus, 1880
- Suborder Nebaliacea Calman, 1904
- Family Nebaliidae Samouelle, 1819
- Genus Nebalia Leach, 1814
- Class Thecostraca Gruvel, 1905
- Subclass Cirripedia Burmeister, 1834
- Superorder Thoracicalcarea Gale, 2015
- Order Balanomorpha Pilsbry, 1916
- Superfamily Elminioidea Chan, Dreyer, Gale, Glenner, Ewers-Saucedo, Pérez-Losada, Kolbasov, Crandall & Høeg, 2021
- Family Elminiidae Foster, 1982
- Genus Austrominius Buckeridge, 1983
- Order Isopoda Latreille, 1817
- Suborder Asellota Latreille, 1802
- Superfamily Janiroidea Sars, 1897
- Family Janiridae Sars, 1897
- Genus Jaera Leach, 1814
- Suborder Cymothoida Wägele, 1989
- Superfamily Cymothooidea Leach, 1814
- Family Cymothoidae Leach, 1818
- Genus Ceratothoa Dana, 1852
- Family Gnathiidae Leach, 1814
- Genus Gnathia Leach, 1814
- Suborder Valvifera Sars, 1883
- Family Idoteidae Samouelle, 1819
- Genus Idotea Fabricius, 1798
- Suborder Sphaeromatidea Wägele, 1989
- Superfamily Sphaeromatoidea Latreille, 1825
- Family Sphaeromatidae Latreille, 1825
- Genus Cymodoce Leach, 1814
Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahyong, S.T.; Lowry, J.K.; Alonso, M.; Bamber, R.N.; Boxshall, G.A.; Castro, P.; Gerken, S.; Karaman, G.S.; Goy, J.W.; Jones, D.S.; et al. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 2011, 3148, 165–191. [Google Scholar] [CrossRef]
- Smaldon, G. Coastal Shrimps and Prawns; Barnes, R.S.K., Crothers, J.H., Eds.; The Linnean Society of London & The Estuarine and Brackish-Water Sciences Association: Shrewsbury, UK, 1993. [Google Scholar]
- Souza, A.T.; Dias, E.; Marques, J.C.; Antunes, C.; Martins, I. Population structure, production and feeding habit of the sand goby Pomatoschistus minutus (Actinopterygii: Gobiidae) in the Minho estuary (NW Iberian Peninsula). Environ. Biol. Fishes 2015, 98, 287–300. [Google Scholar] [CrossRef]
- Mota, M.; Antunes, C. A preliminary characterisation of the habitat use and feeding of Allis shad (Alosa alosa) juveniles in the Minho River tidal freshwater wetlands. Limnetica 2012, 31, 165–172. [Google Scholar]
- Lages, A.; Costa, D.D.A.; Gomes, N.; Antunes, C. Exotic Pumpkinseed Sunfish Lepomis gibbosus (Linnaeus, 1758) in the International Minho River (Iberian Peninsula), and Parasitic Association with Myzobdella lugubris Leidy, 1851 (Annelida, Hirudinea). Oceanogr. Fish. Open Access J. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Naylor, E. British Marine Isopods: Keys and Notes for the Identification of the Species, 2nd ed.; Academic Press: London, UK, 1972; ISBN 0125151500. [Google Scholar]
- Poore, A.G.B.; Campbell, A.H.; Steinberg, P.D. Natural densities of mesograzers fail to limit growth of macroalgae or their epiphytes in a temperate algal bed. J. Ecol. 2009, 97, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Dalei, J.; Sahoo, D. Extraction and characterization of Astaxanthin from the crustacean shell waste from shrimp processing industries. Int. J. Pharm. Sci. Res. 2015, 6, 2532–2537. [Google Scholar] [CrossRef]
- Horton, T. Ceratothoa steindachneri (Isopoda: Cymothoidae) new to British waters with a key to north-east Atlantic and Mediterranean Ceratothoa. J. Mar. Biol. Assoc. UK 2000, 80, 1041–1052. [Google Scholar] [CrossRef]
- Dos Santos, A.; González-Gordillo, J.I. Illustrated keys for the identification of the Pleocyemata (Crustacea: Decapoda) zoeal stages, from the coastal region of south-western Europe. J. Mar. Biol. Assoc. UK 2004, 84, 205–227. [Google Scholar] [CrossRef]
- Costanza, R.; D’Arge, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Mazé, R.A.; Lastra, M.; Mora, J. Macrozoobentos del estuario del Mino (NO de Espana). Publ. Espec. Inst. Español. Ocean. 1993, 11, 283–290. [Google Scholar]
- Sousa, R.; Dias, S.; Freitas, V.; Antunes, C. Subtidal macrozoobenthic assemblages along the River Minho estuarine gradient (north-west Iberian Peninsula). Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 1063–1077. [Google Scholar] [CrossRef]
- Antunes, C.; Weber, M. The glass eel fishery and the by-catch in the Rio Minho after one decade (1981–1982 and 1991–1992). Arch. Pol. Fish. 1996, 4, 131–139. [Google Scholar]
- Picanço, T.C.; Almeida, C.M.R.; Antunes, C.; Reis, P.A. Influence of the abiotic characteristics of sediments on the macrobenthic community structure of the Minho estuary saltmarsh (Portugal). Limnetica 2014, 33, 73–88. [Google Scholar]
- Gomes, N.M.A.; Costa, D.D.A.; Cantallo, H.C.; Ribeiro, T.J.A.; Antunes, C. Isopods (Crustacea, Malacostraca) from International Minho River, Iberian Peninsula. Oceanogr. Fish. Open Access J. 2021, 13, 555866. [Google Scholar] [CrossRef]
- APA. Plano de Gestão de Região Hidrográfica—Região Hidrográfica do Minho e Lima (Rh1); APA: Lisboa, Portugal, 2016. [Google Scholar]
- Dias, E.; Morais, P.; Antunes, C.; Hoffman, J.C. Linking terrestrial and benthic estuarine ecosystems: Organic matter sources supporting the high secondary production of a non-indigenous bivalve. Biol. Invasions 2014, 16, 2163–2179. [Google Scholar] [CrossRef]
- BirdLife International Important Bird Areas Factsheet: Minho and Coura Estuaries. Available online: http://www.birdlife.org (accessed on 11 March 2021).
- Sousa, R.; Guilhermino, L.; Antunes, C. Molluscan fauna in the freshwater tidal area of the River Minho estuary, NW of Iberian Peninsula. Ann. Limnol. 2005, 41, 141–147. [Google Scholar] [CrossRef]
- Mota, M.; Bio, A.; Bao, M.; Pascual, S.; Rochard, E.; Antunes, C. New insights into biology and ecology of the Minho River Allis shad (Alosa alosa L.): Contribution to the conservation of one of the last European shad populations. Rev. Fish Biol. Fish. 2015, 25, 395–412. [Google Scholar] [CrossRef]
- Hayward, P.J.; Ryland, J.S. Handbook of the Marine Fauna of North-West Europe, 2nd ed.; Hayward, P.J., Ryland, J.S., Eds.; Oxford University Press: New York, NY, USA, 2017; ISBN 9780199549443. [Google Scholar]
- Jones, N.S. British Cumaceans. Arthropoda: Crustacea. Keys and Notes for the Identification of the Species. Synopses of the British Fauna No. 7; The Linnean Society of London: London, UK, 1976. [Google Scholar]
- Barnes, R.S.K. The Brackish-Water Fauna of Northeastern Europe; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Zariquiey-Alvarez, R. Crustáceos decápodos ibéricos. Investig. Pesq. Barc. 1968, 32, XV + 1–510. [Google Scholar]
- Anastasiadou, C.; Kitsos, M.S.; Koukouras, A. Redescription of Atyaephyra desmarestii (Millet, 1831) (Decapoda, Caridea, Atyidae) based on topotypical specimens. Crustaceana 2006, 79, 1195–1207. [Google Scholar] [CrossRef]
- Ledoyer, M. Sur quelques espèces nouvelles d’Iphinoe (Crustacea Cumacea). Discussion et description comparative des espèces européennes déjà connues. Recl. Traveaux Stn. Mar. d’Endoume 1965, 39, 253–294. [Google Scholar]
- Mazziotti, C.; Lezzi, M. The cumacean genus Iphinoe (Crustacea: Peracarida) from Italian waters and I. Daphne N. Sp. From the northwestern Adriatic Sea, Mediterranean. Zootaxa 2020, 4766, 331–357. [Google Scholar] [CrossRef]
- Moreira, J.; Díaz-Agras, G.; Candás, M.; Señarís, M.P.; Urgorri, V. Leptostracans (Crustacea: Phyllocarida) from the Ría de Ferrol (Galicia, NW Iberian Peninsula), with description of a new species of Nebalia Leach, 1814. Sci. Mar. 2009, 73, 269–285. [Google Scholar] [CrossRef] [Green Version]
- Makings, P. A guide to the British coastal Mysidacea. Field Stud. Counc. 1977, 4, 575–595. [Google Scholar]
- Zimmer, C. Die Nordischen Schizopoden; Brandt, K., Apstein, C., Eds.; Lipsius und Tischler: Kiel und Leipzig, Germany, 1909. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species; WoRMS and Flanders Marine Institute: Flanders, Belgium, 2021. [Google Scholar]
- GBIF. Home Page. Available online: https://www.gbif.org (accessed on 13 February 2020).
- Lobo, J.; Costa, P.M.; Teixeira, M.A.L.; Ferreira, M.S.G.; Costa, M.H.; Costa, F.O. Enhanced primers for amplification of DNA barcodes from a broad range of marine metazoans. BMC Ecol. 2013, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Hebert, P.D.N.; Ratnasingham, S. The Barcode of Life Data System BOLD. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Benson, D.A. GenBank. Nucleic Acids Res. 2004, 33, D34–D38. [Google Scholar] [CrossRef] [Green Version]
- Girard, C.F. A Revision of the North American Astaci, with Observations on Their Habits and Geographic Distribution. Proc. Acad. Nat. Sci. Phila. 1852, 6, 87–91. [Google Scholar]
- Hagen, H.A. Monograph of the North American Astacidae; University Press: Cambridge, UK, 1870. [Google Scholar]
- Faxon, W. A Revision of the Astacidæ; Museum of Comparative Zoology, Harvard University: Cambridge, UK, 1885. [Google Scholar]
- Campos, E.; Rodríguez-Almaraz, G.A. Distribution of the Red Swamp Crayfish Procambarus clarkii (Girard, 1852) (Decapoda: Cambaridae) in Mexico: An Update. J. Crustac. Biol. 1992, 12, 627–630. [Google Scholar] [CrossRef]
- Holdich, D.M.; Reynolds, J.D.; Sibley, P.J. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2009, 11, 394–395. [Google Scholar] [CrossRef] [Green Version]
- Wizen, G.; Galil, B.S.; Shlagman, A.; Gasith, A. First record of red swamp crayfish, Procambarus clarkii (Girard, 1852) (Crustacea: Decapoda: Cambaridae) in Israel—Too late to eradicate ? Aquat. Invasions 2008, 3, 181–185. [Google Scholar] [CrossRef]
- Harper, D.M.; Smart, A.C.; Coley, S.; Schmitz, S.; Beauregard, G.D.; North, R.; Adams, C.; Obade, P.; Kamau, M. Distribution and abundance of the Louisiana red swamp crayfish Procambarus clarkii Girard at Lake Naivasha, Kenya between 1987 and 1999. Hydrobiologia 2002, 488, 143–151. [Google Scholar] [CrossRef]
- Foster, J.; Harper, D.M. Status and Ecosystem Interactions of the Invasive Louisianan Red Swamp Crayfish Procambarus clarkii in East Africa. In Biological Invaders in Inland Waters: Profiles, Distribution, and Threats; Gherardi, F., Ed.; Springer International Publishing: Dordrecht, The Netherlands, 2007; pp. 91–101. ISBN 9781402060298. [Google Scholar]
- Magalhães, C.; Bueno, S.; Bond-Buckup, G.; Valenti, W.C.; Silva, H.L.M.; Kiyohara, F.; Mossolin, E.C.; Rocha, S.S. Exotic species of freshwater decapod crustaceans in São Paulo, Brazil: Records and possible the state of Sa causes of their introduction. Biodivers. Conserv. 2005, 14, 1929–1945. [Google Scholar] [CrossRef]
- Xu, H.; Qiang, S.; Han, Z.; Guo, J.; Huang, Z.; Sun, H.; He, S.; Ding, H.; Wu, H.; Wan, F. The status and causes of alien species invasion in China. Biodivers. Conserv. 2006, 15, 2893–2904. [Google Scholar] [CrossRef]
- Kawai, T. A review of the spread of Procambarus clarkii across Japan and its morphological observations. Freshw. Crayfish 2017, 23, 41–53. [Google Scholar] [CrossRef]
- Sousa, R.; Freitas, F.E.P.; Mota, M.; Nogueira, A.J.A.; Antunes, C. Invasive dynamics of the crayfish Procambarus clarkii (Girard, 1852) in the international section of the River Minho (NW of the Iberian Peninsula). Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 656–666. [Google Scholar] [CrossRef]
- Bernardo, J.M.; Costa, A.M.; Bruxelas, S.; Teixeira, A. Dispersal and coexistence of two non-native crayfish species (Pacifastacus leniusculus and Procambarus clarkii ) in NE Portugal over a 10-year period. Knowl. Manag. Aquat. Ecosyst. 2011, 401, 28. [Google Scholar] [CrossRef] [Green Version]
- Adao, H.; Marques, J.C. Population Biology of the Red Swamp Crayfish Procambarus Clarkii (Girard, 1852) in Southern Portugal. Crustaceana 1993, 65, 336–345. [Google Scholar] [CrossRef]
- Correia, A.M.; Ferreira, O. Burrowing behaviour of the introduced red swamp crayfish Procambarus clarkii (Decapoda: Cambaridae) in Portugal. J. Crustac. Biol. 1995, 15, 248–257. [Google Scholar] [CrossRef]
- Aquiloni, L.; Ilhéu, M.; Gherardi, F. Habitat use and dispersal of the invasive crayfish Procambarus clarkii in ephemeral water bodies of Portugal. Mar. Freshw. Behav. Physiol. 2005, 38, 225–236. [Google Scholar] [CrossRef]
- Gherardi, F.; Tricarico, E.; Ilhéu, M. Movement patterns of an invasive crayfish, Procambarus clarkii, in a temporary stream of southern portugal. Ethol. Ecol. Evol. 2002, 14, 183–197. [Google Scholar] [CrossRef]
- Geiger, W.; Alcorlo, P.; Baltanás, A.; Montes, C. Impact of an introduced Crustacean on the trophic webs of Mediterranean wetlands. Biol. Invasions 2005, 7, 49–73. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis; Impensis Direct. Laurentii Salvii: Holmiæ [Stockholm], Sweden, 1758. [Google Scholar]
- Herbst, J.F.W. Versuch einer Naturgeschichte der Krabben und Krebse: Nebst einer Systematischen Beschreibung ihrer Verschiedenen Arten; Bei Gottlieb August Lange: Berlin, Germany, 1790. [Google Scholar]
- Fabricius, J.C. Mantissa Insectorum Sistens Eorum Species Nuper Detectas Adjectis Characteribus Genericis Differentiis Specificis, Emendationibus, Observationibus. Tome I; Christ. Gottl. Proft: Hafniae [København], Denmark, 1787. [Google Scholar]
- Montagu, G. Description of several marine animals found on the south coast of Devonshire. Trans. Linn. Soc. Lond. 1804, 7, 61–85. [Google Scholar] [CrossRef]
- Say, T. An account of the Crustacea of the United States. J. Acad. Nat. Sci. Phila. 1817, 1, 97–101. [Google Scholar]
- Leach, W.E. Malacostraca Podophthalmata Britanniae, or; Descriptions of such British Species of the Linnean Genus Cancer as have Their Eyes Elevated on Footstalks; Illustrated with Figures of All the Species by James Sowerby; J. Sowerby: London, UK, 1815. [Google Scholar]
- Kinahan, J.R. On Xantho rivulosa and other decapodous Crustacea occurring at Valentia Island, Co. Kerry. Proc. Dublin Nat. Hist. Soc. 1857, 4, 9–16. [Google Scholar]
- Ahyong, S.T. Range extension of two invasive crab species in eastern Australia: Carcinus maenas (Linnaeus) and Pyromaia tuberculata (Lockington). Mar. Pollut. Bull. 2005, 50, 460–462. [Google Scholar] [CrossRef]
- Carlton, J.T.; Cohen, A.N. Episodic Global Dispersal in Shallow Water Marine Organisms: The Case History of the European Shore Crabs Carcinus maenas and C. aestuarii. J. Biogeogr. 2003, 30, 1809–1820. [Google Scholar] [CrossRef]
- Klassen, G.; Locke, A. A Biological Synopsis of the European Green Crab, Carcinus maenas. Can. Manuscr. Rep. Fish. Aquat. Sci. 2007, 2818, 1–82. [Google Scholar] [CrossRef]
- Sousa, R.; Dias, S.; Antunes, J.C. Spatial subtidal macrobenthic distribution in relation to abiotic conditions in the Lima estuary, NW of Portugal. Hydrobiologia 2006, 559, 135–148. [Google Scholar] [CrossRef]
- Carvalho, A.N.; Santos, P.T. Factors affecting the distribution of epibenthic biodiversity in the Cávado estuary (NW Portugal). Rev. Gestão Costeira Integr. 2013, 13, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Amaral, V.; Cabral, H.N.; Jenkins, S.; Hawkins, S.; Paula, J. Comparing quality of estuarine and nearshore intertidal habitats for Carcinus maenas. Estuar. Coast. Shelf Sci. 2009, 83, 219–226. [Google Scholar] [CrossRef]
- Anastasiadou, C.; Koukouras, A.; Mavidis, M.; Chartosia, N.; Mostakim, M.; Christodoulou, M.; Aslanoglou, C. Morphological variation in Atyaephyra desmarestii (Millet, 1831) within and among populations over its geographical range. Mediterr. Mar. Sci. 2004, 5, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Christodoulou, M.; Antoniou, A.; Magoulas, A.; Koukouras, A. Revision of the freshwater genus Atyaephyra (Crustacea, Decapoda, Atyidae) based on morphological and molecular data. Zookeys 2012, 229, 53–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafinesque, C.S. Précis des Découvertes et Travaux Somiologiques de m.r C. S. Rafinesque-Schmaltz Entre 1800 et 1814 ou Choix Raisonné de Ses Principales Découvertes en Zoologie et en Botanique, Pour Servir D’introduction à Ses Ouvrages Futurs; Royal Typographie Militaire: Palerme, Italy, 1814. [Google Scholar]
- Millet, P.A. Description d’une nouvelle espèce de Crustacé, l’Hippolyte de Desmarets. Mémoires Société D’agriculture Sci. Arts d’Angers 1831, 1, 55–57. [Google Scholar]
- Joly, M. Études sur les moeurs, le développement et les métamorphoses d’une petite salicoque d’eau douce (Caridina desmarestii). Ann. Sci. Nat. Zool. 1843, 19, 34–86. [Google Scholar]
- De Brito Capelo, F. Descripção de Algumas Especies Novas ou Pouco Conhecidas de Crustaceos e Arachnidios de Portugal e Possessões Portuguezas do Ultramar; Typ. da Academia: Lisboa, Portugal, 1866. [Google Scholar]
- Bouvier, E.L. Les variations d’une crevette de la famille des Atyidés, l’Atyaephyra desmaresti Millet. Bull. du Muséum Natl. d’Histoire Nat. 1913, 19, 65–74. [Google Scholar]
- Fidalgo, M.L.; Gerhardt, A. Distribution of the freshwater shrimp, Atyaephyra desmarestii (Millet, 1831) in Portugal (Decapoda, Natantia). Crustaceana 2002, 75, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Fabricius, J.C. Entomologia Systematica emendata et aucta, secundum classes, ordines, genera, species adjectis synonimis locis observationibus descriptionibus. Hafniae: Impensis Christ. Gottl. Proft. 1798, 1–572. [Google Scholar]
- Risso, A. Histoire Naturelle des Crustacés des Environs de Nice; Librairie Grecque—Latine-Allemande: Paris, France, 1816. [Google Scholar]
- Rathke, H. Zur Fauna der Krym. III; Mémoires de l’Académie Impériale des Sciences de Saint Pétersbourg: Tartu, Estonia, 1837. [Google Scholar] [CrossRef] [Green Version]
- Czerniavsky, V. Materialia ad Zoographiam Ponticam Comparatam. Fasc II. Crustacea Decapoda Pontica Littoralia; Universitetskoi Tip: Kharkov, Ukrayne, 1884; Supplement XIII. [Google Scholar]
- Campos, J.; Freitas, V.; Pedrosa, C.; Guillot, R.; van der Veer, H.W. Latitudinal variation in growth of Crangon crangon (L.): Does counter-gradient growth compensation occur? J. Sea Res. 2009, 62, 229–237. [Google Scholar] [CrossRef]
- Quintaneiro, C.; Monteiro, M.; Pastorinho, R.; Soares, A.M.V.M.; Nogueira, A.J.A.; Morgado, F.; Guilhermino, L. Environmental pollution and natural populations: A biomarkers case study from the Iberian Atlantic coast. Mar. Pollut. Bull. 2006, 52, 1406–1413. [Google Scholar] [CrossRef]
- Viegas, I.; Marques, S.C.; Bessa, F.; Primo, A.L.; Martinho, F.; Azeiteiro, U.M.; Pardal, M.Â. Life history strategy of a southern European population of brown shrimp (Crangon crangon L.): Evidence for latitudinal changes in growth phenology and population dynamics. Mar. Biol. 2012, 159, 33–43. [Google Scholar] [CrossRef]
- Moreira, F.; Assis, C.A.; Almeida, P.R.; Costa, J.L.; Costa, M.J. Trophic relationships in the community of the upper Tagus estuary (Portugal): A preliminary approach. Estuar. Coast. Shelf Sci. 1992, 34, 617–623. [Google Scholar] [CrossRef]
- Luttikhuizen, P.C.; Campos, J.; van Bleijswijk, J.; Peijnenburg, K.T.C.A.; van der Veer, H.W. Phylogeography of the common shrimp, Crangon crangon (L.) across its distribution range. Mol. Phylogenet. Evol. 2008, 46, 1015–1030. [Google Scholar] [CrossRef]
- Milne Edwards, H. Histoire Naturelle des Crustacés, Comprenant l´Anatomie, la Physiologie et la Classification de ces Animaux Tome 2; Encyclopédique Roret: Paris, France, 1837. [Google Scholar]
- Pennant, T. British Zoology. A New Edition in Four Volumes. Class V. In Crustacea. Class VI. Vermes; Wilkie & Robinson: London, UK, 1812; Volume IV. [Google Scholar]
- Heller, C. Die Crustaceen des Südlichen Europa. Crustacea Podophthalmia. In Mit Einer Übersicht Über Die Horizontale Verbreitung Sämmtlicher Europäischer Arten; Wilhelm Braumüller: Wien, Austria, 1863. [Google Scholar]
- Fischer, P. Crustacés podopthalmaires et cirrhipèdes du Départment de la Gironde. Actes Société Linnéenne Bordx. 1872, 28, 405–438. [Google Scholar]
- Cartaxana, A. Distribution and migrations of the prawn Palaemon longirostris in the Mira River estuary (southwest Portugal). Estuaries 1994, 17, 685–694. [Google Scholar] [CrossRef]
- Neves, A.; Cabral, H.N.; Gordo, L.S. Distribution and Abundance Patterns of Decapod crustaceans in the Sado estuary, Portugal. Crustaceana 2007, 80, 97–112. [Google Scholar]
- França, S.; Pardal, M.A.; Cabral, H.N. Mudflat nekton assemblages in the Tagus estuary (Portugal): Distribution and feeding patterns. Sci. Mar. 2008, 72, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Williamson, D.I.; Rochanaburanon, T. A new species of Processidae (Crustacea, decapoda, caridea) and the larvae of the north european species. J. Nat. Hist. 1979, 13, 11–33. [Google Scholar] [CrossRef]
- Sampaio, L.; Mamede, R.; Ricardo, F.; Magalhães, L.; Rocha, H.; Martins, R.; Dauvin, J.C.; Rodrigues, A.M.; Quintino, V. Soft-sediment crustacean diversity and distribution along the Portuguese continental shelf. J. Mar. Syst. 2016, 163, 43–60. [Google Scholar] [CrossRef] [Green Version]
- Sars, G.O. Middelhavets Cumaceer. Arch. Math. Og Nat. 1878, 3, 461–512. [Google Scholar]
- Stebbing, T.R.R. Cumacea (Sympoda); R. Friedländer und Sohn: Berlin, Germany, 1913. [Google Scholar]
- Corbera, J.; Garcia-Rubies, A. Cumaceans (Crustacea) of the Medes Islands (Catalonia, Spain) with special attention to the genera Bodotria and Iphinoe. Sci. Mar. 1998, 62, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.A.; Andrade, F. Intertidal communities as indicators of environmental change and their potential use in biomonitoring: The Troia Resort (Portugal), a large-scale tourist development, as a case study. Bol.-Inst. Esp. Oceanogr. 2003, 19, 253–264. [Google Scholar]
- Dexter, D.M. Soft bottom invertebrates of the Portuguese benthos. Buletim Inst. Nac. Investig. Pescas 1992, 17, 61–68. [Google Scholar]
- Carvalho, S.; Barata, M.; Gaspar, M.B.; Pousão-Ferreira, P.; Cancela da Fonseca, L. Enrichment of aquaculture earthen ponds with Hediste diversicolor: Consequences for benthic dynamics and natural productivity. Aquaculture 2007, 262, 227–236. [Google Scholar] [CrossRef]
- Cruz, S.; Gamito, S.; Marques, J.C. Spatial distribution of peracarids in the intertidal zone of the Ria Formosa (Portugal). Crustaceana 2003, 76, 411–431. [Google Scholar] [CrossRef]
- Goodsir, H. Description of the genus Cuma, and of two new genera nearly allied to it. Edinb. New Philos. J. 1843, 34, 119–129. [Google Scholar]
- Bate, C.S. XLI.—On the British Diastylidæ. Ann. Mag. Nat. Hist. 1856, 17, 449–465. [Google Scholar] [CrossRef]
- Reis, C.; Marques, V.; Calvário, J.; Marques, J.C.; Melo, R.; Santos, R. Contribuçao para o estudo dos povoamentos bentónicos (substrato rochoso) da costa ocidental portuguesa. Oecologia Aquat. 1982, 6, 119–145. [Google Scholar]
- Norman, A.M. VI.—Crustacea Cumacea of the ‘Lightning’, ‘Porcupine’, and ‘Valorous’ Expeditions. Ann. Mag. Nat. Hist. 1879, 3, 54–73. [Google Scholar] [CrossRef]
- Sars, G.O. An Account of the Crustacea of Norway, with Short Descriptions and Figures of All the Species, Cumacea; Bergen Museum: Bergen, NJ, USA, 1900; Volume 3. [Google Scholar]
- Boeck, A. Beskrivelse og fremlagde Tegninger af 4 norske Decapoder, undersogte af Overlaege Danielssen og ham. Forhandlinger i Videnskabs-Selskabet i Christiana. Forh. Vidensk. Christ. 1863, 1962–1963, 189–190. [Google Scholar]
- Sars, G.O. Om den aberrante Krebsdyrgruppe Cumacea og dens nordiske Arter. Forh. Vidensk. Christ. 1864, 1864–65, 128–208. [Google Scholar]
- Norman, A.M. On British Mysidae, a family of Crustacea Schizopoda. Ann. Mag. Nat. Hist. Zool. Bot. Geol. 1892, 10, 242–263. [Google Scholar] [CrossRef]
- Goës, A. Crustacea decapoda podophthalma marina Sueciæ, interpositis speciebus norvegicus aliisque vicinis, enumerat. In Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar; Norstedt & Soner: Stockholm, Sweeden, 1864; Volume 20, pp. 161–180. [Google Scholar]
- Sim, G. Stalk-eyed Crustacea of the north-east coast of Scotland. Scotitsh Nat. 1872, 1, 182–190. [Google Scholar]
- Stebbing, T.R.R. Gastrosaccus spiniferus Goës, newly described and figured. Ann. Mag. Nat. Hist. Zool. Bot. Geol. 1880, 6, 114–118. [Google Scholar] [CrossRef]
- Cunha, M.; Sorbe, J.; Moreira, M. Spatial and seasonal changes of brackish peracaridan assemblages and their relation to some environmental variables in two tidal channels of the Ria de Aveiro (NW Portugal). Mar. Ecol. Prog. Ser. 1999, 190, 69–87. [Google Scholar] [CrossRef] [Green Version]
- Tattersall, W.M.; Tattersall, O.S. The British Mysidacea; Ray Society: London, UK, 1951. [Google Scholar]
- Leach, W.E. Crustaceology. In The Edinburgh Encyclopaedia; Brewster, D., Ed.; Balfour: Edinburgh, UK,, 1814. [Google Scholar]
- Thompson, J.V. Zoological Researches and Illustrations; or Natural History of Nondescript or Imperfectly Known Animals, in a Series of Memoirs; King and Ridings: Cork, Ireland, 1829. [Google Scholar]
- Czerniavsky, V. Monographia Mysidarum inprimis Imperii Rossici. Fasc. 1, 2. Tr. St.-Peterbg. Obs. Estestvoispyt. 1882, 12, 1–170. [Google Scholar]
- Remerie, T.; Vierstraete, A.; Weekers, P.H.H.; Vanfleteren, J.R.; Vanreusel, A. Phylogeography of an estuarine mysid, Neomysis integer (Crustacea, Mysida), along the north-east Atlantic coasts. J. Biogeogr. 2009, 36, 39–54. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Meireles, S.; Pereira, T.; Gama, A.; Quintino, V. Spatial patterns of benthic macroinvertebrates in intertidal areas of a Southern European estuary: The Tagus, Portugal. Hydrobiologia 2006, 555, 99–113. [Google Scholar] [CrossRef]
- Sars, G.O. Undersøgelser over Christiania-fjordens Dybvansfauna anstillede paa en i Sommeren 1868 foretagen Zoologisk Reise. Nyt. Mag. Nat. 1869, 16, 305–362. [Google Scholar]
- Keeble, F.; Gamble, F.W.X. The colour-physiology of higher crustacea. Philos. Trans. R. Soc. B Biol. Sci. 1904, 196, 295–388. [Google Scholar]
- Cardoso, P.G.; D’Ambrosio, M.; Marques, S.C.; Azeiteiro, U.M.; Coelho, J.P.; Pereira, E. The effects of mercury on the dynamics of the peracarida community in a temperate coastal lagoon (Ria de Aveiro, Portugal). Mar. Pollut. Bull. 2013, 72, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Norman, C.A.M. XXII.—A new Heterotanais and a new Eurydice, genera of Isopoda. Ann. Mag. Nat. Hist. 1906, 17, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Holdich, D.M.; Jones, J.A. The distribution and ecology of British shallow-water tanaid crustaceans (Peracarida, tanaidacea). J. Nat. Hist. 1983, 17, 157–183. [Google Scholar] [CrossRef]
- Krøyer, H. Nye Arter af Slaegten Tanais. Nat. Tidsskr. Ser. I 1842, 4, 167–188. [Google Scholar]
- Müller, F. Tanais rhynchites und balticus, neue Arten aus der Ostsee. Arch. Nat. 1852, 18, 87–90. [Google Scholar]
- Risso, A. Histoire Naturelle des Principales Productions de l’Europe Méridionale et Particulièrement de Celles des Environs de Nice et des Alpes Maritimes; Tome V; Chez F.G. Levrault, Libraire: Paris, France, 1826. [Google Scholar]
- Dahl, E. Crustacea Leptostraca, principles of taxonomy and a revision of european shelf species. Sarsia 1985, 70, 135–165. [Google Scholar] [CrossRef]
- Moreira, J.; Quintas Pérez, P.; Souza Troncoso, J. Sobre la presencia de Nebalia strausi Risso, 1826 (Crustacea, Leptostraca) en la Península Ibérica. Boletín Real Soc. Española Hist. Nat. Sección Biológica 2004, 99, 83–92. [Google Scholar]
- Moreira, J.; Abad, L.M.; Riera, R. Presencia de Nebalia strausi Risso, 1826 (Crustacea: Leptostraca) en las Islas Canarias. Rev. Acad. Canar. Ciencias 2009, 21, 99–108. [Google Scholar]
- McCormack, E.; Ashelby, C.W.; McGrath, D. A review of the Leptostraca of the British Isles with discussion of the genus Sarsinebalia Dahl. Nauplius 2016, 24, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Moreira, J.; Gestoso, L.; Troncoso, J.S. Two new species of Sarsinebalia (Crustacea, Leptostraca) from the Northeast Atlantic, with comments on the genus. Sarsia 2003, 88, 189–209. [Google Scholar] [CrossRef]
- Darwin, C. A Monograph on the Sub-Class Cirripedia; The Ray Society: London, UK, 1854. [Google Scholar]
- Foster, B.A. The Marine Fauna of New Zealand: Barnacles (Cirripedia: Thoracica). Mem. N. Z. Oceanogr. Inst. 1978, 69, 1–143. [Google Scholar]
- Hutton, F.W. List of the New Zealand Cirripedia in the Otago Museum. Trans. Proc. N. Z. Inst. 1879, 11, 285–293. [Google Scholar]
- O’Riordan, R.M.; Ramsay, N.F. The current distribution and abundance of the Australasian barnacle Elminius modestus in Portugal. J. Mar. Biol. Assoc. UK 1999, 79, 937–939. [Google Scholar] [CrossRef]
- Wirtz, P.; Araújo, R.; Southward, A.J. Cirripedia of Madeira. Helgol. Mar. Res. 2006, 60, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Kussakin, O.G. Morskye I Solonovatovodnye Ravnonogie Rakoobrasnye (Isopoda) Cholodnix I Umerennix Vod Severnogo Polushariya. In [Marine and Brackish Isopods (Isopoda) of Cold and Temperate Waters of the Northern Hemisphere] Podotryad Asellota. Chast 2 Janiridae, Santiidae, 1st ed.; Semeistva Opredeliteli po Faune SSSR, Izdavaemye Zoologicheskim Institutom Academii Nauk SSSR, 152; Nauka: Leningrad, Russia, 1988. [Google Scholar]
- Hansen, H.J. Crustacea Malacostraca. III–V. The order Isopoda. In The Danish Ingolf–Expedition; H. Hagerup: Copenhagen, Denmark, 1916; Volume 3, pp. 3–262. [Google Scholar]
- Fabricius, O. Fauna Groenlandica, Systematice Sistens Animalia Groenlandiae Occidentalis Hactenus Indagata, Quoad Nomen Specificum, Triviale, Vernaculumque; Synonyma Auctorum Plurium, Descriptionem, Locum, Victum, Generationem, Mores, Usum, Cap-turamque Singuli, Proutd; Ioannis Gottlob Rothe: Hafniae & Lipsiae, Germany, 1780. [Google Scholar]
- Kröyer, H. Grönlands Amfipoder. Det Konigelige Danske Vidensk. Selsk. Nat. Og Math. Afh. Ser. 4 1838, 7, 229–326. [Google Scholar]
- Sars, G.O. An Account of the Crustacea of Norway, 2, Isopoda; Cammermeyer: Christiania, Denmark, 1899. [Google Scholar]
- Richardson, H. A monograph on the isopods of North America. Bulletin of the United States. Bull. USA Natl. Mus. 1905, 54, 583–717. [Google Scholar]
- Forsman, B. Weitere Studien über die Rassen von Jaera albifrons Leach. Zool. Bidr. Fran Upps. 1949, 27, 449–463. [Google Scholar]
- Pereira, S.C.G. Diversidade e Biogeografia de Isópodes Intertidais de Substrato Rochoso na Costa Continental Portuguesa; Universidade do Porto: Porto, Portugal, 2004. [Google Scholar]
- Meinert, F.V.A.; Schiødte, J.C. Symbolæ ad Monographiam Cymotharum Crustaceorum Isopodum Familiæ; Scripserunt J. C. Schidte et Fr. Meinert; Typis H. H. Thiele: Hauniæ, Denmark, 1879. [Google Scholar]
- Carus, J.V. Prodromus Faunae Mediterraneae, Sive Descriptio Animalium Maris Mediterranei incolarum, Quam Comparata Silva Rerum Quatenus Innotuit, Adiectis Locis et Nominibus Vulgaribus Eorumque Auctoribus; E. Schweizerbart: Stuttgart, Germany, 1885. [Google Scholar]
- Barrois, T. Catalogue des Crustacés Marins Recuellis Aux Açores. In Durant les Mois D’août et de Septembre 1887; Le Bigot Frères: Lille, France, 1888. [Google Scholar]
- Sociedad Española de Historia Natural. Actas de la Sociedad Española de Historia Natural. Anales de la Sociedad Española de Historia Natural; Real Jardín Botánico: Madrid, Spain, 1890; Volume 19, pp. 1–197.
- Gourret, P. Les Lemodipodes et les Isopodes du golfe de Marseille. Ann. Musée d’Histoire Nat. Marseille Zool. 1891, 4, 1–44. [Google Scholar]
- Koelbel, C. Beitraege zur Kenntnis der Crustaceen der Canarischen Inseln. Ann. Nat. Mus. Wien 1892, 7, 105–116. [Google Scholar]
- Graeffe, E. Ubersicht der fauna des Golfes Von Triest. Arb. Zool. Inst. Univ. Wien Zool. Stn. Triest 1902, 13, 33–80. [Google Scholar]
- Brian, A. Noti su alcuni Crostacei parassiti dei pesci del Mediterraneo. Boll. Musei Zool. Anat. Comp. Della R. Univ. Genova 1902, 115, 1–16. [Google Scholar]
- Nobre, A. Subsídios para o estudo da fauna marinha do norte Portugal. Ann. Sci. Nat. 1903, 8, 37–94. [Google Scholar]
- Nobre, A. Subsídios para o estudo da fauna marinha do sul de Portugal. Ann. Sci. Nat. 1903, 8, 153–160. [Google Scholar]
- Trilles, J.P. Les Cymothoidae (Crustacea: Isopoda) du Monde (Prodrome pour une faune). Stud. Mar. 1991, 21, 5–288. [Google Scholar]
- Hadfield, K.A.; Bruce, N.L.; Smit, N.J. Redescription of poorly known species of Ceratothoa Dana, 1852 (Crustacea, Isopoda, Cymothoidae), based on original type material. Zookeys 2016, 592, 39–91. [Google Scholar] [CrossRef] [PubMed]
- Milne Edwards, H. Histoire Naturelle des Crustacés: Comprenant L’anatomie, la Physiologie et la Classification de ces Animaux Tome 3; Librairie Encyclopédique de Roret: Roret, Kenya; Paris, France, 1840. [Google Scholar]
- Lucas, H. Histoire naturelle des Animaux Articules. Exploration scientifique de l’Algerie pendant les annees 1840, 1841, 1842. Sci. Phys. Zool. 1849, 1, 1–403. [Google Scholar]
- Nierstrasz, H.F. Die Isopoden-Sammlung im Natuurhistorischen Reichsmuseum zu Leiden: I. Cymothoidae. Zool. Meded. 1915, 1, 71–108. [Google Scholar]
- Trilles, J.P. Les Cymothoidae (Isopoda, Flabellifera) des côtes françaises. (Systématique, faunistique, écologie et répartition géographique) I. Les Ceratothoinae Schiœdte et Meinert, 1883. Bull. Muséum Natl. D’histoire Nat. 1972, 91, 1191–1228. [Google Scholar]
- Trilles, J.P.; Raibaut, A. Sur les Cymothoidae (Isopoda, Flabellifera) parasites de poissons marins de Tunisie (2e note). Bull. Muséum Natl. D’histoire Nat. 1973, 114, 273–281. [Google Scholar]
- Hadfield, K.A.; Smit, N.J. Review of the global distribution and hosts of the economically important fish parasitic isopod genus Ceratothoa (Isopoda: Cymothoidae), including the description of Ceratothoa springbok n. sp. from South Africa. Int. J. Parasitol. 2020, 50, 899–919. [Google Scholar] [CrossRef]
- Naylor, E. Isopoda. Sub-Order: Flabellifera, Family: Gnathidae, Genera: Paragnathia, Gnathia, Family: Cirolanidae, Genus: Eurydice. In Fiches D’identification du Zooplancton; ICES, 78: Copenhagen, Denmark, 1957. [Google Scholar]
- Naylor, E. The comparative external morphology and revised taxonomy of the British species of Idotea. J. Mar. Biol. Assoc. UK 1955, 34, 467–493. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.; Marques, J.C.; Banha, M.M.; Cancela da Fonseca, L. Macrobenthic Crustacea of the Bay of S. Torpes (Portugal). Rev. Biol. 2003, 21, 57–70. [Google Scholar]
- Gonzalez, P.; Sanchez, M.I.; Chirivella, J.; Carbonell, E.; Riera, F.; Grau, A. A preliminary study on gill metazoan parasites of Dentex dentex (Pisces: Sparidae) from the western Mediterranean Sea (Balearic Islands). J. Appl. Ichthyol. 2004, 20, 276–281. [Google Scholar] [CrossRef]
- Tattersall, W.M. Die Nordischen Isopoden; Lipsius & Tischer: Kiel, Germany, 1911. [Google Scholar]
- Cabral, H.N. Comparative feeding ecology of sympatric Solea solea and S. senegalensis, within the nursery areas of the Tagus estuary, Portugal. J. Fish Biol. 2000, 57, 1550–1562. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Costa, A.; Cunha, R.; Gabriel, R.; Gonçalves, V.; Martins, A.; Melo, I.; Parente, M.; Raposeiro, P.; Rodrigues, P.; et al. Listagem dos Organismos Terrestres e Marinhos dos Açores—A List of the Terrestrial and Marine Biota from the Azores; Princípia: Cascais, Portugal, 2010; ISBN 9789898131751. [Google Scholar]
- Leach, W.E. A tabular view of the external characters of four classes of animals, which Linné arranged under Insecta; with the distribution of the genera composing three of these classes into orders, &c. and descriptions of several new genera and species. Trans. Linn. Soc. Lond. 1815, 11, 306–400. [Google Scholar]
- Dollfus, A. Les Idoteidae des côtes de France. Feuille Des Jeunes Nat. 1895, 25, 1–5, 17–18, 38–40, 53–56. [Google Scholar]
- Bos, J.R. Bijdrage Tot de Kennis van de Crustacea Hedriophthalmata van Nederland en Zijne Kusten/Door J. Ritzema Bos; J. B. Huber: Groningen, The Netherlands, 1874. [Google Scholar]
- Pereira, S.G.; Lima, F.P.; Queiroz, N.C.; Ribeiro, P.A.; Santos, A.M. Biogeographic patterns of intertidal macroinvertebrates and their association with macroalgae distribution along the Portuguese coast. Hydrobiologia 2006, 555, 185–192. [Google Scholar] [CrossRef]
- Khalaji-Pirbalouty, V.; Bruce, N.L.; Wägele, J.W. The genus Cymodoce Leach, 1814 (Crustacea: Isopoda: Sphaeromatidae) in the Persian Gulf with description of a new species. Zootaxa 2013, 3686, 501. [Google Scholar] [CrossRef] [Green Version]
- Dumay, D. Révision systématique du genre Cymodoce (Isopoda Flabellifera). I. Introduction et decription de deux espèces: Cymodoce truncata (Montagu) et C. pilosa Milne Edwards. Téthys 1972, 3, 638–654. [Google Scholar]
- Dumay, D. Révision systématique du genre Cymodoce (Isopoda Flabellifera), II. Descriptions de Cymodoce hanseni nov. sp. des cotes Méditerranéenes. Crustaceana 1972, Supplement 3, 198–206. [Google Scholar]
- Dumay, D. Révision systématique du genre Cymodoce (Isopoda Flabellifera) 3. Description de C. spinosa Risso et de C. emarginata sensu Torelli. Téthys 1972, 4, 127–144. [Google Scholar]
- Dumay, D. Révision systématique du genre Cymodoce (lsopoda Flabellifera) 4. Description de C. tattersalli Torelli, C. rubropunctata (Grube), C. tuberculata Costa. Clef systematique et conclusion générale. Téthys 1972, 4, 457–480. [Google Scholar]
- Mladineo, I.; Šegvić, T.; Grubišić, L. Molecular evidence for the lack of transmission of the monogenean Sparicotyle chrysophrii (Monogenea, Polyopisthocotylea) and isopod Ceratothoa oestroides (Crustacea, Cymothoidae) between wild bogue (Boops boops) and cage-reared sea bream (Sparus aurata). Aquaculture 2009, 295, 160–167. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, N.; Costa, D.A.; Cantallo, H.; Antunes, C. Crustaceans (Malacostraca and Thecostraca) from the International Minho River, Iberian Peninsula. Hydrobiology 2022, 1, 47-75. https://doi.org/10.3390/hydrobiology1010005
Gomes N, Costa DA, Cantallo H, Antunes C. Crustaceans (Malacostraca and Thecostraca) from the International Minho River, Iberian Peninsula. Hydrobiology. 2022; 1(1):47-75. https://doi.org/10.3390/hydrobiology1010005
Chicago/Turabian StyleGomes, Nuno, Dimítri A. Costa, Harold Cantallo, and Carlos Antunes. 2022. "Crustaceans (Malacostraca and Thecostraca) from the International Minho River, Iberian Peninsula" Hydrobiology 1, no. 1: 47-75. https://doi.org/10.3390/hydrobiology1010005





